- 1Department of Urology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 2Department of Urology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
- 3Yangzhou University, Yangzhou, China
- 4People's Liberation Army of China Rocket Force Characteristic Medical Center, Department of Urology, Beijing, China
Objective: Overactive bladder (OAB) is a disease characterized by the presence of urinary urgency. We carried out a meta-analysis to assess the effectiveness and safety of trigonal-involved injection of onabotulinumtoxinA (BoNT-A) in comparison with the trigonal-sparing technique in cases with OAB [neurogenic detrusor overactivity (NDO) and idiopathic detrusor overactivity (IDO)].
Methods: Randomized controlled trials (RCTs) of BoNT-A injection for OAB were searched systematically by using EMBASE, MEDLINE, and the Cochrane Controlled Trials Register. The datum was calculated by RevMan version 5.3.0. The original references of relating articles were also reviewed.
Results: In total, six RCTs involving 437 patients were included in our analysis. For OAB, the trigone-including group showed a different patient symptom score (p = 0.03), complete dryness rate (p = 0.002), frequency of incontinence episodes (p = 0.01), detrusor pressure at maximum flow rate (p = 0.01), and volume at the first desire to void (p = 0.0004) compared with the trigone-sparing group. Also, a trigone-including intradetrusor injection demonstrated a significant improvement in the patient symptom score (p = 0.0004), complete dryness rate (p = 0.0002), frequency of incontinence episodes (p = 0.0003), detrusor pressure at maximum flow rate (p = 0.01), and volume at the first desire to void (p = 0.00006) compared with the trigone-sparing group for treatment of NDO. The adverse events rates were similar in both groups.
Conclusions: The meta-analysis has demonstrated that trigone-including BoNT-A injection was more effective compared with the trigone-sparing injection for the treatment of OAB, especially for NDO.
Introduction
According to the International Continence Society (ICS), overactive bladder (OAB) is a disease characterized by the presence of urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection (UTI) or other clear pathology (1). OAB is a common disease that threatens the quality of human life severely. The symptoms of OAB were disturbing chronic disease and imposed an economic burden in many patients (2). The incidence of OAB increased significantly with age from 11% up to 20% between the ages of 40 and 60 years for both men and women (3, 4). OAB was considered as a symptom complex, and it includes idiopathic detrusor overactivity (IDO) or neurogenic detrusor overactivity (NDO). According to the ICS, IDO was defined as the occurrence of involuntary detrusor contractions during filling cystometry without infected and pathological changes. NDO was detrusor overactivity (DO) caused by various neurologic diseases such as brain tumors, dementia, and spinal cord injury (SCI) (5). Although the causes of NDO and IDO are different, they show many similar symptoms and treatment methods.
For treating this disease, there are many methods, including bladder and behavioral training, drug treatment, onabotulinumtoxinA (BoNT-A), neuromodulation, and surgical therapies. Bladder and behavioral training was the first-line treatment for OAB, and drug treatment, such as the use of anticholinergics, was the second-line treatment; low compliance can affect the treatment result in the mentioned two treatments (3). In these circumstances, onabotulinumtoxinA has been advised as an alternative treatment for patients.
BoNT-A is a poisonous potent neurotoxin produced by the anaerobic bacterium clostridium botulinum and related species (6). According to antigenic and serological classification, there are eight distinguishable exotoxins of BoNT (A, B, C1, C2, D, E, F, and G), and type A had a better effect in durative time (7). BoNT-A inhibits muscle contractions on muscle by binding strongly to presynaptic cholinergic nerve terminals at the neuromuscular junction to inhibit acetylcholine release.
According to the American Urological Association and European Association of Urology guidelines recommendations, BoNT-A has been recommended as an alternative treatment (8, 9). Intravesical BoNT-A injections have achieved the satisfactory clinical effect; it could increase bladder capacity, decreased sense of urgency, and improved other symptoms in patients with NDO or IDO (10–13). In the past 10 years, the drugs were injected into different sites of the bladder wall with a cystoscope. The most common adverse reactions following the administration of the toxin reported in the clinical research were urinary retention and urinary tract infection (14). The trigone of the bladder has a large number of sensory nerve fibers (15), which might improve effectiveness with trigonal injections. Recent research also showed that trigonal BoNT-A injection had a less adverse effect and shorter treatment time compared to that of bladder body injections (16).
BoNT-A injection is an effective alternative treatment. Unfortunately, there is no standardized way of injecting BoNT-A for the treatment of OAB. Therefore, a systematic review and a meta-analysis of randomized controlled trials (RCTs) were performed to assess the effectiveness and safety of trigonal-involved injection of BoNT-A comparison with the trigonal-sparing technique in cases with OAB (NDO and IDO).
Materials and Methods
Study Design
A systematic review of RCTs was carried out using the preferred reporting items for the meta-analyses (PRISMA) checklist (17). Main analyses included trigonal-including vs. trigonal-sparing. The subgroup analysis was IDO vs. NDO.
Search Strategy
We searched relevant RCTs looking at the use of BoNT-A intravesical injection for patients with OAB on PubMed (1997 to Oct. 2020), EMBASE (1997 to Oct. 2020), and the Cochran Central Register for Controlled Trials. Keywords and medical subjects were as follows: “onabotulinumtoxinA (BoNT-A),” “overactive bladder,” “neurogenic detrusor overactivity,” “idiopathic detrusor overactivity,” “randomized controlled trials,” and “meta-analysis.” Two authors completed the whole screening process independently, and if there was any dispute, articles would be sent to another author for evaluation. Relevant reference articles were also included. Furthermore, we reviewed original references of included texts.
Inclusion Criteria
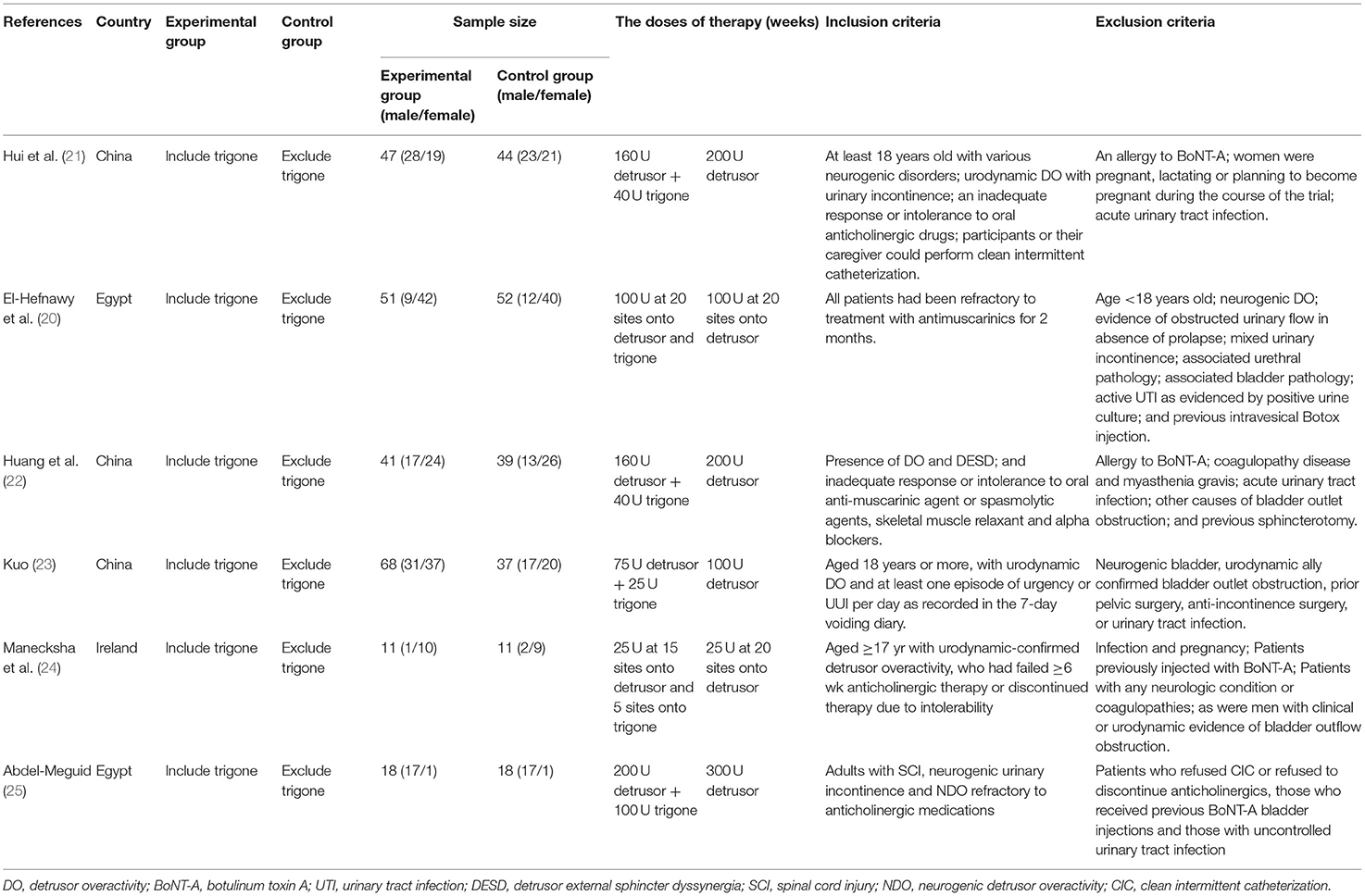
All of the included RCTs meet the following criteria: (a) the studies should have a connection with the topic “The effect of BoNT-A injections for patients with overactive bladder”; (b) the full useful texts are on RCTs; (c) accurate data could be obtained, and there are similar indicators between the trigone-including group and trigone-sparing group in every RCT. The following studies were excluded: (a) the data were incomplete; (b) the type of study was an abstract, review, comment, case-control, and cohort study. Furthermore, we included the most recently published studies if they described identical experiments. Every study was included if different measures were evaluated.
Quality Assessment
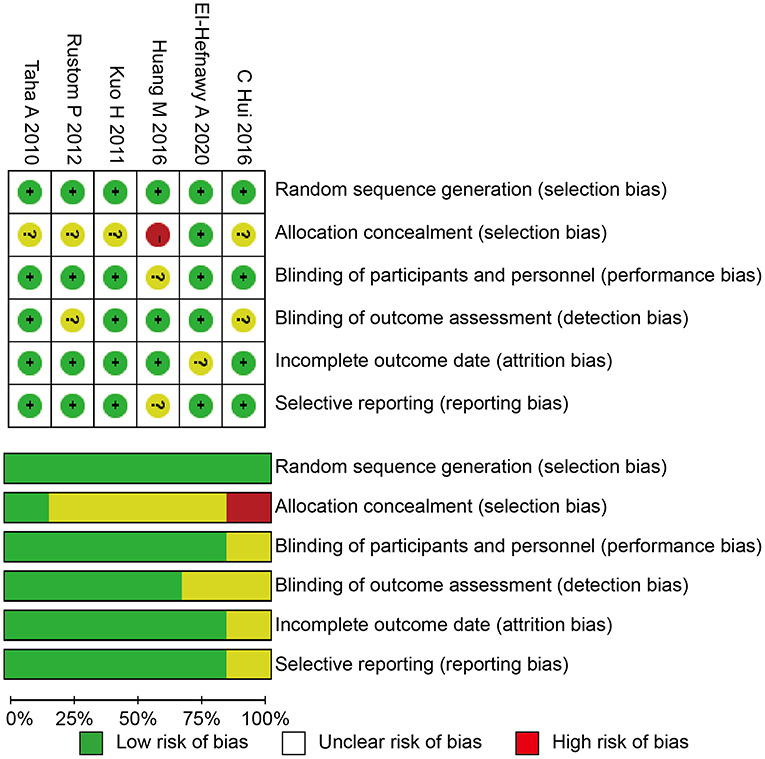
We used the Cochrane risk of bias tool to determine the quality of the retrieved RCTs (18). The quality items were selective outcome reporting, blinding, allocation concealment, incomplete outcome data, random sequence generation, and other sources of bias. A graph summarizing the risk of bias was generated based on discussions among the authors (shown in Figure 1). Then, according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions v.5.3.0, the studies were classified qualitatively (19). All of the authors participated in the quality assessment of all RCTs and agreed with the results. Meanwhile, the differences between each RCT were bridged through discussion among authors. All authors participated in the evaluation process and reached a consensus on the final results.
Data Extraction
The following information was collected for RCTs: (a) the general data in the test; (b) name of the first author; (c) published time; (d) the design of study and size of the sample; (e) the efficacious data in every article that changes in the following parameters, such as the impact on patient symptom score; impact on complete dryness rate; impact on change in the number of incontinence episodes; impact on detrusor pressure at maximum flow rate; impact on volume at the first desire to void; impact in maximum cystometric capacity; impact on post-void residual volume. Finally, another author checked the data extracted from the text. Meanwhile, our team cross-checked references and data for each included study to ensure there were no overlapping data and to maintain the meta-analysis integrity.
Statistical Analysis and Meta-Analysis
The abstracted data were analyzed with Review Manager 5.1.0 (The Cochrane Collaboration, London, UK) (19). The mean difference (MD) with a 95% confidence interval (CI) was utilized to analyze the continuous data and the odds ratio (OR), and 95% CI was applied to analyze the dichotomous data among the different groups. The chi-square based on Q statistic was performed to check the heterogeneity among the studies, and the result was recognized as significant at P < 0.05. The fixed-effects model was used and considered to be homogeneous if the result was p-value > 0.05. We utilized the I2 statistic to analyze inconsistent results, and it can reflect the proportion of heterogeneity across trials. In this meta-analysis, it is not necessary to have ethical approval and patient consent because all the data were acquired from articles that have already been published. When the I2 < 50%, this indicated that there was no significant heterogeneity and the fixed-effects model (Mantel-Haenszel method) would be used. And we performed the random-effects model (DerSimonian and Laird method) when the heterogeneity of the data could not be explained (p < 0.05, I2 > 50%).
Results
Characteristics of the Individual Studies
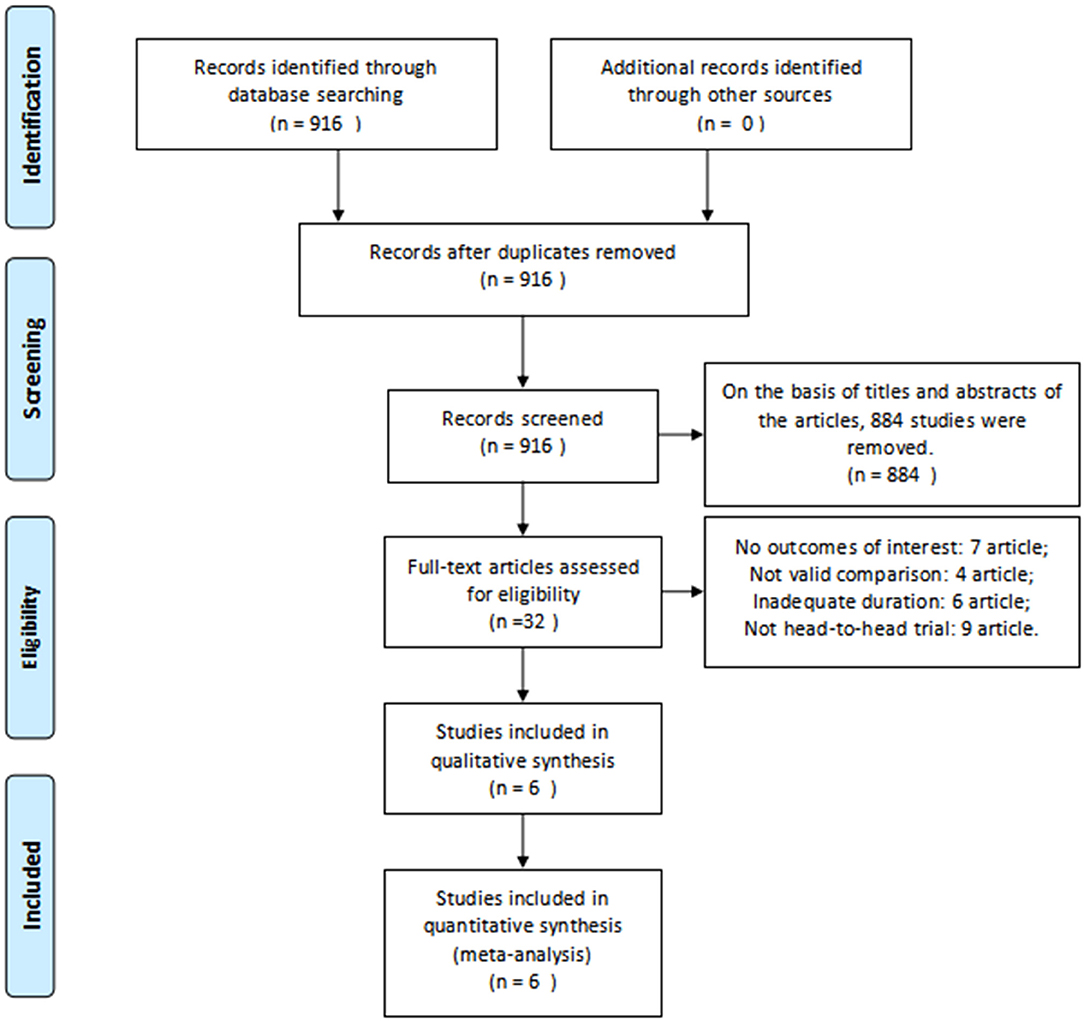
Based on the inclusion criteria above, we found 916 articles in the database. After reviewing the abstracts, 578 duplicate articles and 306 studies were removed after reviewing the titles and abstracts of the articles. In total, 26 studies were ruled out for a lack of useful data. Finally, six RCTs (20–25) involving 437 patients were included in our analysis. A detailed flowchart showing the selection process is shown in Figure 2. Table 1 shows the baseline characteristics of studies.
Quality of the Individual Studies
All of the included studies in the systematic review and meta-analysis were high-quality RCTs. Figure 1 presents a graphical summary of the risk bias. We have found that their randomization process has been developed in all studies. All RCTs were effective and determined the best sample size. The funnel plot displayed the conclusion of a qualitative estimation of publication bias of each RCT (Figure 3). Table 2 showed the specific inclusion and exclusion criteria.
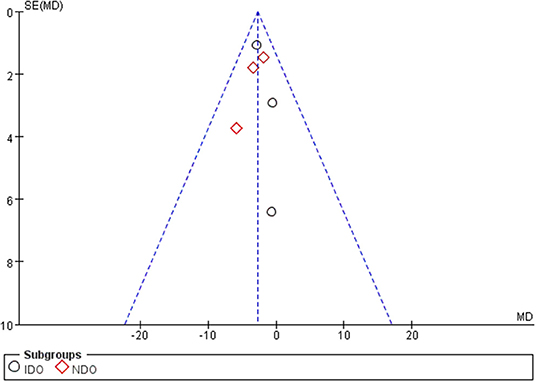
Figure 3. Funnel plot of the studies included in our meta-analysis. MD, mean difference; SE, standard error.
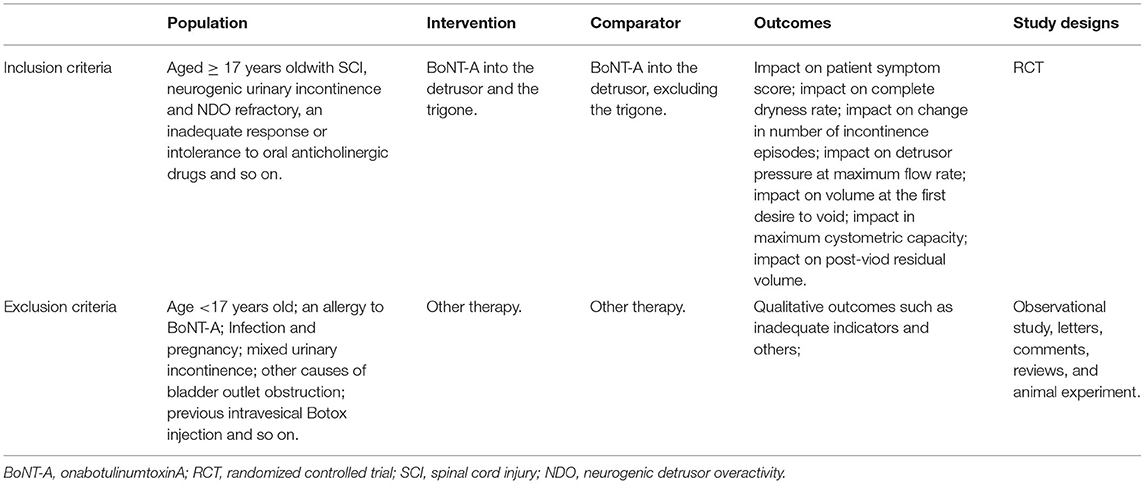
Table 2. Criteria for considering studies for the review based on the population, intervention, comparator, outcomes, and study designs (PICOS) structure.
Effectiveness
We analyzed the differences in the mean score of each domain for OAB to identify the efficacy of treatment with BoNT-A. Also, we analyzed the differences in treatment effects of BoNT-A for NDO and IDO.
Impact on Patient Symptom Score
Five RCTs involving 401 patients (218 in the trigone-including group and 183 in the trigone-sparing group) recorded the changes in impact on patient symptom score (Figure 4A). Since P < 0.05, we employed a random-effects model, which reflected an MD of −1.79 (95 CI%: −3.41 to −0.16, P = 0.03). The results suggest that the trigone-including group showed statistical differences in the impact on patient symptom score compared with the trigone-sparing group for OAB. A subgroup analysis revealed that trigone-including intradetrusor injection had no marked difference in the impact on patient symptom score compared with the trigone-sparing group for IDO (MD = −0.91, 95% CI: −2.28 to −0.70, P = 0.27). For NDO, it showed a significant difference between the two groups in the change of the impact on patient symptom score (MD = −6.97, 95% CI: −10.83 to −3.11, P = 0.0004).
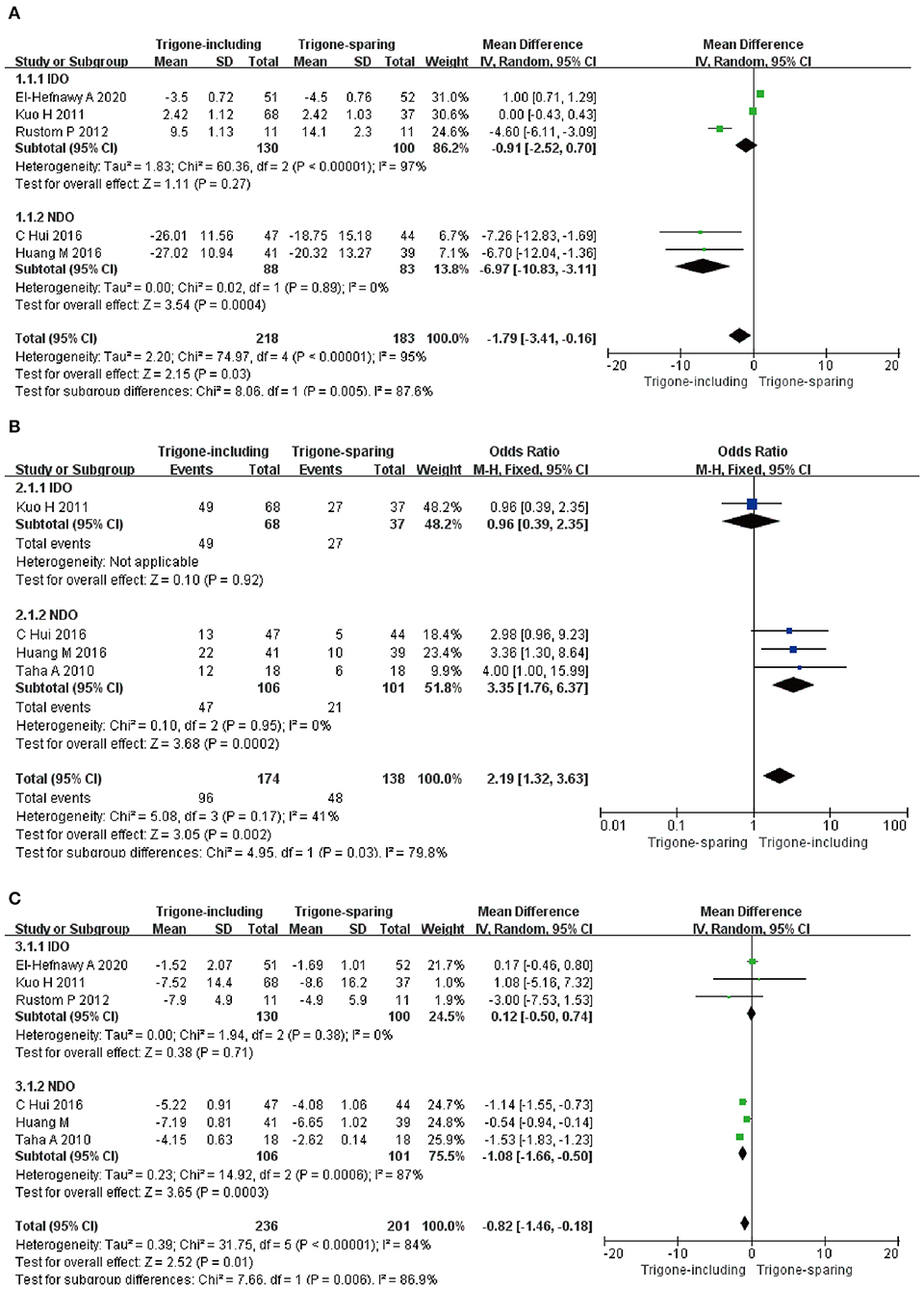
Figure 4. Forest plots showing changes between two groups in the impact on (A) patient symptom score, (B) complete dryness rate, and (C) change in the number of incontinence episodes; NDO, neurogenic detrusor overactivity; IDO, idiopathic detrusor overactivity; SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Impact on Complete Dryness Rate
Four RCTs involving 312 patients (174 in trigone-including group and 138 in trigone-sparing group) recorded the change in impact on complete dryness rate (Figure 4B). Since P > 0.05, we employed a fixed-effects model, which reflected an MD of 2.19 (95% CI: 1.32 to 3.63, P = 0.002). The results suggest that the trigone-including group showed statistical differences in the impact on complete dryness rate compared to the trigone-sparing group for OAB. A subgroup analysis revealed that trigone-including intradetrusor injection showed a significantly different impact on patient symptom score compared with the trigone-sparing group for NDO (MD = 3.35, 95% CI: 1.76 to 6.37, P = 0.0002).
Impact on Change in Number of Incontinence Episodes
A total of six RCTs involving 437 patients (236 in the trigone-including group and 201 in the trigone-sparing group) recorded the changes in impact on change in the number of incontinence episodes (Figure 4C). A random-effects model showed an MD of −0.82 (95% CI: −1.46 to 0.18, P = 0.01). It proved that the trigone-including group showed statistical differences in terms of the impact on change in the number of incontinence episodes for OAB. For IDO, a subgroup analysis revealed that trigone-including intradetrusor injection showed no differences in the impact on patient symptom score compared with the trigone-sparing group for IDO (MD = 0.12, 95% CI: −0.50 to 0.74, P = 0.71). For NDO, it showed statistical differences between the two groups (MD = −1.08, 95% CI: −1.66 to −0.50, P = 0.0003).
Impact on Detrusor Pressure at the Maximum Flow Rate
A total of six RCTs involving 437 patients (236 in the trigone-including group and 201 in the trigone-sparing group) recorded the changes in impact on detrusor pressure at the maximum flow rate (Figure 5A). Since P < 0.05, we employed a random-effects model, which reflected an MD of −4.47 (95% CI: −7.97 to −0.96, P = 0.01). The results suggest that the trigone-including group showed statistical differences in the impact on detrusor pressure at maximum flow rate compared with the trigone-sparing group for OAB. A subgroup analysis revealed that a trigone-including intradetrusor injection showed no marked differences in the impact on detrusor pressure at the maximum flow rate compared with the trigone-sparing group for IDO (MD = −9.01, 95% CI: −19.31 to 1.29, P = 0.09). Also, there were statistical differences in terms of impact on detrusor pressure at the maximum flow rate between the two groups for NDO (MD = −2.82, 95% CI: −4.95 to −0.68, P = 0.01).
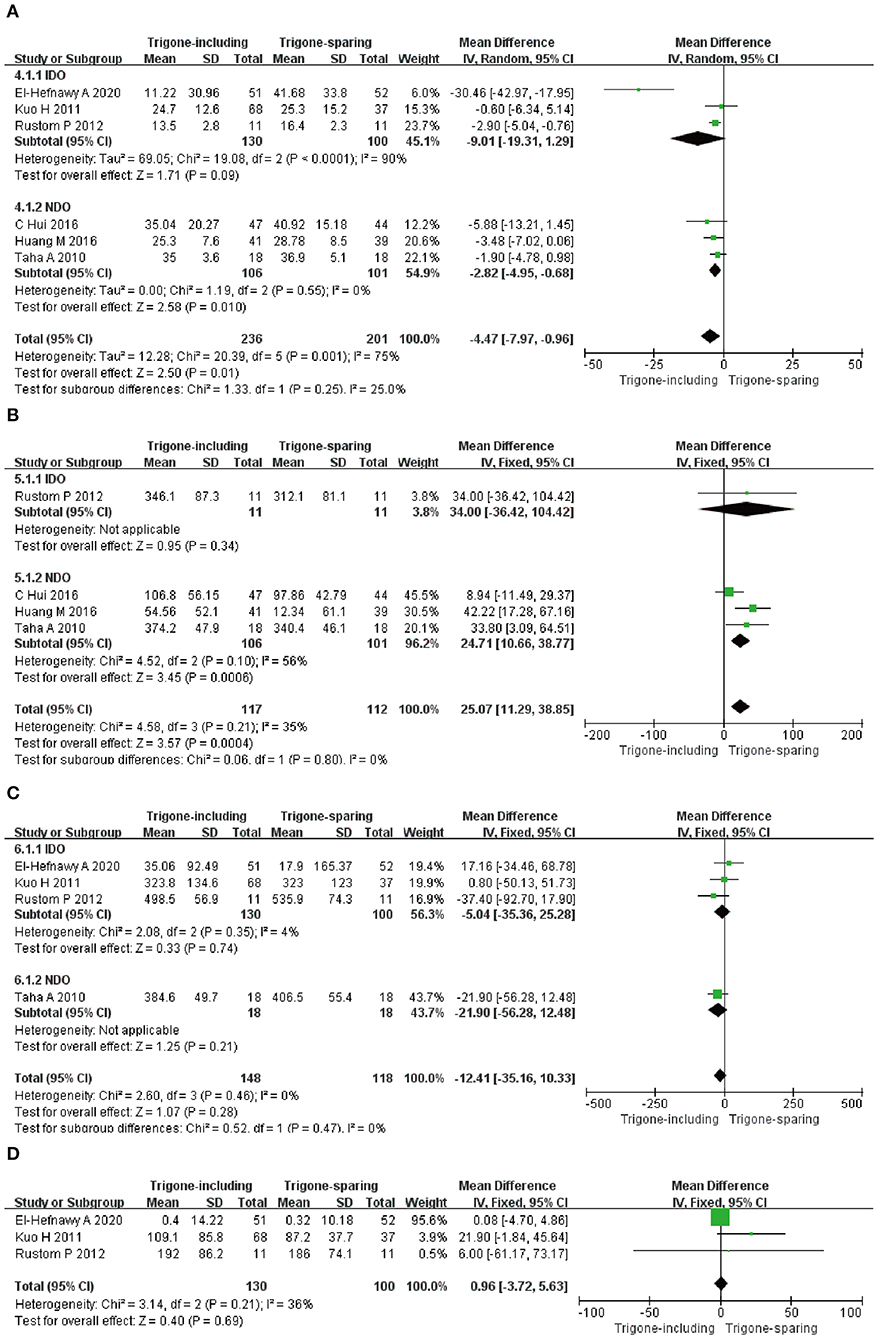
Figure 5. Forest plots showing changes between two groups in (A) impact on detrusor pressure at maximum flow rate, (B) impact on volume at the first desire to void, (C) impact in maximum cystometric capacity, (D) impact on post-void residual volume; NDO, neurogenic detrusor overactivity; IDO, idiopathic detrusor overactivity; SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
The Changes in Differences in the Impact on Volume at the First Desire to Void
A total of four RCTs involving 318 patients (117 in the trigone-including group and 201 in the trigone-sparing group) recorded the changes in impact on volume at the first desire to void (Figure 5B). Since P > 0.05, we employed a fixed-effects model, which reflected an MD of 25.07 (95% CI: 11.29 to 38.85, P = 0.0004). The results suggest that the trigone-including group showed statistical differences in the impact on the volume of the first desire to void compared with the trigone-sparing group for OAB. For NDO, a subgroup analysis showed statistical differences between the two groups (MD = 24.71, 95% CI: 10.66 to 38.77, P = 0.0006).
Impact in Maximum Cystometric Capacity
A total of four RCTs involving 266 patients (148 in trigone-including group and 118 in trigone-sparing group) recorded the changes in impact in terms of maximum cystometric capacity (Figure 5C). A fixed-effects model showed an MD of −12.41, 95% CI: −35.16 to 10.33, P = 0.28. It proved that the trigone-including group showed no differences in terms of the change in impact in maximum cystometric capacity. A fixed-effects model also showed that the trigone-including group showed no marked differences in the impact in maximum cystometric capacity with the trigone-sparing group for IDO (MD = −5.04, 95% CI: −35.36 to 25.28, P = 0.74).
Impact on Post-void Residual Volume
A total of three RCTs involving 230 participants (130 in the trigone-including group and 100 in the trigone-sparing group) recorded the changes in impact on the post-void residual volume (Figure 5D). A fixed-effects model showed an MD of 0.96, 95% CI: −3.72 to −5.63, P = 0.69. There were no statistical differences in terms of impact on post-void residual volume between the two groups.
Safety
Hematuria; General Weakness; Bladder Discomfort; Incidence of Large Post-void Residual; Urinary Tract Infection; Difficulty of Voiding
A total of five RCTs, including 301 participants (152 in the trigone-including group and 149 in the trigone-sparing group) were involved in the research for Hematuria. The OR of the study was 1.34, and the 95% CI was 0.63–2.85 (P = 0.45); three RCTs analyzed the incidence of the general weakness of 207 patients (106 in the trigone-including group and 101 in the trigone-sparing group) (OR = 1.15; 95%CI = 0.36–3.68; P = 0.82); four RCTs including 221 participants (111 in the trigone-including group and 110 in trigone-sparing group) were involved in the research for bladder discomfort (OR = 0.85; 95%CI = 0.40–1.79; P = 0.66); four RCTs including 233 patients (115 in the trigone-including group and 118 in the trigone-sparing group) were involved in the research for the incidence of large post-void residual (OR = 1.69; 95%CI = 0.54–5.26; P = 0.37); three RCTs analyzed the incidence of urinary tract infection of 197 patients (97 in the trigone-including group and 100 in the trigone-sparing group) (OR = 1.35, 95%CI = 0.60–3.03 P = 0.47); and three RCTs including 197 participants (97 in the trigone-including group and 100 in the trigone-sparing group) were involved in the research for difficulty of voiding (OR = 1.87, 95%CI = 0.88–4.00 P =0.10). These results indicate that there is no significant difference between the two groups in terms of Hematuria; general weakness; bladder discomfort; incidence of large post-void residual; urinary tract infection; difficulty of voiding. There were no statistical differences in terms of side-effects between the two groups (Figure 6).
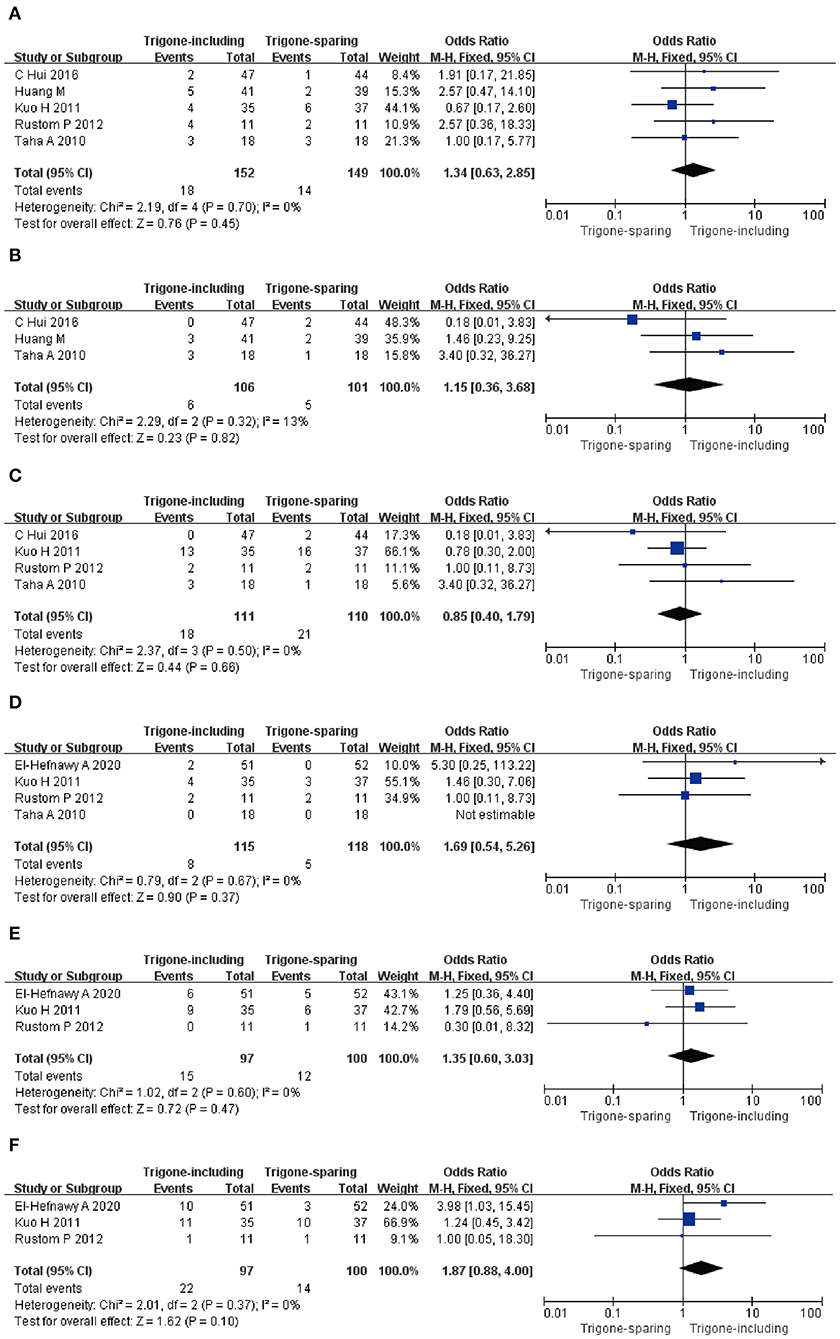
Figure 6. Forest plots showing changes between two groups in (A) hematuria, (B) general weakness, (C) bladder discomfort, (D) incidence of large post-void residual, (E) urinary tract infection, and (F) difficulty of voiding; SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Discussion
OAB is a series of clinical symptoms by urgency frequency with or without UUI that are caused by neurogenic or non-neurogenic factors (4). Behavioral and bladder training, drug therapy, botulinum toxin, electrical stimulation, biofeedback, or surgery can be used to treat patients with OAB. OAB is an illness that greatly compromises the quality of life of patients. Due to less effectiveness and poor adherence to first and second-line treatments, the main objective is the search for new treatments in OAB therapy. Nowadays, literature research reveals that BoNT-A intravesical injection was recommended as a third-line treatment of OAB (26, 27). Intravesical BoNT-A injections may represent a treatment modality for patients with NDO and IDO. Local drug delivery is one of the efficacious ways to lessen the side effects of systemic administration compared with anticholinergic therapy (inadequate efficacy or intolerable side effects).
In summary, for OAB, the trigone-including group showed differences in the patient symptom score (p = 0.03), complete dryness rate (p = 0.002), frequency of incontinence episodes (p = 0.01), detrusor pressure at maximum flow rate (p = 0.01), and volume at the first desire to void (p = 0.0004) compared with the trigone-sparing group. A trigone-including injection can improve the patient symptom score. Meanwhile, the higher complete dryness rate and a lower frequency of incontinence episodes in patients with a trigone-including intradetrusor injection can also increase the quality of life of patients. At the same time, the analysis demonstrated that the trigone-including group showed a similar result to the trigone-sparing group in terms of the aspects of maximum cystometric capacity (p = 0.28) and post-void residual volume (p = 0.69).
The dose of BoNT-A can affect the therapeutic effect. In this study, the high dose (150–300 U) of BoNT-A was used for the treatment of NDO, and it showed marked differences between the trigone-including group and trigone-sparing group. At the same time, there were no differences between the groups for treatment of IDO when a low dose (25–150 U) of BoNT-A was used. The dose of BoNT-A was different for the treatment of NDO and IDO, and we cannot be sure which one dose of BoNT-A was suitable for the treatment of OAB. The dose of 50 U demonstrated consistently lower non-sustained efficacy than doses of 100 U or higher (28). Meanwhile, the 150 U does or higher had the same clinical curative effect and not provided additional effect. Previously research can also show the high doses of BoNT-A can increase the risk of post-void residual(PVR) and associated urinary tract infection(UTI) in patients with OAB (29). Thus, we recommend to use a dose of 100 U for treatment of unexplained OAB.
In subgroup analyses, a trigone-including intradetrusor injection demonstrated significant improvement in patient symptom score (p = 0.0004), complete dryness rate (p = 0.0002), frequency of incontinence episodes (p = 0.0003), detrusor pressure at maximum flow rate (p = 0.01), and volume at the first desire to void (p = 0.0006) compared with the trigone-sparing group for treatment of NDO. That, the two methods did not differ for the treatment of IDO. This result showed that the trigone is potentially the optimal region where therapy should be directed.
BoNT-A, a potent neurotoxic protein, is a kind of neurotoxin that comes from the aerobic bacterium botulinum. There are several types of botulinum toxin, and type A is the most effective regarding duration. There are two influencing mechanisms: (a) it can regulate acetylcholine release from presynaptic neurons and inhibit the contractions of the detrusor or urethral sphincter; (b) it also regulates sensory nerve function by blocking the release of various noxious neurotransmitters, including adenosine triphosphate, calcitonin gene-related peptide, calcitonin gene-related peptide, and substance P (14). The BoNT-A consists of two pieces: 50-kDa light chain and a 100-kDa heavy endocytosis (30). The light chain of BoNT-A has biological activities. It cleaves the synaptosome-associated protein in the presynaptic nerve terminal and inhibits the release of acetylcholine by disrupting the fusion of vesicles with the neuron's cell membrane. This could finally lead to flaccid paralysis of muscles (31, 32). BoNT-A was injected into multiple sites within the bladder wall directly by cystoscopy. The most common adverse reactions following the administration of the toxin are urinary retention and urinary tract infection.
NDO was considered in relation to neural dysfunction. The motor neurons of the bladder include: (a) the sympathetic nerves, which are associated with an increment of bladder outlet obstruction during the storage phase of the micturition cycle; and (b) the parasympathetic nerves, which can produce spontaneous action potential and initiating spontaneous contraction of the bladder. New research indicates that the trigone was most densely innervated in the bladder. These nerves respond to passive distension and active contraction of the bladder and are the sensory component during a normal micturition cycle (33). Botulinum toxins have a direct role in the sensory nerve endings as well as the synaptic nerve junctions. Intravesical injections of BTX-A are useful for the treatment of bladder overactivity without the systemic adverse effects associated with pharmacotherapy (34). Thus, using trigonal injection might play a central role in treating NDO.
This meta-analysis found that the inclusion of the trigone in the injection pattern improves the outcome. Moreover, other studies reported that pressure directly on the trigone results in sensations of urgency, and trigonal injection of lidocaine or surgical denervation of the trigone relieved urgency in patients with OAB (35). This makes sense, as it is known that there is a rich source of afferent innervation in the trigone. These afferents originate primarily via the hypogastric nerve, suggesting a sympathetic nerve origin for the afferents responsible for urgency sensation arising from the trigone, whereas filling sensations arising from the bladder body are likely mixed in origin (parasympathetic and sympathetic) (36). Also, this meta-analysis revealed a greater effect in NDO than IDO, where it well-known that sensory nerve function is altered. We know that the sensory nerves innervate the bladder and are tuned to be mechanosensitive and that this signal activates areas of the spinal cord responsible for relaying sensation (37). Furthermore, studies have shown that the vortex infused into the bladder lumen is able to significantly attenuate this mechanosensitive bladder nerve activity without affecting the pressure/volume relationship during bladder filling (38). Thus, the analysis and conclusions of this meta-analysis are consistent with previous anatomical and physiological findings.
The incidence of adverse events between the trigone-including group and trigone-sparing group included hematuria (p = 0.45) and general weakness (p = 0.82); bladder discomfort (p = 0.66); incidence of large post-void residual (p = 0.37); urinary tract infection (p = 0.47); and difficulty of voiding (p = 0.10). The adverse events rates were similar in both groups. It is worth mentioning that trigone-including injections of BoNT-A may cause vesicoureteral reflux in theory; however, Mascarenhas et al. showed it did not cause vesicoureteral reflux in patients with OAB (39, 40). Meanwhile, the single injection of BTX-A only inflicts minor damage and does not induce bladder fibrosis or cause bladder tissue damage. The adverse reactions from long-term treatment remain to be elucidated (41).
One final but important point is that the results of injecting BoNT-A for treatment of OAB are affected by the dose of BoNT-A and the depth of injection. The work of Jo et al. presents a subgroup analysis according to the dose of BoNT-A. When a dosage of 200–300 units of BoNT-A was used, the endpoints of the symptom score, complete dryness, and frequency of incontinence episodes improved significantly in the trigone-including group. The depth of injection was determined by the surgeon with flexible or rigid cystoscopy. Jo et al. also reported that the depth of injection had no significant impact on the efficacy and safety between submucosal and detrusor injection (42). Submucosal injections have some advantages. It is easy to operate, does not require deep injection of the needle, and reduces the damage of the blood vessel. Thus, we recommend submucosal injection as a standard approach for the treatment of OAB (43).
This meta-analysis included six RCTs and concentrating on the efficacy and safety of BoNT-A for the treatment of overactive bladder. Compared with previous studies, our study had some advantages; the data were derived from randomized, double-blind controlled trials. Meanwhile, we also separately analyze the differences between IDO and NDO. However, this study also has some limitations, which reflect the common limitations of other systematic reviews and meta-analyses. This article did not include numerous patients and RCTs, which may affect our meta-analysis quality. Meanwhile, we will need more appropriate high-quality trials to improve the accuracy of results.
Conclusions
In summary, our meta-analysis has demonstrated that trigone-including BoNT-A injection was more effective compared with the trigone-sparing injection for the treatment of OAB, especially for NDO. There had been similar safety between the two methods.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TC and TD: conceptualization and methodology. ZZ: data curation. TC and YW: writing—original draft preparation. YL: visualization. ZG: investigation. XZ: supervision. YZ: software. JW and LD: validation. YC: writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Nature Science Foundation of China (Nos. 81870525; 81801429; and 81572835), Taishan Scholars Program of Shandong Province (No. tsqn201909199), Beijing Municipal Administration of Hospitals' Ascent Plan (Code: DFL20190502), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Code: ZYLX201820).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
OAB, overactive bladder; UUI, urgency urinary incontinence; UTI, urinary tract infection; NDO, neurogenic detrusor overactivity; DO, overactivity; SCI, spinal cord injury; BoNT-A, onabotulinumtoxinA; IDO, idiopathic detrusor overactivity; RCT, randomized controlled trial; MD, mean difference; SE, standard error; SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom; OR, odds ratio.
References
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. (2002) 21:167–78. doi: 10.1002/nau.10052
2. Onukwugha E, Zuckerman IH, McNally D, Coyne KS, Vats V, Mullins CD. The total economic burden of overactive bladder in the United States: a disease-specific approach. Am J Managed Care. (2009) 15(4Suppl.):S90–7.
3. Garely AD, Burrows LJ. Current pharmacotherapeutic strategies for overactive bladder. Expert Opin Pharmacother. (2002) 3:827–33. doi: 10.1517/14656566.3.7.827
4. Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. (2003) 20:327–36. doi: 10.1007/s00345-002-0301-4
5. Hagen EM Eide GE, Rekand T, Gilhus NE, Gronning M. Traumatic spinal cord injury and concomitant brain injury: a cohort study. Acta neurologica Scandinavica Supplementum. (2010) 190:51–7. doi: 10.1111/j.1600-0404.2010.01376.x
6. Montecucco C, Molgó, J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. (2005) 5:274–9. doi: 10.1016/j.coph.2004.12.006
7. Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. (2010) 55:8–14. doi: 10.4103/0019-5154.60343
8. Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. (2012) 188(6Suppl.):2455–63. doi: 10.1016/j.juro.2012.09.079
9. Lucas MG, Bosch RJ, Burkhard FC, Cruz F, Madden TB, Nambiar AK, et al. EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur Urol. (2012) 62:1130–42. doi: 10.1016/j.eururo.2012.08.047
10. Reitz A, Stöhrer M, Kramer G, Del Popolo G, Chartier-Kastler E, Pannek J, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. (2004) 45:510–5. doi: 10.1016/j.eururo.2003.12.004
11. Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: a systematic review and meta-analysis. Neurourol Urodyn. (2015) 34:413–9. doi: 10.1002/nau.22598
12. Yuan H, Cui Y, Wu J, Peng P, Sun X, Gao Z. Efficacy and adverse events associated with use of onabotulinumtoxina for treatment of neurogenic detrusor overactivity: a meta-analysis. Int Neurourol J. (2017) 21:53–61. doi: 10.5213/inj.1732646.323
13. Zhou X, Yan HL, Cui YS, Zong HT, Zhang Y. Efficacy and safety of onabotulinumtoxinA in treating neurogenic detrusor overactivity: a systematic review and meta-analysis. Chinese Med J. (2015) 128:963–8. doi: 10.4103/0366-6999.154318
14. Duthie JB, Vincent M, Herbison GP, Wilson DI, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Datab Syst Rev. (2011) 12:CD005493. doi: 10.1002/14651858.CD005493.pub3
15. Andersson KE. Bladder activation: afferent mechanisms. Urology. (2002) 59(5Suppl.1):43–50. doi: 10.1016/S0090-4295(01)01637-5
16. Kuo HC. Comparison of effectiveness of detrusor, suburothelial and bladder base injections of botulinum toxin a for idiopathic detrusor overactivity. J Urol. (2007) 178:1359–63. doi: 10.1016/j.juro.2007.05.136
17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
18. Vader JP. Randomised controlled trials: a user's guide. BMJ. (1998) 317:1258. doi: 10.1136/bmj.317.7167.1258
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
20. El-Hefnawy AS, Elbaset MA, Taha DE, Wadie BS, Kenawy M, Shokeir AA, Badry ME. Trigonal-sparing versus trigonal-involved Botox injection for treatment of idiopathic overactive bladder: a randomized clinical trial. Lower urinary tract symptoms. Adv Onl Publ. (2020) 13:22–30. doi: 10.1111/luts.12321
21. Hui C, Keji X, Chonghe J, Ping T, Rubiao O, Jianweng Z, et al. Combined detrusor-trigone BTX-A injections for urinary incontinence secondary to neurogenic detrusor overactivity. Spinal Cord. (2016) 54:46–50. doi: 10.1038/sc.2015.143
22. Huang M, Chen H, Jiang C, Xie K, Tang P, Ou R, et al. Effects of botulinum toxin A injections in spinal cord injury patients with detrusor overactivity and detrusor sphincter dyssynergia. J Rehabil Med. (2016) 48:683–7. doi: 10.2340/16501977-2132
23. Kuo HC. Bladder base/trigone injection is safe and as effective as bladder body injection of onabotulinumtoxinA for idiopathic detrusor overactivity refractory to antimuscarinics. Neurourol Urodyn. (2011) 30:1242–8. doi: 10.1002/nau.21054
24. Manecksha RP, Cullen IM, Ahmad S, McNeill G, Flynn R, McDermott TE, et al. Prospective randomised controlled trial comparing trigone-sparing versus trigone-including intradetrusor injection of abobotulinumtoxinA for refractory idiopathic detrusor overactivity. Eur Urol. (2012) 61:928–35. doi: 10.1016/j.eururo.2011.10.043
25. Abdel-Meguid TA. Botulinum toxin-A injections into neurogenic overactive bladder–to include or exclude the trigone? A prospective, randomized, controlled trial. J Urol. (2010) 184:2423–8. doi: 10.1016/j.juro.2010.08.028
26. Kasman A, Stave C, Elliott CS. Combination therapy in overactive bladder-untapped research opportunities: A systematic review of the literature. Neurourol Urodyn. (2019) 38:2083–92. doi: 10.1002/nau.24158
27. Huang AJ, Grady D, Mendes WB, Hernandez C, Schembri M, Subak LL, et al. Randomized controlled trial of device guided, slow-paced respiration in women with overactive bladder syndrome. J Urol. (2019) 202:787–94. doi: 10.1097/JU.0000000000000328
28. Dmochowski R, Chapple C, Nitti VW, Chancellor M, Everaert K, Thompson C, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. (2010) 184:2416–22. doi: 10.1016/j.juro.2010.08.021
29. Cohen BL, Barboglio P, Rodriguez D, Gousse AE. Preliminary results of a dose-finding study for botulinum toxin-A in patients with idiopathic overactive bladder: 100 versus 150 units. Neurourol Urodyn. (2009) 28:205–8. doi: 10.1002/nau.20611
30. Franciosa G, Floridi F, Maugliani A, Aureli P. Differentiation of the gene clusters encoding botulinum neurotoxin type A complexes in Clostridium botulinum type A, Ab, and A(B) strains. Appl Environ Microbiol. (2004) 70:7192–9. doi: 10.1128/AEM.70.12.7192-7199.2004
31. Dolly JO, O'Connell MA. Neurotherapeutics to inhibit exocytosis from sensory neurons for the control of chronic pain. Curr Opin Pharmacol. (2012) 12:100–8. doi: 10.1016/j.coph.2011.11.001
32. Rummel A. The long journey of botulinum neurotoxins into the synapse. Toxicon. (2015) 107:9–24. doi: 10.1016/j.toxicon.2015.09.009
33. Purves JT, Spruill L, Rovner E, Borisko E, McCants A, Mugo E, et al. A three dimensional nerve map of human bladder trigone. Neurourol Urodyn. (2017) 36:1015–9. doi: 10.1002/nau.23049
34. Chancellor MB, Elovic E, Esquenazi A, Naumann M, Segal KR, Schiavo G, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of urologic conditions. Toxicon. (2013) 67:129–40. doi: 10.1016/j.toxicon.2013.01.020
35. Westney OL, Lee JT, McGuire EJ, Palmer JL, Cespedes RD, Amundsen CL. Long-term results of Ingelman-Sundberg denervation procedure for urge incontinence refractory to medical therapy. J Urol. (2002) 168:1044–7. doi: 10.1016/S0022-5347(05)64571-5
36. Yamaguchi K, Kobayashi M, Kato T, Akita K. Origins and distribution of nerves to the female urinary bladder: new anatomical findings in the sex differences. Clin Anat. (2011) 24:880–5. doi: 10.1002/ca.21186
37. Grundy L, Harrington AM, Caldwell A, Castro J, Staikopoulos V, Zagorodnyuk VP, et al. Translating peripheral bladder afferent mechanosensitivity to neuronal activation within the lumbosacral spinal cord of mice. Pain. (2019) 160:793–804. doi: 10.1097/j.pain.0000000000001453
38. Collins VM, Daly DM, Liaskos M, McKay NG, Sellers D, Chapple C, et al. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int. (2013) 112:1018–26. doi: 10.1111/bju.12266
39. Mascarenhas F, Cocuzza M, Gomes CM, Leão N. Trigonal injection of botulinum toxin-A does not cause vesicoureteral reflux in neurogenic patients. Neurourol Urodyn. (2008) 27:311–4. doi: 10.1002/nau.20515
40. Karsenty G, Elzayat E, Delapparent T, St-Denis B, Lemieux MC, Corcos J. Botulinum toxin type a injections into the trigone to treat idiopathic overactive bladder do not induce vesicoureteral reflux. J Urol. (2007) 177:1011–4. doi: 10.1016/j.juro.2006.10.047
41. Apostolidis A, Jacques TS, Freeman A, Kalsi V, Popat R, Gonzales G, et al. Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol. (2008) 53:1245–53. doi: 10.1016/j.eururo.2008.02.037
42. Jo JK, Kim KN, Kim DW, Kim YT, Kim JY, Kim JY. The effect of onabotulinumtoxinA according to site of injection in patients with overactive bladder: a systematic review and meta-analysis. World J Urol. (2018) 36:305–17. doi: 10.1007/s00345-017-2121-6
Keywords: onabotulinumtoxinA (BoNTA), overactive bladder (OAB), neurogenic detrusor overactivity (NDO), idiopathic detrusor overactivity, randomized controlled trials (RCT), meta-analysis
Citation: Cui Y, Cai T, Dong T, Zhang X, Zhou Z, Lu Y, Zhang Y, Wu J, Gao Z, Wang Y and Dong L (2021) Trigonal-Sparing vs. Trigonal-Involved OnabotulinumtoxinA Injection for the Treatment of Overactive Bladder: A Systematic Review and Meta-Analysis. Front. Neurol. 12:651635. doi: 10.3389/fneur.2021.651635
Received: 10 January 2021; Accepted: 06 September 2021;
Published: 08 October 2021.
Edited by:
Rizwan Hamid, Royal National Orthopaedic Hospital, United KingdomReviewed by:
Matthew O. Fraser, Duke University, United StatesLuke Grundy, Flinders University, Australia
Copyright © 2021 Cui, Cai, Dong, Zhang, Zhou, Lu, Zhang, Wu, Gao, Wang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongqiang Wang, ZG9jdG9yY3VpeXNAMTYzLmNvbQ==; Liying Dong, RG9jdG9ybGl5aW5nQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yuanshan Cui
Yuanshan Cui Tong Cai
Tong Cai Tiantian Dong1†
Tiantian Dong1† Zhongbao Zhou
Zhongbao Zhou