
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 30 March 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.646613
This article is part of the Research Topic Antiplatelet Agents in Stroke Prevention View all 13 articles
Objective: To evaluate the association between aspirin use and the risks of unruptured intracranial aneurysm (UIA) growth and aneurysmal subarachnoid hemorrhage (aSAH).
Methods: We searched PubMed and Scopus from inception to 1 September 2020. Studies evaluating the associations between aspirin prescription and the risk of UIA growth or the risk of aSAH were included. The study only included patients with intracranial aneurysms. We assessed the quality of included studies using the Newcastle-Ottawa scale. Random-effects meta-analysis was conducted to pool the estimates of effect size quantitatively. Sensitivity analyses using the leave-one-out strategy were performed to identify any potential source of heterogeneity.
Results: After a review of 2,226 citations, five cohort studies, two case-control studies, and one nested case-control study involving 8,898 participants were included. Pooled analyses showed that aspirin use, regardless of frequency and duration, was associated with a statistically significantly lower risk of UIA growth (OR 0.25, 95% CI 0.11–0.54; I2 = 0.0%, p = 0.604) and aSAH (OR, 0.37, 95% CI, 0.23–0.58; I2 = 79.3%, p = 0.001) in patients presented with intracranial aneurysms. The results did not significantly change in sensitivity analyses.
Conclusions: Summarizing available evidence in the literature, our findings indicate that aspirin use, regardless of frequency and duration, was associated with a statistically significantly lower risk of UIA growth and aSAH in patients with UIA. Well-designed and large-scale clinical trials are needed to help define the role of aspirin as a protective pharmaceutical for UIAs.
According to global statistics, it is estimated that 3% of the adult population has an unruptured intracranial aneurysm (UIA) (1). With the development of non-invasive imaging techniques, an increasing number of UIAs are being detected (2). Despite the further expansion of endovascular techniques and surgical clipping in recent years, the incidence of aneurysmal subarachnoid hemorrhage (aSAH) is relatively unchanged worldwide (3). Small aneurysms (<7 mm) are often left untreated because these patients cannot benefit from existing treatments, and the risk of aneurysm rupture does not outweigh the risk of morbidity and mortality from treatment complications for these aneurysms. Due to the non-negligible rate of aneurysm growth, regular follow-up with imaging surveillance to assess change in size and morphology is indicated (4–6). However, the continuous growth of an intracranial aneurysm results in subarachnoid hemorrhage (SAH), which has a mortality of 35%, and leads to serval serious complications (7). Thus, there is an urgent need for a non-invasive pharmaceutical treatment that can mitigate the risk of UIA growth.
Recently, accumulative evidence has suggested that inflammation plays a critical role in the structural deterioration of the IA wall and its subsequent rupture (8). Several observational studies have linked a representative non-steroidal anti-inflammatory drug-aspirin use with a slower rate of IA growth and lower risk of aSAH (4, 9–15). Aspirin has been widely prescribed as a standard secondary preventative agent in patients with risks of cardio- and cerebrovascular diseases. If aspirin is proved to have a beneficial effect on the risk of UIA growth with an acceptable safety profile, it could be a promising treatment option for this indication. As such, we conducted this systematic review and meta-analysis including patients with intracranial aneurysms to evaluate the association between aspirin use and risk of UIA growth and aSAH.
We conducted this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines 2009 (16). This systematic review and meta-analysis was not registered in the PROSPERO database. We thoroughly searched PubMed and Scopus from inception to 1 September 2020. A combination of search terms related to aspirin use (i.e., acetylsalicylic acid,) and outcomes of interest (i.e., occurrence of aSAH, growth of UIA) were used in the search strategy. We also searched the references of the included articles for further information. The details of the search strategy for each of the databases are included in Supplementary Materials.
Two collaborators (SY. and LX) individually screened the studies from two databases for eligibility according to predefined selection criteria: (i) the research design was cohort, case-control, or cross-sectional study; (ii) the study population was patients with UIAs and aspirin was the exposure factor; (iii) the primary outcome contained the prevalence of UIA growth or aSAH; and (iv) the study reported the odds ratio (OR) and corresponding 95% confidence intervals (CIs) (or OR and 95%CI can be manually derived from the study). Reviews, animal studies, clinical trials, case reports, commentaries were excluded. Disagreements were solved in a discussion with a senior author (XY.).
Two investigators attentively screened the titles and abstracts of articles and excluded irrelevant studies after duplicates were removed. After the first-round review, the same investigators retrieved full reports of those potentially eligible studies for details independently and then included studies that met the inclusion criteria. The disagreement was resolved in discussions with a third reviewer.
Data were extracted from retrieved articles by two reviewers independently. Details on the name of the first author, year of publication, region, study design, age and gender ratio of participants, exposures, primary outcomes, controls, OR with 95% Cis, and covariates adjusted rates, if available, were recorded.
We appraised included studies using the Newcastle-Ottawa Scale 10, which is a nine-point scoring system used to assess the quality of non-randomized studies included in a systematic review/meta-analysis. A high-quality study was defined as a study with at least seven points. All items were independently assessed by two investigators with disagreements resolved by group discussion.
We preferred to pool adjusted ORs from the primary studies; otherwise, we used the unadjusted estimates. A random-effects model was used to pool the effect estimates and I2 statistic was used to evaluate heterogeneity (0–100%). We considered I2 < 50% as low heterogeneity, I2 of 50–75% as moderate heterogeneity, and I2 > 75% as statistically high heterogeneity. We performed sensitivity analyses using a “leave-one-out” strategy to clarify the potential sources of the heterogeneity between included studies which may result from differences in the study population, intervention, or comparators. Also, we planned to assess for publication bias by the Egger test and funnel plots. All analyses were conducted in Stata version 11.
Figure 1 displays the flow chart of our study. We identified 2,226 citations from PubMed and Scopus. Eight studies met the inclusion criteria and provided data with 8,898 distinct participants: one prospective cohort study reported associations between aspirin use and UIA growth/rupture; four retrospective studies of either a prospectively maintained database, a patient cohort, or a consecutive series, indicated a negative relationship between aspirin use and UIA growth or aSAH; two case-control studies and 1 nested case-control study discussed the relationship between aspirin use and risk of aSAH. Table 1 illustrates the detailed characteristics of the included studies, whose quality was carefully assessed by the Newcastle-Ottawa Scale (see Table 2).
Three studies reported associations between aspirin use and UIA growth. Although Serrone et al. identified a relatively lower risk in aspirin users (OR 0.72, 95% CI 0.29–1.81), their primary outcome was UIA growth or de novo aneurysm formation (14). Thus, we excluded it from the pooled analyses. Combining findings from the other two studies suggested that aspirin use, regardless of frequency and duration, was associated with a significantly lower risk of UIA growth (OR 0.25, 95% CI 0.11–0.54) (Figure 2). No significant heterogeneity was observed (p = 0.604).

Figure 2. Forest plot for an association between aspirin use and growth of intracranial aneurysm. OR, odds ratio; CI, confidence interval.
Five studies reported on the association between aspirin use and risk of aSAH in patients with UIA. A meta-analysis was conducted to pool estimates of aspirin use and the risk of aSAH in UIA patients, resulting in an OR of 0.37 (95% CI, 0.23–0.58) (Figure 3). Significant heterogeneity was tested out in the included studies (p = 0.001). We then conducted a sensitivity analysis using a leave-one-out strategy. Figure 4 showed the corresponding pooled ORs when one study was excluded from the final analysis. The results remained stable when any specific study was excluded from the pooled analysis, indicating that aspirin use was associated with a lower risk of aSAH in patients with UIA despite the high heterogeneity in studies.
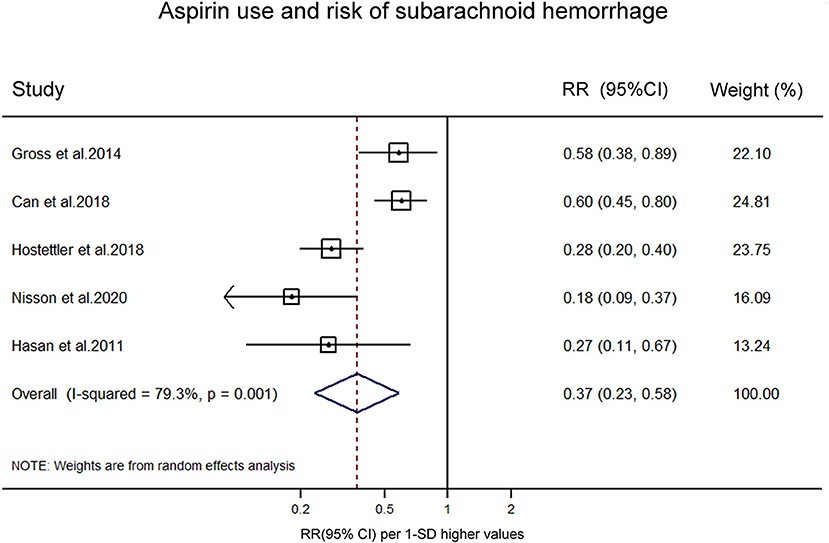
Figure 3. Forest plot for an association between aspirin use and risk of subrachnoid hemorrhage. OR, odds ratio; CI, confidence interval.
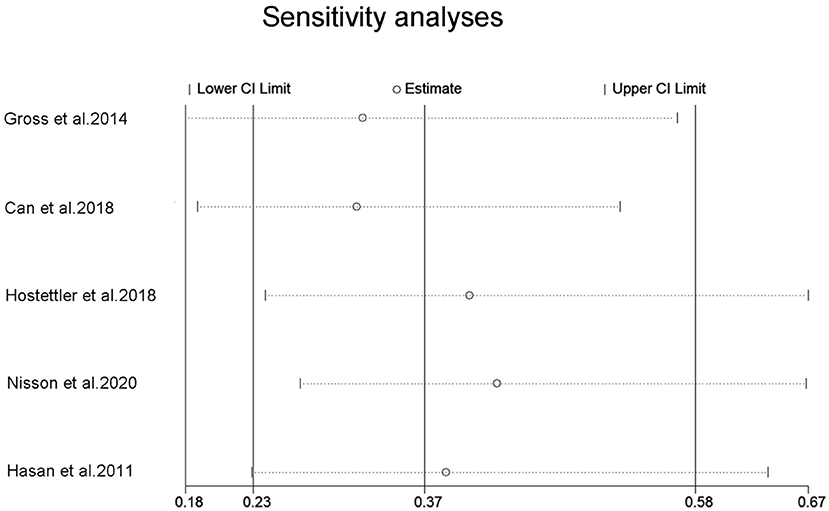
Figure 4. Sensitivity analysis for an association between aspirin use and risk of subrachnoid hemorrhage. OR, odds ratio; CI, confidence interval.
In the present systematic review and meta-analysis, we summarized all available epidemiological evidence, using data from 8,898 participants involving 581 cases in the aspirin users to help clarify the association between aspirin use and UIA growth or aSAH in UIA patients. Our results showed that aspirin use, regardless of frequency and duration, was associated with a statistically significant decreased risk of UIA growth (OR 0.25, 95% CI 0.11–0.54; I2 = 0.0%, p = 0.604) and a significantly lower risk of aSAH (OR, 0.37, 95% CI, 0.23–0.58; I2 = 79.3%, p = 0.001) in patients with UIAs. The results of this study suggest that aspirin could play a role in reducing the risk of intracranial aneurysm expansion and the risk of aSAH, and aspirin could be a potential drug to treat intracranial aneurysms.
Two previous meta-analyses have discussed the effect of aspirin prescription on the risk of aSAH (6, 19). Both meta-analyses found no significant difference between aspirin users and non-aspirin users regarding the risk of aSAH (OR, 1.00; 95% CI, 0.81–1.24, p = 0.99, and OR, 0.981,95% CI, 0.773–1.312, p = 0.897). However, neither of the two meta-analyses focused on the risk of aSAH in the specific patient group with intracranial aneurysms, which may attenuate the possible protective effect of aspirin on IA rupture and aSAH in UIA patients. Moreover, better concomitant risk factor management in the UIA patients, particularly blood pressure control, might contribute to the lower risk of UIA progression. Besides, Phan et al. reported a significant association between short-term use of aspirin (<3 months) and risk of aSAH (OR, 1.61; 95% CI, 1.20–2.18, p = 0.002) (6). Qian et al. also reported that short-term use of aspirin (<3 months) was associated with an elevated risk of aSAH (OR, 1.697, 95% CI, 1.175–2.452, p = 0.005) (19). They concluded that when prescribing aspirin for prophylactic use, particularly with known UIAs, its inherent bleeding risks should be taken into consideration, especially in the short term. Several population-based studies have explored the association between antiplatelet therapy and SAH, reaching conflicting results (20, 21). Recently, Weng et al. provided Class III evidence in a prospective, multicenter cohort that for patients harboring UIAs <7 mm with ischemic cerebrovascular disease, aspirin does not increase the risk of aneurysm rupture (17). Together with our findings, we believe that the benefit of aspirin uses in this specific population outweighs the possibly increased risk of aSAH.
Both animal experiments and human clinical studies indicate that vascular remodeling and inflammatory cascades are crucial in the formation, progression, and rupture of IAs (22). Abnormal wall shear stress-activated the PGE2 (prostaglandin E2) -EP2 (prostaglandin E receptor 2) pathway in endothelial cells (ECs) at the early stage of cerebral aneurysm formation (23, 24). Subsequently, vascular smooth muscle cell apoptosis and migration, accompanied by inflammatory cell infiltration, resulted in degradation of the vascular wall, leading to the progression, and eventual rupture of IAs (22). Hasan et al. found in a small patient group that cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) are expressed in human cerebral aneurysms and expression increases in ruptured aneurysms (25). Thus, drugs targeting molecules involved in the above process might have potential therapeutic effects. As a commonly used preventative agent in patients with risks of cardio- and cerebrovascular diseases, aspirin has been shown to have inhibitory effects on several inflammatory mediators such as COX-2 and mPGES-1, making it one of the promising drugs for decreasing UIA growth and rupture (10). Several groups have proved that acetylsalicylic acid (ASA) was associated with a slower IA growth rate and lower IA rupture or aSAH rate in mice IA-induction models, suggesting the protective effect of ASA against IA rupture (8). Moreover, Hasan et al. demonstrated a decreased expression of inflammatory cells and markers such as COX-2 in a small randomized sample of patients with unruptured aneurysms who underwent microsurgical clipping after 3 months of aspirin treatment (18). More researches should be conducted to further elucidate the underlying mechanisms of this issue.
The present study was constrained by several limitations. Firstly, the number of included studies was relatively low, especially for the meta-analysis on UIA growth. Secondly, all eligible data included in the meta-analysis were extracted from observational studies and most studies were retrospective. Last but not least, heterogeneity among studies suggests that the effect of aspirin on UIA growth and rupture should be further confirmed by clinical trials. Re-analyzing existing non-randomized data using advanced statistical techniques (i.e., inverse probably of treatment weighting) could better explore this association as well.
Summarizing available evidence in the literature, our findings indicate that aspirin use, regardless of frequency and duration, was associated with a statistically significant decreased risk of UIA growth and aSAH in UIA patients. Aspirin might be a potential drug for the treatment of intracranial aneurysms. Well-designed, large-scale clinical trials are needed to help definitively define aspirin's role as a protective pharmaceutical for UIAs.
SY, LX, and XY contributed to the conception or design of the work and contributed to the acquisition, analysis, or interpretation of data for the work. SY and LX drafted the manuscript. TL, YW, and NX critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
This work was supported by the Hubei Natural Science Foundation (2019CFB465).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank all the authors of the original articles.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.646613/full#supplementary-material
1. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. (2011) 10:626–36. doi: 10.1016/S1474-4422(11)70109-0
2. Gabriel RA, Kim H, Sidney S, McCulloch CE, Singh V, Johnston SC, et al. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke. (2010) 41:21–6. doi: 10.1161/STROKEAHA.109.566018
3. Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among medicare beneficiaries, 2000 to 2010. Stroke. (2015) 46:2452–7. doi: 10.1161/STROKEAHA.115.009777
4. Weng JC, Wang J, Li H, Jiao YM, Fu WL, Huo R, et al. Aspirin and Growth of Small Unruptured Intracranial Aneurysm: Results of a Prospective Cohort Study. Stroke. (2020) 51:3045–54. doi: 10.1161/STROKEAHA.120.029967
5. Malhotra A, Wu X, Gandhi D, Sanelli P, Matouk CC. Management of small, unruptured intracranial aneurysms. World Neurosurg. (2020) 135:379–80. doi: 10.1016/j.wneu.2019.12.139
6. Phan K, Moore JM, Griessenauer CJ, Ogilvy CS, Thomas AJ. Aspirin and risk of subarachnoid hemorrhage: systematic review and meta-analysis. Stroke. (2017) 48:1210–7. doi: 10.1161/STROKEAHA.116.015674
7. Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. (2020). doi: 10.1007/s12975-020-00867-0. [Epub ahead of print].
8. Hudson JS, Marincovich AJ, Roa JA, Zanaty M, Samaniego EA, Hasan DM. Aspirin and intracranial aneurysms. Stroke. (2019) 50:2591–6. doi: 10.1161/STROKEAHA.119.026094
9. Zanaty M, Roa JA, Nakagawa D, Chalouhi N, Allan L, Kasab SA, et al. Aspirin associated with decreased rate of intracranial aneurysm growth. J Neurosurg. (2019) 133:1–8. doi: 10.3171/2019.6.JNS191273
10. Hasan DM, Mahaney KB, Brown RD Jr, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. (2011) 42:3156–62. doi: 10.1161/STROKEAHA.111.619411
11. Gross BA, Rosalind Lai PM, Frerichs KU, Du R. Aspirin and aneurysmal subarachnoid hemorrhage. World Neurosurg. (2014) 82:1127–30. doi: 10.1016/j.wneu.2013.03.072
12. Can A, Rudy RF, Castro VM, Yu S, Dligach D, Finan S, et al. Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: a case-control study. Neurology. (2018) 91:e1175–81. doi: 10.1212/WNL.0000000000006200
13. Hostettler IC, Alg VS, Shahi N, Jichi F, Bonner S, Walsh D, et al. Characteristics of unruptured compared to ruptured intracranial aneurysms: a multicenter case-control study. Neurosurgery. (2018) 83:43–52. doi: 10.1093/neuros/nyx365
14. Serrone JC, Tackla RD, Gozal YM, Hanseman DJ, Gogela SL, Vuong SM, et al. Aneurysm growth and de novo aneurysms during aneurysm surveillance. J Neurosurg. (2016) 125:1374–82. doi: 10.3171/2015.12.JNS151552
15. Nisson PL, Meybodi T, Secomb TW, Berger GK, Roe DJ, Lawton MT. Patients taking antithrombotic medications present less frequently with ruptured aneurysms. World Neurosurg. (2020) 136:e132–40. doi: 10.1016/j.wneu.2019.12.045
16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
17. Weng J-C, Wang J, Du X, et al. Safety of aspirin use in patients with stroke and small unruptured aneurysms. Neurology. (2020) 96:e19–29. doi: 10.1212/WNL.0000000000010997
18. Hasan DM, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, et al. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J Am Heart Assoc. (2013) 2:e000019. doi: 10.1161/JAHA.112.000019
19. Qian C, He Y, Li Y, Chen C, Zhang B. Association between aspirin use and risk of aneurysmal subarachnoid hemorrhage: a meta-analysis. World Neurosurg. (2020) 138:299–308. doi: 10.1016/j.wneu.2020.01.120
20. García-Rodríguez LA, Gaist D, Morton J, Cookson C, González-Pérez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. (2013) 81:566–74. doi: 10.1212/WNL.0b013e31829e6ffa
21. Pottegård A, García Rodríguez LA, Poulsen FR, Hallas J, Gaist D. Antithrombotic drugs and subarachnoid haemorrhage risk. A nationwide case-control study in Denmark. Thromb Haemost. (2015) 114:1064–75. doi: 10.1160/TH15-04-0316
22. Liu Z, Ajimu K, Yalikun N, Zheng Y, Xu F. Potential therapeutic strategies for intracranial aneurysms targeting aneurysm pathogenesis. Front Neurosci. (2019) 13:1238. doi: 10.3389/fnins.2019.01238
23. Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Aziz HA, Shono M, et al. Endothelial injury and inflammatory response induced by hemodynamic changes preceding intracranial aneurysm formation: experimental study in rats. J Neurosurg. (2007) 107:405–11. doi: 10.3171/JNS-07/08/0405
24. Aoki T, Nishimura M, Matsuoka T, Yamamoto K, Furuyashiki T, Kataoka H, et al. PGE2-EP2 signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol. (2011) 163:1237–49. doi: 10.1111/j.1476-5381.2011.01358.x
Keywords: aspirin, intracranial aneuryms, aneurysmal subarachnoid hemorrhage, prevention, meta-analysis
Citation: Yang S, Liu T, Wu Y, Xu N, Xia L and Yu X (2021) The Role of Aspirin in the Management of Intracranial Aneurysms: A Systematic Review and Meta-Analyses. Front. Neurol. 12:646613. doi: 10.3389/fneur.2021.646613
Received: 27 December 2020; Accepted: 08 March 2021;
Published: 30 March 2021.
Edited by:
Peter Klivenyi, University of Szeged, HungaryReviewed by:
Seana Gall, University of Tasmania, AustraliaCopyright © 2021 Yang, Liu, Wu, Xu, Xia and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyu Yu, eXV4aW55dTdAaHVzdC5lZHUuY24=; orcid.org0000-0002-3689-980X; Liangtao Xia, eGlhbGlhbmd0YW9AaHVzdC5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.