- 1Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Biostatistics, School of Public Health, Shandong University, Jinan, China
- 4Department of Clinical Epidemiology, Cheeloo College of Medicine, Qilu Hospital, Shandong University, Jinan, China
- 5Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Metabolic syndrome (MetS) can worsen cerebral arterial atherosclerosis stenosis in patients with stroke; however, its effect on patients without stroke remains ambiguous. This study explored the association of MetS and its individual components with asymptomatic intracranial arterial stenosis (aICAS) and asymptomatic extracranial arterial stenosis (aECAS) among older Chinese adults. A total of 1988 participants from the Kongcun Town study aged ≥40 years and without a history of stroke were enrolled. The baseline data were obtained via face-to-face interviews. MetS was defined according to International Diabetes Federation criteria. Detection of aICAS was conducted using transcranial Doppler ultrasound, followed by diagnosis via magnetic resonance angiography. The evaluation of aECAS was performed using bilateral carotid ultrasonography. The aICAS and aECAS groups were 1:1 matched separately to the non-stenosis group by age and sex. The association between MetS and aICAS or aECAS was analyzed using multivariate logistic regression. Among the 1988 participants, 909 were diagnosed with MetS. The prevalence of MetS was higher in the aICAS group than in the non-stenosis group (P <0.001), but did not differ significantly between the aECAS and non-stenosis groups. The prevalence of aICAS increased with the number of MetS components from 3.4% in the ≤ 1 component group to 12.7% in the ≥4 components group (P for trend <0.001). After adjusting for confounding factors, MetS components associated with aICAS included central obesity, elevated triglyceride levels, and elevated blood pressure. None of the MetS components was associated with aECAS. MetS was positively associated with aICAS, but not with aECAS. Further, different components play different roles in the pathological process leading to aICAS.
Introduction
Ischemic stroke caused by cerebral arterial atherosclerotic stenosis is a serious health and social issue worldwide, often leading to disability and mortality (1, 2). Some traditional vascular risk factors are involved in cerebral arterial atherogenesis, and they play different roles in the pathogenesis of intracranial and extracranial atherosclerosis (3). For instance, hyperlipidemia is more closely associated with extracranial arterial stenosis (ECAS), while diabetes mellitus (DM) and hypertension are associated with intracranial arterial stenosis (ICAS) (3, 4). Identifying different risk factors for ICAS and ECAS is helpful for efficient stroke prevention.
Metabolic syndrome (MetS) is a constellation of several metabolic risk factors, including central obesity, hypertension, elevated fasting blood glucose, and hyperlipidemia. The prevalence of MetS is gradually increasing with the aging population and changing lifestyle (5). In recent years, there has been a widespread concern regarding MetS as a novel risk factor for atherosclerosis (6).
MetS and its individual components are closely associated with ICAS or ECAS in patients with stroke, and this relationship is more significant with respect to ICAS than to ECAS (7–11). Some retrospective studies have evaluated the association of MetS with different locations (extracranial vs. intracranial) of asymptomatic cerebral arterial stenosis. A study in an asymptomatic Caucasians population found that MetS was an independent risk factor for moderate to severe (defined as ≥50% stenosis) intracranial atherosclerotic disease, but not for moderate to severe extracranial atherosclerotic disease (12). Another study involving a racially and ethnically diverse population found that the impact of MetS on the distribution of intracranial and extracranial atherosclerosis varied by race and ethnicity (13). Two community-based studies from China have separately reported a significant association between MetS and asymptomatic ICAS (aICAS) or asymptomatic ECAS (aECAS) (14, 15). However, no study has compared the association between MetS, including its individual components, and aICAS or aECAS in the Chinese population.
This study aimed to explore whether there is a differential profile in the association of MetS and its individual components with aICAS and aECAS among middle-aged and older adults living in rural communities in China.
Materials and Methods
Study Design and Population
This study was based on the Kongcun Town study (16), a population-based study targeting 2,311 rural residents aged ≥40 years with no history of clinical stroke. Data on demographics, medical history, and physical examinations were obtained through face-to-face interviews. aICAS was detected using a two-phase procedure: screening using transcranial Doppler and diagnosis via magnetic resonance angiography. aECAS was evaluated using bilateral carotid ultrasonography. Among the 2,311 participants, 305 were excluded owning to incomplete information, two because of abnormal waist circumference, and 16 with combined aICAS/aECAS were excluded owning to the small number of cases. Finally, data from the 1988 eligible participants were analyzed. Participants were categorized into the following three groups according to the site of stenosis: (1) non-stenosis (n = 1813) (2) isolated aICAS (n = 132); and (3) isolated aECAS (n = 43).
The study protocol was approved by the Ethical Standards Committee on Human Experimentation at Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University. This study was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided a written informed consent.
Definitions of Vascular Risk Factors
Baseline data on demographics and risk factors were collected via interviews, clinical examinations, and laboratory tests in a similar manner as reported in our previous study (16). Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, use of antihypertensive drugs, or self-reported hypertension. DM was defined as a fasting plasma glucose level of ≥7.0 mmol/L (126.0 mg/dL), use of blood glucose-lowering drugs, receipt of insulin injection, or a self-reported history of diabetes. Based on smoking habits, participants were classified into current smokers (smoked at least one cigarette per day for more than 1 year) and former smokers (quit smoking <6 months earlier). Based on their drinking habits, participants were classified into current drinkers (consumed alcohol at least once a week for at least 6 months) and former drinkers (quit <6 months earlier).
Definition of MetS
MetS was defined using the criteria previously published by the International Diabetes Federation (17). The definition included the presence of central obesity (waist circumference ≥90 cm for Chinese men and ≥80 cm for Chinese women), plus any two of the following: (1) triglyceride (TG) level ≥1.7 mmol/L (150 mg/dL) or receiving specific treatment for this lipid abnormality (2) high-density lipoprotein cholesterol (HDL-C) level <1.03 mmol/L (40 mg/dL) in men and <1.29 mmol/L (50 mg/dL) in women or receiving specific treatment for this lipid abnormality; (3) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or receiving treatment for previously diagnosed arterial hypertension; and (4) fasting plasma glucose level ≥5.6 mmol/L (100 mg/dL) or previously diagnosed DM.
Assessment of aICAS and aECAS
The protocol for the evaluation of aICAS and aECAS has been described in detail in our previous study (16). In brief, aICAS was detected through a two-phase procedure: a screen phase using transcranial Doppler and the diagnostic phase using magnetic resonance angiography. Transcranial Doppler was performed by two physicians using a portable machine (VIASYS Companion III). The bilateral middle cerebral artery, internal carotid artery, anterior cerebral artery, posterior cerebral artery, vertebral artery, and basilar artery were examined with a 2-MHz probe via temporal, occipital, and eye windows. Stenosis of participants with poor temporal windows in the bilateral vertebral artery, posterior cerebral artery, and basilar artery was examined via the occipital window. In this study, aICAS was defined according to the previously published criteria for the identification of one or more stenotic lesions of any degree in any one of the analyzed intracranial vessels on magnetic resonance angiography (18). The diagnosis and grading of aICAS was performed by a stroke specialist and clinical neuroradiologist. The degree of stenosis in the evaluated arteries (bilateral middle cerebral artery, bilateral intracranial internal carotid artery, anterior cerebral artery, posterior cerebral artery, and basilar artery) was classified into five grades by consensus as normal, mild (signal reduction <50%), moderate (signal reduction ≥50% and <70%), severe (signal reduction ≥70%), or occlusion (focal signal loss with the presence of distal signal). aECAS was diagnosed via carotid ultrasonography examination, which has a high sensitivity and specificity (19, 20), and was performed by two experienced physicians. aECAS was defined according to established carotid ultrasonography criteria (21) as the identification of one or more stenotic lesions of any degree in any one of the analyzed vessels, including the carotid artery, internal carotid artery, and external carotid artery. The degree of aECAS was classified into four grades: mild (<50% stenosis), moderate (50–69% stenosis), severe (70–99% stenosis), and total occlusion.
Statistical Analyses
All analyses were conducted using IBM Statistical Package for the Social Sciences Statistics V22.0 for Windows (IBM Corp., released 2013, Armonk, NY, USA). Baseline population statistics and continuous laboratory-based variables are expressed as terms of mean and standard deviation, and categorical variables are expressed as frequencies and percentages. Continuous variables were compared using the t-test or analysis of variance with post hoc tests, while categorical variables were compared using the chi-square test. The Bonferroni adjustment was performed to assess the statistical significance of the intergroup differences. The aICAS and aECAS groups were 1:1 matched separately to the non-stenosis group by age and sex. The multivariate logistic regression was used to determine the association between MetS and its individual components with aICAS or aECAS. The variables with a P-value of <0.1 in the univariate analysis were included in the logistic regression models. The associations of different cerebral arterial stenosis were reported as odds ratio (OR) values and their 95% confidence intervals (CI). All statistical tests were two-tailed, and P < 0.05, indicated statistical significance.
Results
Baseline Characteristics of the Study Population
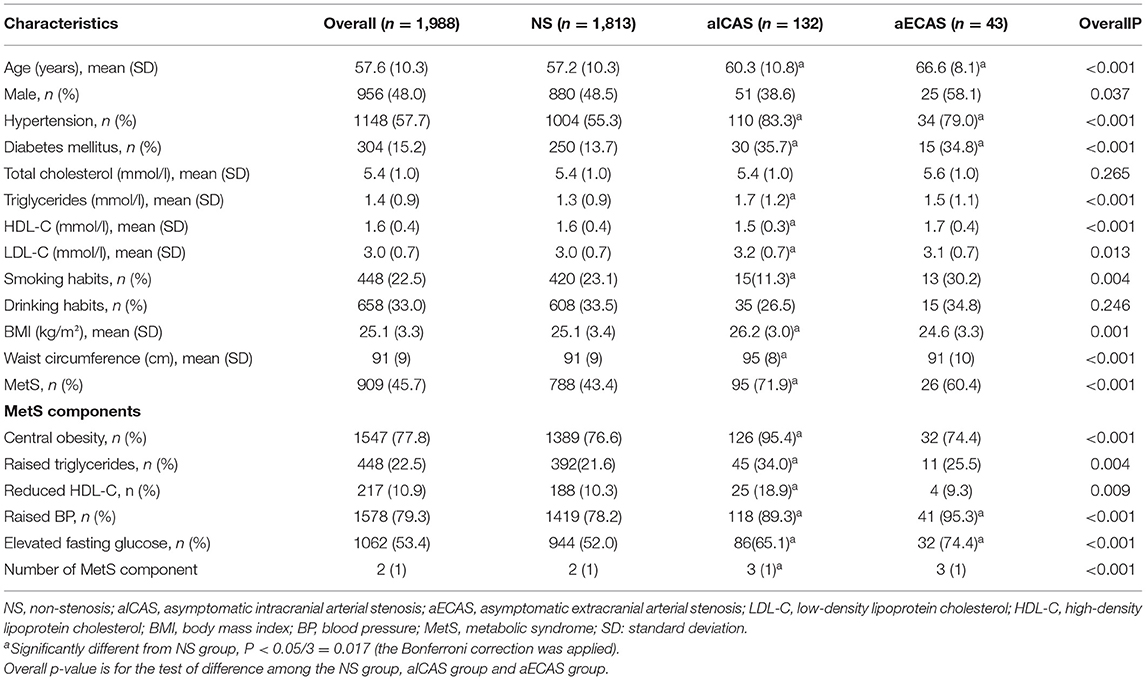
The demographic and clinical characteristics of the study participants are shown in Table 1. Compared with the non-stenosis group, the aICAS group had significantly higher mean age, body mass index, waist circumference, and TG and low-density lipoprotein cholesterol levels and a significantly lower mean HDL-C level. The prevalence of hypertension, DM, and MetS was also higher in the aICAS group than in the non-stenosis group. The mean age and prevalence of hypertension and DM were also higher in the aECAS group than in the non-stenosis group. Of the 1988 participants, 909 (45.7%) were diagnosed with MetS. Compared with the non-stenosis group, the prevalence of MetS and all its individual components (all P < 0.017) was higher in the aICAS group and that of elevated blood pressure and elevated fasting glucose (all P < 0.017) was higher in the aECAS group.
Demographic and Clinical Characteristics of the Participants After Matching for Age and Sex
Table 2 shows the demographic and clinical characteristics of the participants after matching for age and sex. Age, sex, smoking habits, and drinking habits showed no significant difference between the aICAS/aECAS and control groups. Compared with the control 1 group, the aICAS group had significantly higher body mass index, waist circumference, and a significantly lower mean HDL-C level. The prevalence of hypertension and MetS was also higher in the aICAS group than in the control 1 group. The HDL-C level was lower in the aECAS group than in the control 2 group.
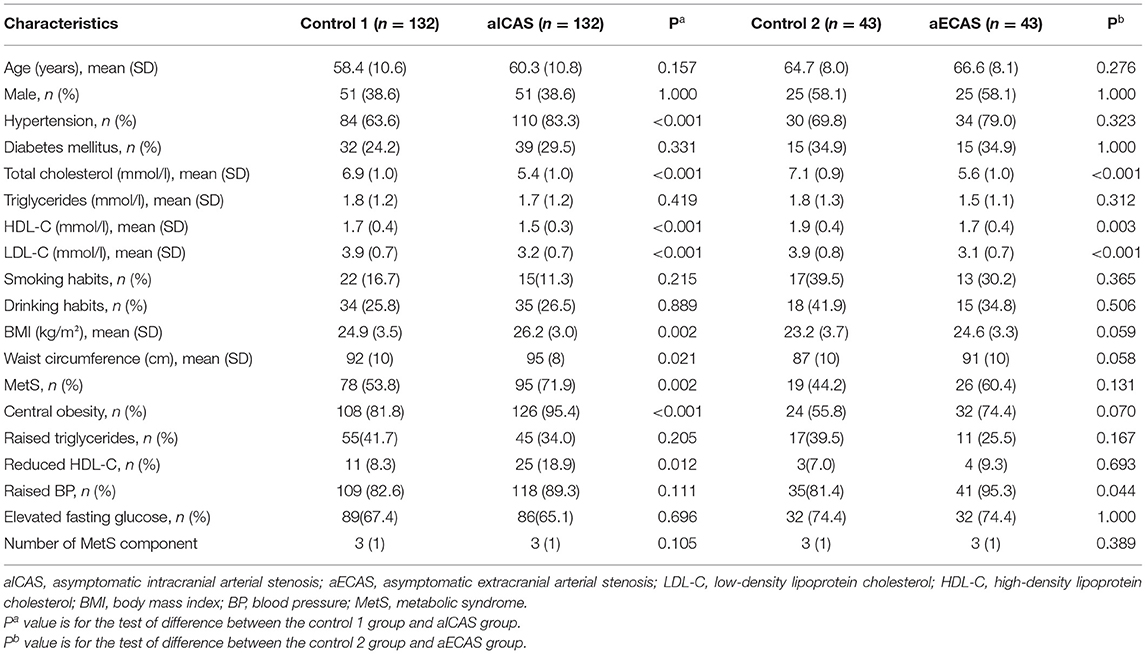
Table 2. Demographic and clinical characteristics of the participants after matching for age and sex.
Associations of MetS and Its Components With aICAS or aECAS
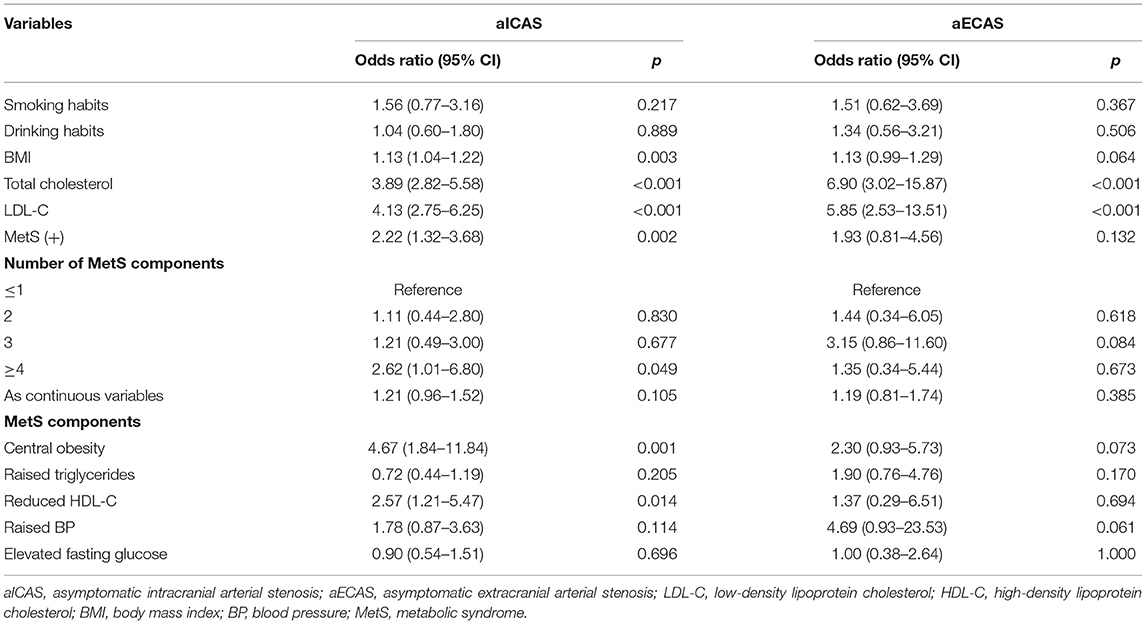
In the multivariate logistic regression analysis (Figure 1), MetS was significantly associated with aICAS (OR: 4.01; 95% CI: 1.84, 8.75) after adjusting for total cholesterol level, body mass index, and low-density lipoprotein cholesterol level, which were significantly related to aICAS or ECAS in the univariate logistic regression analysis (Table 3, all P <0.1). Participants with more severe MetS components were more likely to have aICAS (P for linear trend =0.011). The following MetS components were significantly associated with aICAS: central obesity, elevated TG levels, and elevated blood pressure. However, no significant association between aECAS, MetS and its components was observed.
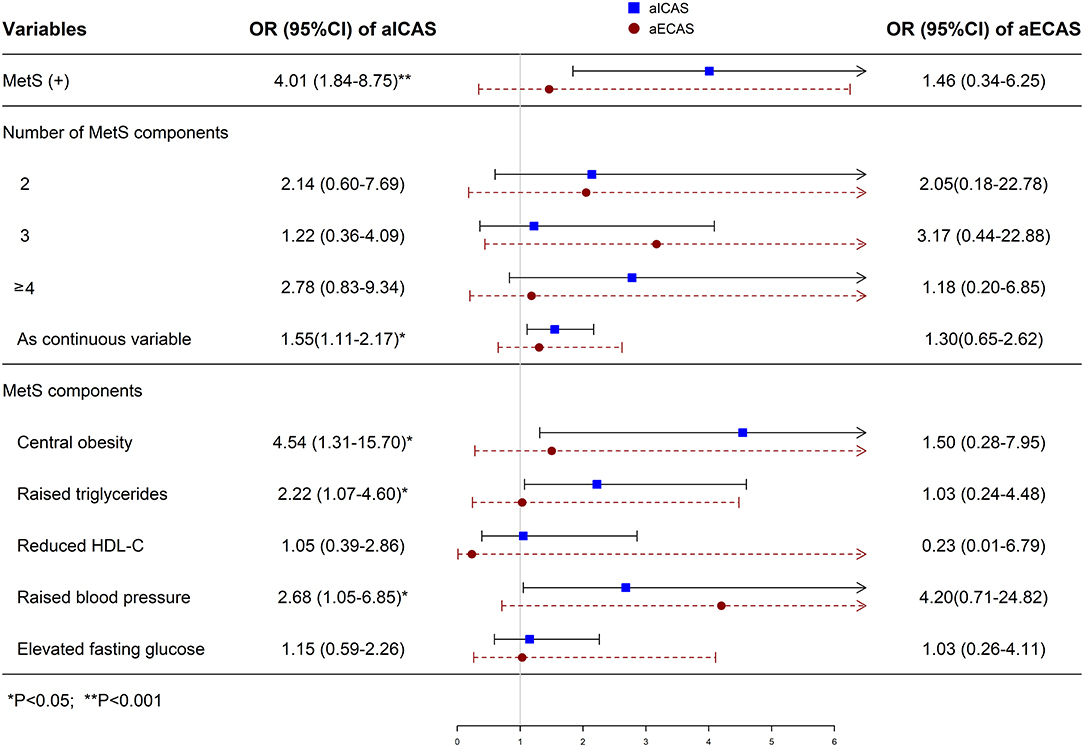
Figure 1. Multivariate logistic regression analysis of the association of MetS with aICAS or aECAS. aICAS, asymptomatic intracranial arterial stenosis; aECAS, asymptomatic extracranial arterial stenosis; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; BP, blood pressure; MetS, metabolic syndrome; OR, odds ratio. ORs were calculated using logistic regression model after adjusting total cholesterol, BMI, and LDL-C.
Prevalence of aICAS and aECAS According to the Number of MetS Components
The prevalence of aICAS increased significantly in proportion to the number of MetS components from 3.4% in the ≤ 1 component group to 12.7% in the ≥4 components group (P for trend <0.001). However, the same association was not found for aECAS (Figure 2).

Figure 2. Prevalence of aICAS and aECAS according to the number of MetS components. aICAS, asymptomatic intracranial arterial stenosis; aECAS, asymptomatic extracranial arterial stenosis; MetS, metabolic syndrome.
Discussion
This study found that MetS was associated with aICAS, but not with aECAS, and different components play different roles in the pathological process leading to aICAS. Among MetS components, central obesity, elevated TG levels, and elevated blood pressure were significantly associated with aICAS. To the best of our knowledge, this is the first study to investigate the association between MetS and aICAS or aECAS among middle-aged and older adults living in rural communities in China.
MetS is a proinflammatory and hypercoagulable state, which is mainly mediated by insulin resistance. It has been suggested that accelerated atherosclerosis in MetS is associated with defective insulin signaling pathways (22). In addition, oxidative stress is associated with MetS and plays a role in endothelial dysfunction and subsequent atherosclerosis (23). Previous hospital-based studies reported that MetS was an independent risk factor for stroke, and that patients with MetS were more likely to experience ICAS than ECAS (7–11). A prospective Korean study revealed that MetS was more prevalent in ICAS patients than in ECAS patients with posterior circulation stroke (3). These studies concluded that MetS could play an important role in promoting cerebral arterial stenosis and increasing the risk of subsequent stroke. Furthermore, several studies have focused on investigating the association between MetS and aICAS or aECAS in the stroke-free population. The Asymptomatic Polyvascular Abnormalities in Community study targeting employees and retirees of the Kailuan (Group) Co. Ltd (a large coal mine industry) reported significant associations between MetS and aICAS (14), which is consistent with our results. Another study involving asymptomatic Chinese people reported significant associations between MetS and aECAS in menopausal women (15). The reason for the inconsistent results may be that the participants investigated were different. Regarding severity, the Barcelona-Asymptomatic Intracranial Atherosclerosis study found that MetS was significantly associated with moderate to severe intracranial atherosclerotic disease and not with moderate to severe extracranial atherosclerotic disease (12). This finding was apparently corroborated by the finding that MetS may be independently associated with the early stage rather than the advanced stages of intracranial arterial atherosclerosis (24). To the best of our knowledge, some studies have reported that ECAS is common in Caucasian population, whereas ICAS is more frequent in Asian and African populations (25–27). A previous study involving a racially and ethnically diverse population found that the impact of MetS on the distribution of intracranial and extracranial atherosclerosis varied by race and ethnicity (13). The race-specific impact of MetS on the distribution of cerebral arterial atherosclerosis may be caused by racial-specific differences in the prevalence of MetS components and in the host response to the presence of specific components. This finding may partly explain the well-known differences in race-ethnic predilection to intracranial or extracranial atherosclerosis. Future studies with genotyping may be important to define the relationships between the biological race differences between ICAS and ECAS.
In this study, central obesity was associated with aICAS, but not with aECAS. Central obesity can lead to an increase in the free fatty acids, which play an important role in the pathogenesis of insulin resistance (28, 29). Furthermore, insulin resistance can damage intracranial vasodilatory function by increasing the stiffness of the vascular wall and reducing the buffering ability, which increases its susceptibility to arterial oxidative stress (30, 31). Therefore, early intervention for central obesity may delay the progression of cerebral arterial stenosis, especially aICAS.
In this study, elevated TG levels were significantly associated with aICAS. High TG levels can promote the formation of low-density lipoprotein particles (32); high levels of low-density lipoprotein cholesterol, especially its oxidized form, can facilitate endothelial dysfunction, which is the first step in atherosclerotic plaque formation (33).
The significant association between elevated blood pressure and aICAS detected in this study is consistent with previous studies (34, 35). Hypertension is a well-known risk factor for arteriosclerosis (36). Compared with the extracranial arteries, the thickness and elasticity of the tunica media is inferior in the intracranial arteries; therefore, it may be more vulnerable to the changes in vascular stress and blood flow caused by hypertension (36).
Among MetS components, the association between reduced HDL-C and elevated fasting glucose levels with MetS was not found. However, previous hospital-based studies found that the components (reduced HDL-C and elevated fasting glucose levels) constituting MetS were related to aICAS (9, 11). This suggests that MetS can affect intracranial arterial atherosclerosis in different pathological states via different metabolic pathways. In addition, this finding may reveal that the association between aICAS and MetS may be derived from the specific components of MetS, such as central obesity, elevated TG levels, and elevated blood pressure, especially in the asymptomatic phase of intracranial arterial stenosis. More metabolism-related basic research is needed to confirm this inference in the future.
The reasons for the aforementioned differential effects of MetS on the distribution of cerebral arterial stenosis are not well-understood. The potential reasons for this are as follows: first, the differential responses of intracranial and extracranial arteries to oxidative stress may explain our finding that most components constituting MetS were associated with aICAS, since oxidative stress has been reported to be associated with MetS (23). Compared with the extracranial arteries, the intracranial arteries were found to be susceptible to oxidative stress with increasing age (37). Second, the histological differences between the intracranial and extracranial arteries should be considered. The extracranial arteries are elastic arteries whose tunica media are rich in elastin filaments. However, the intracranial arteries with a fewer elastic fibers may be more vulnerable to the circulatory abnormalities caused by MetS (36).
Some potential limitations of our study are worth mentioning. First, a cross-sectional study cannot prove the existence of a causal relationship between MetS and aICAS/aECAS; further studies using a prospective study are needed to confirm this relationship. Second, owning to the relatively small sample size, this study was unable to evaluate the association between MetS and distribution of stenosis in various strata of severity of stenosis. Finally, the findings of this study may not be generalizable to other populations since it included only Chinese adults living in rural areas. Nevertheless, to the best of our knowledge, this is the first study to investigate the differences in the associations between certain MetS components and the distribution of cerebral arterial stenosis.
In conclusion, the study findings indicate that MetS is associated with aICAS, but not with aECAS, and different components play different roles in the pathological process of aICAS. These differences may prompt the employment of individualized preventive measures during the asymptomatic stage of cerebral arterial stenosis; thereby, reducing the incidence of stroke.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QS, YD, and FX conceived and designed the research. SL, YZ, XW, XJ, SSa, SSh, and YX acquired the data. SL, XS, YZ, XW, XJ, SSa, SSh, YX, and GW analyzed and interpreted the data. SL and XS draft the manuscript. XW, ML, FX, QS, and YD made critical revisions of the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the grants from Jinan Science and Technology Bureau [Grant Number 201704101], Department of Science and Technology of Shandong Province [Grant Numbers 2014GSF118106, 2016GSF201062, and ZR2017MH114], the National Nature Science Foundation of China [Grant Numbers 8171101298 and 81971128], the China Ministry of Sciences and Technology [Grant Numbers 2017YFC1310100 and 2017YFC0907003], and the International Science and Technology Cooperation Programme [Grant Number 2014DFA 32830].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the study participants, staff of the participating hospitals, and the Steering Committee Members of this study.
References
1. Collaborators GN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/s1474-4422(18)30499-x
2. Xu Y, Yuan C, Zhou Z, He L, Mi D, Li R, et al. Co-existing intracranial and extracranial carotid artery atherosclerotic plaques and recurrent stroke risk: a three-dimensional multicontrast cardiovascular magnetic resonance study. J Cardiovasc Magnet Resonance. (2016) 18:90. doi: 10.1186/s12968-016-0309-3
3. Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. (2012) 43:3313–8. doi: 10.1161/strokeaha.112.658500
4. Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. (2014) 383:984–98. doi: 10.1016/s0140-6736(13)61088-0
5. Li Y, Zhao L, Yu D, Wang Z, Ding G. Metabolic syndrome prevalence and its risk factors among adults in China: A nationally representative cross-sectional study. PLoS ONE. (2018) 13:e0199293. doi: 10.1371/journal.pone.0199293
6. Bang OY. Intracranial atherosclerotic stroke: specific focus on the metabolic syndrome and inflammation. Curr Atherosc Rep. (2006) 8:330–6. doi: 10.1007/s11883-006-0012-1
7. Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. (2005) 65:296–8. doi: 10.1212/01.wnl.0000168862.09764.9f
8. Park JH, Kwon HM, Roh JK. Metabolic syndrome is more associated with intracranial atherosclerosis than extracranial atherosclerosis. Eur J Neurol. (2007) 14:379–86. doi: 10.1111/j.1468-1331.2007.01682.x
9. Park JH, Kwon HM. Association between metabolic syndrome and previous ischemic lesions in patients with intracranial atherosclerotic stroke. Clin Neurol Neurosurg. (2008) 110:215–21. doi: 10.1016/j.clineuro.2007.10.016
10. Rincon F, Sacco RL, Kranwinkel G, Xu Q, Paik MC, Boden-Albala B, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis. (2009) 28:65–71. doi: 10.1159/000219299
11. Liu C, Yang X, Chen C. Correlation between Metabolic Syndrome and Intracranial versus Extracranial Arteriosclerosis among Chinese Patients with Stroke. Iran J Public Health. (2019) 48:1997–2006.
12. López-Cancio E, Galán A, Dorado L, Jiménez M, Hernández M, Millán M, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke. (2012) 43:2712–9. doi: 10.1161/strokeaha.112.661702
13. Bang OY, Saver JL, Liebeskind DS, Pineda S, Yun SW, Ovbiagele B. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci. (2009) 284:40–5. doi: 10.1016/j.jns.2009.03.033
14. Wang A, Li Z, Luo Y, Liu X, Guo X, Wu S, et al. Asymptomatic intracranial arterial stenosis and metabolic syndrome: the APAC study. PLoS ONE. (2014) 9:e113205. doi: 10.1371/journal.pone.0113205
15. Zhu B, Zhang L, Cheng XP, Wang L, Tian Y, Li XX, et al. The association between metabolic syndrome and asymptomatic carotid artery stenosis in menopausal women: a cross-sectional study in a Chinese population. Therap Clin Risk Manage. (2018) 14:2183–8. doi: 10.2147/tcrm.S177265
16. Sun Q, Wang Q, Wang X, Ji X, Sang S, Shao S, et al. Prevalence and cardiovascular risk factors of asymptomatic intracranial arterial stenosis: the Kongcun Town Study in Shandong, China. Eur J Neurol. (2020) 27:729–35. doi: 10.1111/ene.14144
17. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
18. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. (2000) 21:643–6. doi: 10.1016/S0925-4927(99)00050-5
19. Honish C, Sadanand V, Fladeland D, Chow V, Pirouzmand F. The reliability of ultrasound measurements of carotid stenosis compared to MRA and DSA. Can J Neurol Sci. (2005) 32:465–71. doi: 10.1017/s0317167100004455
20. Mozzini C, Roscia G, Casadei A, Cominacini L. Searching the perfect ultrasonic classification in assessing carotid artery stenosis: comparison and remarks upon the existing ultrasound criteria. J Ultrasound. (2016) 19:83–90. doi: 10.1007/s40477-016-0193-6
21. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of radiologists in ultrasound consensus conference. Radiology. (2003) 229:340–6. doi: 10.1148/radiol.2292030516
22. González-Navarro H, Vinué A, Vila-Caballer M, Fortuño A, Beloqui O, Zalba G, et al. Molecular mechanisms of atherosclerosis in metabolic syndrome: role of reduced IRS2-dependent signaling. Arterioscl Thromb Vasc Biol. (2008) 28:2187–94. doi: 10.1161/atvbaha.108.175299
23. Ahmadnezhad M, Arefhosseini SR, Parizadeh MR, Tavallaie S, Tayefi M, Darroudi S, et al. Association between serum uric acid, high sensitive C-reactive protein and pro-oxidant-antioxidant balance in patients with metabolic syndrome. BioFactors. (2018) 44:263–71. doi: 10.1002/biof.1424
24. Leng XY, Chen XY, Chook P, Xiong L, Lin WH, Liu JY, et al. Association between metabolic syndrome and carotid atherosclerosis: a community-based study in Hong Kong. Metab Syndr Relat Disord. (2013) 11:109–14. doi: 10.1089/met.2012.0099
25. Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. (1996) 27:1974–80. doi: 10.1161/01.str.27.11.1974
26. Sacco RL, Roberts JK, Boden-Albala B, Gu Q, Lin IF, Kargman DE, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. (1997) 28:929–35. doi: 10.1161/01.str.28.5.929
27. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
28. Arner P, Rydén M. Fatty Acids, Obesity and Insulin Resistance. Obesity Facts. (2015) 8:147–55. doi: 10.1159/000381224
29. Zhai W, Xu C, Ling Y, Liu S, Deng J, Qi Y, et al. Increased lipolysis in adipose tissues is associated with elevation of systemic free fatty acids and insulin resistance in perilipin null mice. Hormone Metab Res. (2010) 42:247–53. doi: 10.1055/s-0029-1243599
30. Menezes VP, Cohen C, Del-Rei J, Oigman W, Neves MF, Medeiros FJ. Evaluation of endothelial function and arterial stiffness in obese individuals with insulin resistance. Nutr Health. (2019) 25:85–92. doi: 10.1177/0260106018819374
31. Watt NT, Gage MC, Patel PA, Viswambharan H, Sukumar P, Galloway S, et al. Endothelial SHIP2 Suppresses Nox2 NADPH Oxidase-Dependent Vascular Oxidative Stress, Endothelial Dysfunction, and Systemic Insulin Resistance. Diabetes. (2017) 66:2808–21. doi: 10.2337/db17-0062
32. Lei C, Wu B, Liu M, Chen Y. Risk factors and clinical outcomes associated with intracranial and extracranial atherosclerotic stenosis acute ischemic stroke. J Stroke Cerebrovasc Dis. (2014) 23:1112–7. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.024
33. Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci. (2011) 342:135–42. doi: 10.1097/MAJ.0b013e318224a147
34. Zhang S, Zhou Y, Zhang Y, Gao X, Zhang Q, Wang A, et al. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur J Neurol. (2013) 20:1479–85. doi: 10.1111/ene.12210
35. Jin H, Peng Q, Nan D, Lv P, Liu R, Sun W, et al. Prevalence and risk factors of intracranial and extracranial artery stenosis in asymptomatic rural residents of 13 villages in China. BMC Neurol. (2017) 17:136. doi: 10.1186/s12883-017-0924-0
36. Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. (2014) 130:1407–14. doi: 10.1161/circulationaha.114.011147
Keywords: asymptomatic intracranial arterial stenosis, asymptomatic extracranial arterial stenosis, metabolic syndrome, asymptomatic cerebral arterial stenosis, stroke primary prevention
Citation: Li S, Sun X, Zhao Y, Wang X, Ji X, Sang S, Shao S, Xiang Y, Wang G, Lv M, Xue F, Sun Q and Du Y (2021) Association Between Metabolic Syndrome and Asymptomatic Cerebral Arterial Stenosis: A Cross-Sectional Study in Shandong, China. Front. Neurol. 12:644963. doi: 10.3389/fneur.2021.644963
Received: 22 December 2020; Accepted: 08 April 2021;
Published: 12 May 2021.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Mohamed Osman, St. Vincent Mercy Medical Center, United StatesPhilipp Gruber, Aarau Cantonal Hospital, Switzerland
Copyright © 2021 Li, Sun, Zhao, Wang, Ji, Sang, Shao, Xiang, Wang, Lv, Xue, Sun and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinjian Sun, c3FqMTIxMEAxNjMuY29t; Yifeng Du, ZHV5aWZlbmcyMDEzQDE2My5jb20=
†These authors have contributed equally to this work
 Shan Li
Shan Li Xiao Sun1†
Xiao Sun1† Xiang Wang
Xiang Wang Yuanyuan Xiang
Yuanyuan Xiang Ming Lv
Ming Lv Fuzhong Xue
Fuzhong Xue Qinjian Sun
Qinjian Sun Yifeng Du
Yifeng Du