- Department of Neurology, University of Campinas (UNICAMP), Campinas, Brazil
The last years have evinced a remarkable growth in neuroimaging studies around the world. All these studies have contributed to a better understanding of the cerebral outcomes of dementia, even in the earliest phases. In low- and middle-income countries, studies involving structural and functional neuroimaging are challenging due to low investments and heterogeneous populations. Outstanding the importance of diagnosing mild cognitive impairment and dementia, the purpose of this paper is to offer an overview of neuroimaging dementia research in Brazil. The review includes a brief scientometric analysis of quantitative information about the development of this field over the past 10 years. Besides, discusses some peculiarities and challenges that have limited neuroimaging dementia research in this big and heterogeneous country of Latin America. We systematically reviewed existing neuroimaging literature with Brazilian authors that presented outcomes related to a dementia syndrome, published from 2010 to 2020. Briefly, the main neuroimaging methods used were morphometrics, followed by fMRI, and DTI. The major diseases analyzed were Alzheimer's disease, mild cognitive impairment, and vascular dementia, respectively. Moreover, research activity in Brazil has been restricted almost entirely to a few centers in the Southeast region, and funding could be the main driver for publications. There was relative stability concerning the number of publications per year, the citation impact has historically been below the world average, and the author's gender inequalities are not relevant in this specific field. Neuroimaging research in Brazil is far from being developed and widespread across the country. Fortunately, increasingly collaborations with foreign partnerships contribute to the impact of Brazil's domestic research. Although the challenges, neuroimaging researches performed in the native population regarding regional peculiarities and adversities are of pivotal importance.
Introduction
The majority of people with dementia live in low- and middle-income nations, as is the case of Brazil, the largest and the most populated country in Latin America (LA). LA is experiencing an unprecedented and fast demographic change in the last decades, with the increasing aging of the population (1). As well, Brazil has experienced significant changes in the population age pyramid. Nowadays, the country counts more than 30 million people over 60 years old (14% of the population), and by 2060 this number is projected to increase to 73 million (2). Such a consequence is the increase in the prevalence of dementia cases. In LA is expected a four-fold rise in subjects with dementia by 2050 (3). In Brazil, a recent meta-analysis, which included seven Brazilian studies, found a pooled dementia prevalence of 14.3% (6.8–23.9), but with substantial heterogeneity (4).
Neuroimaging research can provide useful diagnostic images and experimental outcomes that report and support evidence-based clinical practice (5). Moreover, is an essential part of dementia workup to exclude non-neurodegenerative causes of cognitive impairment, as well as to evaluate possible patterns of brain atrophy and cerebrovascular disease (6). Since the creation of the multicentric study Alzheimer's disease Neuroimaging Initiative (ADNI) in the United States in 2004, there was a significant increase both in the number of studies and Magnetic Resonance Imaging (MRI) techniques that have contributed to better understand the cerebral repercussions of the disease, even in the earliest phases (7). After then, different techniques have been improved, like brain volumetry (automated, manual, semi-automated), voxel-based morphometry (VBM), cortical thickness analyses, diffusion tensor imaging (DTI), and functional MRI (fMRI), especially functional connectivity, among others (8).
Outstanding the importance of neuroimaging examinations in dementia, especially in Alzheimer's disease (AD) and mild cognitive impairment (MCI), we aimed to evaluate the scientometric characteristics of Brazilian research in this field in the native population. We analyzed studies published on structural and functional neuroimaging in the last decade in a manner to assess the Brazilian scientific production in this relevant area, especially regarding original research papers. Questions addressed in this review included: journals nationalities and their impact factors, if international coauthorships, authors' gender, location of the neuroimaging research centers in Brazil, the main research funding agencies, number of publications per year, number of total citations for each paper, pathologies studied, and neuroimaging techniques utilized. Moreover, we discussed the peculiarities and challenges that this kind of research could found in a miscegenated population and a resource-limited country.
Methods
PubMed (https://pubmed.ncbi.nlm.nih.gov/) was queried using the search strategy described in Supplementary Material 1. The results were inspected by IKA to select relevant matches. In brief, research papers were selected if they: (a) had a Brazilian author; (b) presented some kind of neuroimaging result, either quantitative or qualitative; (c) either concerned a primary or secondary neurological disease presenting with a dementia syndrome or represented cognitive aspects of the aging process; and (d) were published during or after the year of 2010 until to the date of access in the year of 2020.
Papers were classified according to their nature and design (e.g., review, longitudinal design, controlled trial), international participation in authorship, and journal nationality (Brazilian or international), first author gender, and the number of male and female authors. Web of Science (webofknowledge.com) was consulted for the number of citations received by each paper and the journal's impact factor (Journal Citation Reports™-JCR). Original research papers were further inspected and tabulated as to their MRI and other imaging methods (e.g.,18-FDG-PET), number of participants in each group (e.g., AD, MCI, controls), AD biomarker reporting, the Brazilian state where the study was performed, and funding agencies (the latter two were only accessed if the study concerned Brazilian participants).
Statistical analyses were performed using SciPy 1.5.3 (9), pandas 1.1.4 (10), and statsmodels 0.12.1 (11).
Results
Article Selection
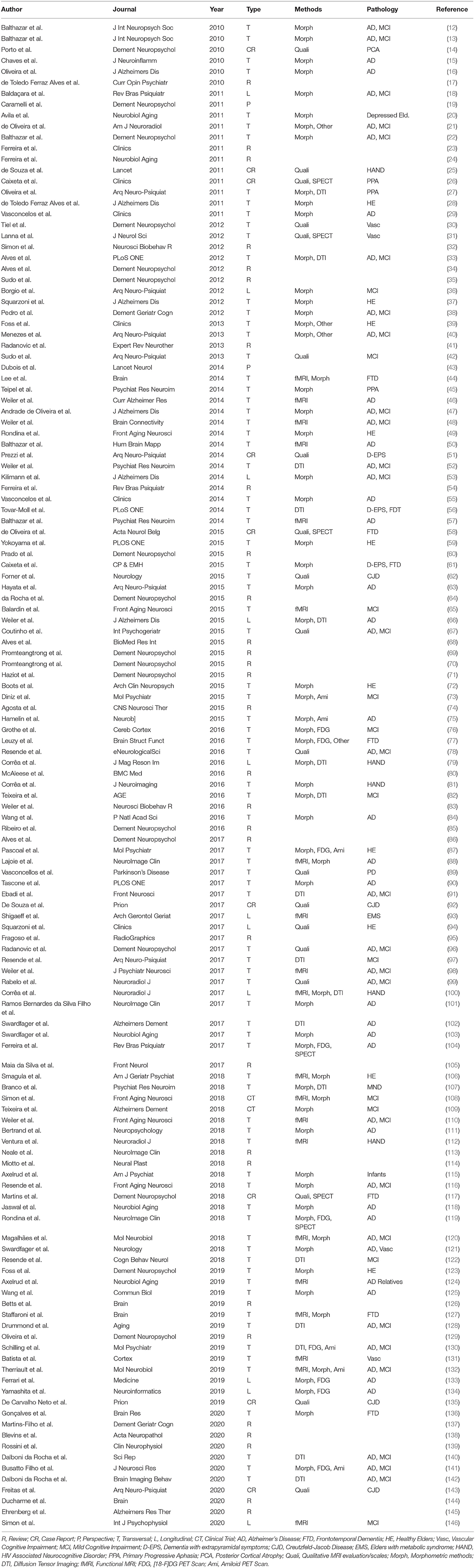
Figure 1 shows schematically the article selection process. The PubMed search resulted in 300 matches from which 135 met the aforementioned criteria. Thirty-two reviews or perspective articles were selected for a separate analysis. From the remaining 103 original research papers, 74 studied Brazilian subjects, among them: 9 case reports, 55 transversal studies, 8 longitudinal studies, and 2 controlled trials. Case reports were excluded from the main analyses. Selected articles are listed in Table 1 with the main findings, and in Supplementary Material 2 with all findings.
Reviews
Review papers found covered a wide range of topics. Nineteen out of 32 papers were published in non-Brazilian journals and 16/32 were coauthored by non-Brazilians. Concerning gender, males were the first authors in 20/32 papers, the median number of male and female authors were 5 and 2, respectively. Publication in international journals was correlated with international coauthorship (χ2 = 4.66, p = 0.031) and marginally correlated with a female first author (χ2 = 3.12, p = 0.077). The number of publications per year is presented in Figure 2. Time was not associated with an increasing number of publications during these years (Spearman ρ = 0.42, p = 0.19).
The median number of citations per article was 7 (IQR 2.75–23.75). A multivariate linear model showed a negative correlation of citation number with the Publication Year (p = 0.045). International Coauthorship, Journal Nationality, and First Author Gender showed no correlation. Due to the latency expected for an article to be cited, we repeated this analysis with papers published up to 2015, resulting in a median of 7 (IQR 6–33) citations. Regression results were non-significant. The journal's impact factor (JIF) was available for 21/32 papers, with a median of 4.35 (IQR 3.093–8.329). The multivariate regression showed no correlation with other variables.
Original Research
Figure 3 shows the characteristics of the selected papers. Concerning the number of publications per year, there was no trend toward increasing or decreasing the number of publications (Spearman ρ = 0.13, p = 0.70) (Figure 3A). The most studied pathologies were AD (54%, n = 35) and MCI (48%, n = 31), followed by vascular dementia (4.6%, n = 3) (Figure 3B). Most studies used morphometric methods (58%, n = 38) followed by fMRI (23%, n = 15) and closely by DTI (18%, n = 12) (Figure 3C). Some methods addressed by only a single study nonetheless worth mentioning included spectroscopy (40), texture analysis (21), magnetization transfer ratio, and relaxometry (39).
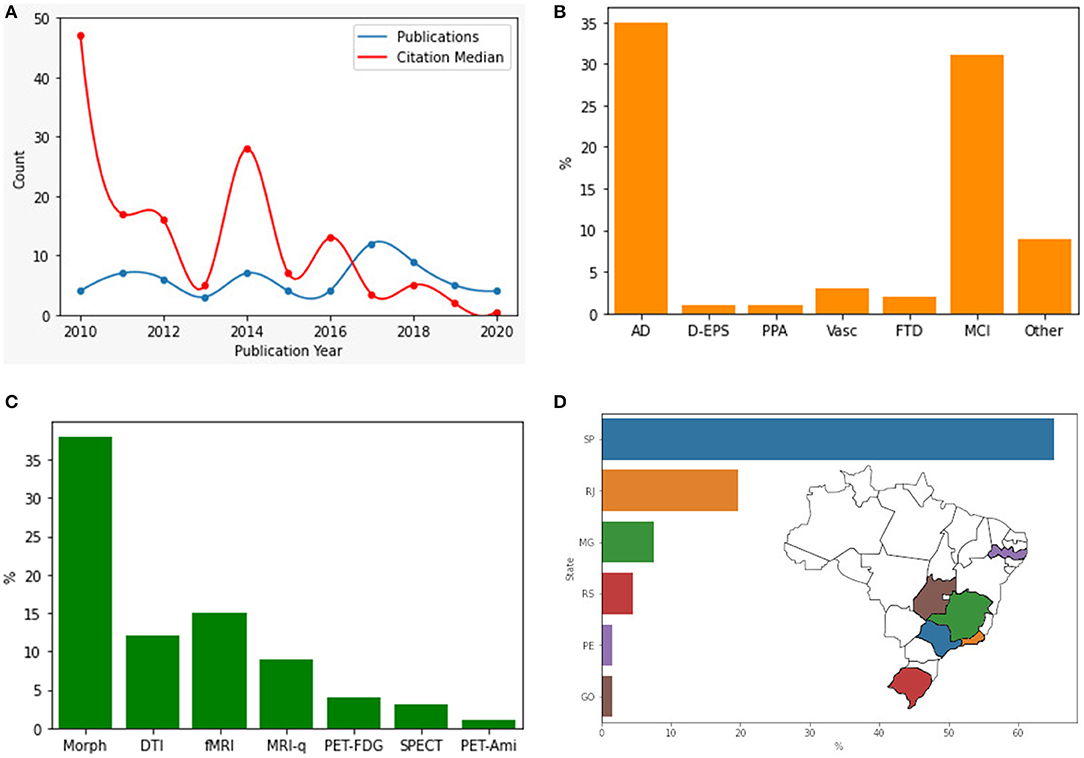
Figure 3. Research articles in different categories. (A) Original research articles published by year and citations median. (B) Pathology addressed by the article. (C) Methodology used. (D) Location of main neuroimaging research centers in Brazil. AD, Alzheimer's Disease; D-EPS, Dementia with extrapyramidal symptoms; PPA, Primary Progressive Aphasia; Vasc, Vascular Dementia; FTD, Frontotemporal Dementia; MCI, Mild Cognitive Impairment; Morph, Morphometric; DTI, Diffusion Tensor Imaging; fMRI, functional MRI; MRI-q, MRI qualitative analysis; PET-FDG, 18-Fluorodeoxyglucose positron emission tomography; PET-Ami: amyloid marker positron emission tomography.
Regarding gender analyses of original research papers, we found that females are more frequently first-authors (60%). 26/65 of the first authors are male, with a significant time effect for female authorship (Wilcoxon rank-sum test, p = 0.022). However, when considering all co-authors, males are more frequent (5/4 ratio). The median number of male and female authors was 5 and 4, respectively, with significantly more male authors per paper (Wilcoxon sign-rank test, p = 0.001). These findings might indicate that gender inequalities are less relevant in this specific field. Nineteen-out-of-sixty-five articles were co-authored by non-Brazilians. The most common nationalities among those were North-Americans (n = 14), British (n = 3), German (n = 2), Chilean (n = 2) and Swiss (n = 2).
There is great heterogeneity in the distribution of the research centers in the country. Research activity in Brazil has been restricted almost entirely to a few centers in the Southeast of Brazil. The vast majority of studies were set in the state of São Paulo (65%, n = 43), with studies also from Rio de Janeiro (20%, n = 13), Minas Gerais (7.6%, n = 5), Rio Grande do Sul (4.5%, n = 3), Pernambuco and Goiás (each with 1.5%, n = 1) (Figure 3D). Funding could be the main driver for publications. The São Paulo Research Foundation (FAPESP) was the most common funding agency, supporting 33 studies, followed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), responsible for the funding of 28 studies, and Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), with 14 studies being supported. Other agencies worth mentioning include Fundação de Apoio a Pesquisa do Estado do Rio de Janeiro (FAPERJ, 3 studies), Fundação de Apoio a Pesquisa do Estado de Minas Gerais (FAPEMIG, 4 studies), and the Welcome Thrust (3 studies). Seventeen studies did not report the source of resources.
The median number of citations received by original research papers was 5 (IQR 2–18). Considering only articles published up to 2015, the median was 17 (IQR 5–28). We produced three multivariate linear models to better understand what drives citation: (a) a regression for author and journal variables; (b) a regression for imaging technique; and (c) a model for the disease studied. All models were repeated restricting the sample to papers published up to 2015. The first model included Publication Year, International Coauthorship, First Author Gender, and Journal Nationality, showing a significant effect for publication in an international journal (p = 0.001) and the publication year (p < 0.001). Repeating the analysis with the papers up to 2015, only the effect of publication in an international journal remained significant (p = 0.037). None of the imaging techniques were associated with citation numbers either with the full or restricted sample (all ps non-significant). AD studies were associated with a higher number of citations (p = 0.003) and MCI studies showed a correlation with fewer citations (p = 0.04). In the restricted sample, only AD studies remained significant (p = 0.017).
JIF was available for 55/65 papers, with a median of 2.94 (IQR 1.90–4.35). The same models described for citations were used to predict JIF. In the first model, omitting Journal Nationality as a regressor, International Coauthorship was marginally associated with a higher JIF (p = 0.055). For imaging technique, Amyloid PET (p = 0.077) and fMRI (p = 0.061) showed a marginal positive correlation with JIF. None of the specific pathologies were associated with JIF.
Peculiarities and Challenges That Hinder Neuroimaging Dementia Research in Brazil
Dementia research in low- and middle-income regions is challenging. Like other countries in LA, due to different historical processes that have occurred since the end of the fifteenth century, Brazil has its own social, cultural, racial, and regional peculiarities (147). The heterogeneity makes the diagnosis of dementia and mild cognitive impairment particularly challenging in comparison with developed countries (148). Regarding specific biological characteristics, for example, we far from understand the particularities of Brazilians miscegenated population. The regional genomic distribution of Brazilians is linked with the different colonization history of each region. Genetic admixture has been influenced by the colonization process, resulting in Brazil becoming a genetically trihybrid population (genomic inheritance of European, African, and Amerindian groups have been traced) (147). Previous epidemiological studies have highlighted that overall dementia prevalence can vary substantially across different ethnic groups and geographical regions (149). These differences in dementia prevalence rates have been attributed to different susceptibility to pathological brain changes in each ethnicity (150). In this sense, neuroimaging research in Brazil should consider these aspects. Neuroimaging studies are required to better characterize how subclinical brain changes might differ among ethnicities, and whether such differences may help explain differences in cognitive performance.
Neuroimaging research has provided evidence that previous or current adversities, such as low socioeconomic status or low levels of educational attainment, may reflect on interindividual variations in brain imaging measurements. Analysis from elderly individuals, recruited in an economically underprivileged area of São Paulo, showed reductions in both regional brain volumes and glucose metabolism in subjects with disadvantageous socioeconomic backgrounds (151, 152). Furthermore, education has a great impact on cognitive performance in older adults (153). A population census found that in 2018 nearly 52.6% of Brazilians over 25 years old did not have finished elementary school, and around 7.2% were unable to read or write (2). Variations in regional brain volumes were verified depending on the level of previous educational attainment (154). In this sense, ecological cognitive tests adapted to Brazilian characteristics (ex: including a wide range of schooling levels, illiterates, and stratified into groups of age and education) are important to be applied to more sophisticated methods, like body fluid biomarkers and neuroimaging.
Among chronic non-communicable diseases, those of the circulatory system are also the main cause of mortality worldwide, including Brazil, which has one of the highest rates in LA (155). Cerebrovascular damage, produced by midlife hypertension, diabetes, dyslipidemia, among other factors, may contribute to the onset and progression of cognitive dysfunction and dementia (156). Besides, Brazilians may have more cerebrovascular damage than other populations, as shown by Grinberg et al. (157) in a clinicopathological study with 1,291 individuals. In Brazil, cerebrovascular damage is one of the most neglected diseases, due to poor control of cardiovascular factors, especially hypertension, the main risk factor (155). In this context, it is surprising that only 4.6% of Brazilian original neuroimaging research was focused on vascular cognitive impairment. Dementia neuroimaging research in Brazil is highly focused on AD. Although AD is the most prevalent form of dementia, our results showed a disproportionate predominance to dementia epidemiology (158). The widespread interest in new drugs for AD may partially explain this finding (159). However, our study also showed that research involving AD was more likely to be cited, potentially feeding a vicious cycle. The underrepresentation of vascular dementia is particularly worrisome, as vascular risk factors and vascular pathology–either exclusive or mixed–are highly prevalent in Brazil. Once improvements in neuroimaging techniques allow detailed and sophisticated evaluation of many manifestations of cerebrovascular diseases, this topic must be considered a priority among Brazilian researchers.
The need for studies with the Brazilian population in this research field is an urgent matter. Scientific research, in general, is far from being fully developed and widespread across the country. Nowadays, even though Brazil is the 13th largest producer of research publications globally, its citation impact has historically been below the world average (160). The present work highlights some of the virtues and faults of the dementia neuroimaging research scenario in Brazil. Most of our findings are consistent with the Brazilian general scientific research background: a significant growth during the first decade of the twenty first century followed by relative stability. Furthermore, the trend toward a highly concentrated scientific production in the Southeast region along with average-to-low research impact also reflects the national tendency (160). Finally, health research is particularly affected by spatial restriction in the national territory, as the cultural, ethnic, and socioeconomic diversity is not captured by the published depictions of our reality.
Brazil has limited wherewithals, sequential financial crises, bad investment of financial resources, and a lack of priority in investing in science in the different governments. All these factors limit the quality of scientific research performed in Brazil and delay the incorporation of novelties to generate original scientific data of global relevance. One of the consequences of these facts was the failure to implement Brazilian ADNI. Lack of fundings, heterogeneity of resources, and lack of specialized centers across the different regions of the country have hampered the implementation of a large national multicenter study. Besides, only recently Brazilian researchers have started studying molecular neuroimaging, with only five amyloid PET studies, and no Tau PET studies in the last decade. Despite these difficulties, Brazilians are studying and refining new neuroimaging methods, such as functional and structural connectivity, DTI, and surface-based morphometry. Two Brazilian centers in São Paulo and Rio Grande do Sul are studying amyloid PET, and collaborative studies are taking place. Comparisons of Brazilian neuroimaging studies with other countries of Latin America are difficult, due to the lack of relevant studies in this research area as they share the same problems found in Brazil. However, our neighbor Argentina is moving forward in the field, with the establishment of the first ADNI of Latin America (161). This program currently accounts for approximately sixty participants that are evaluated by structural MRI analysis, and metabolic and amyloid PET scan (FDG and PiB). This kind of multicentric program notably will assist the development of neuroimaging studies in low- and middle-income nations in the future.
Fortunately, increasingly Brazilian researchers are working across country borders, within foreign partnerships, and the resulting papers contribute to the impact of Brazil's domestic research. Although the majority of foreign partnerships analyzed in this review were derived from North America and Europe, there are efforts to develop collaborations with our neighbors of LA. One promising group is the Latin America and Caribbean Consortium on Dementia (LAC-CD), which is a regional organization that oversees and promotes clinical and research activities on dementia. Collaborations like this certainly can set new networks to support research and increase the supply of regional and international grant proposals (162). Taken together, suggests that knowledge and technological exchange can drive the Brazilian research scenario toward a richer production. All the above-mentioned challenges require efforts toward solutions involving clinicians, researchers, and policymakers, to better understand and investigate the dementia context in a continental country such as Brazil.
Concluding Remarks
As illustrated along with this manuscript, neuroimaging research carried out in low- and middle-income countries, such as Brazil, are challenging. Nonetheless, they are extremely important to increase the global knowledge about brain impacts derived from the inherent characteristics of the population, and their relationship with the development of dementia. Neuroimaging researches performed in the native population regarding regional peculiarities and adversities are of pivotal importance, especially in a resource-limited country facing economic and political adversities. In this sense, neuroimaging studies should address dementia not merely from a clinical perspective, but also in a societal context, considering individuals' environment and peculiarities. Despite the aforementioned limitations, Brazilian researchers in dementia should be encouraged to deepen neuroimaging studies in Alzheimer's spectrum and other prevalent conditions, such as vascular dementia.
Because our focus was neurodegenerative diseases that primarily affect cognition, we did not evaluate normal aging or other conditions that may secondarily lead to dementia, such as Parkinson's disease, Motor Neuron diseases, Epilepsy, or infectious/parasitic diseases common in Brazil. Further studies might consider the whole spectrum of dementias.
Author Contributions
All authors contributed to the preparation and writing manuscript and approved the submitted version.
Funding
This work was supported by grants from the São Paulo Research Foundation (FAPESP) (grants numbers: 18/15571-7 and 2019/23028-4).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.640525/full#supplementary-material
References
1. Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: epidemiological evidence and implications for public policy. Front Aging Neurosci. (2017) 9:221. doi: 10.3389/fnagi.2017.00221
2. IBGE. Instituto Brasileiro de Geografia e Estatística. (2020) Available online at: https://www.ibge.gov.br/ (accessed November 10, 2020).
3. Parra MA, Baez S, Allegri R, Nitrini R, Lopera F, Slachevsky A, et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology. (2018) 90:222–31. doi: 10.1212/WNL.0000000000004897
4. Farina N, Ibnidris A, Alladi S, Comas-Herrera A, Albanese E, Docrat S, et al. A systematic review and meta-analysis of dementia prevalence in seven developing countries: a STRiDE project. Glob Public Health. (2020) 15:1878–93. doi: 10.1080/17441692.2020.1792527
5. Márquez F, Yassa MA. Neuroimaging biomarkers for Alzheimer's d ase. Mol Neurodegener. (2019) 14:21. doi: 10.1186/s13024-019-0325-5
6. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. (2019) 322:1589–99. doi: 10.1001/jama.2019.4782
7. Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. (2008) 27:685–91. doi: 10.1002/jmri.21049
8. Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, et al. Impact of the Alzheimer's disease neuroimaging initiative, 2004 to 2014. Alzheimer's Dement. (2015) 11:865–84. doi: 10.1016/j.jalz.2015.04.005
9. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. (2020) 17:261–72. doi: 10.1038/s41592-019-0686-2
10. McKinney W, et al. Data structures for statistical computing in python. Proc Python Sci Conf. (2010) 445:51–6. doi: 10.25080/Majora-92bf1922-00a
11. Seabold S, Perktold K. Statsmodels J. Econometric and statistical modeling with python. Proc Python Sci Conf. (2010) 445:92–6. doi: 10.25080/Majora-92bf1922-011
12. Balthazar ML, Yasuda CL, Pereira FR, Bergo FP, Cendes F, Damasceno BP. Coordinated and circumlocutory semantic naming errors are related to anterolateral temporal lobes in mild AD, amnestic mild cognitive impairment, and normal aging. J Int Neuropsychol Soc. (2010) 16:1099–107. doi: 10.1017/S1355617710000998
13. Balthazar ML, Yasuda CL, Cendes F, Damasceno BP. Learning, retrieval, and recognition are compromised in aMCI and mild AD: are distinct episodic memory processes mediated by the same anatomical structures? J Int Neuropsychol Soc. (2010) 16:205–9. doi: 10.1017/S1355617709990956
14. Porto FHG, Machado GCL, Morillo LS, Brucki SMD. Progressive posterior cortical dysfunction. Dement Neuropsychol. (2010) 4:75–8. doi: 10.1590/S1980-57642010DN40100013
15. Chaves ML, Camozzato AL, Ferreira ED, Piazenski I, Kochhann R, Dall'Igna O, et al. Serum levels of S100B and NSE proteins in Alzheimer's disease patients. J Neuroinflammation. (2010) 7:6. doi: 10.1186/1742-2094-7-6
16. Oliveira PP, Nitrini R, Busatto G, Buchpiguel C, Sato JR, Amaro E. Use of SVM methods with surface-based cortical and volumetric subcortical measurements to detect Alzheimer's disease. J Alzheimers Dis. (2010) 19:1263–72. doi: 10.3233/JAD-2010-1322
17. de Toledo Ferraz Alves TC, Ferreira LK, Busatto GF. Vascular diseases and old age mental disorders: an update of neuroimaging findings. Curr Opin Psychiatry. (2010) 23:491–7. doi: 10.1097/YCO.0b013e32833e339c
18. Baldaçara L, Borgio JG, Moraes WA, Lacerda AL, Montaño MB, Tufik S, et al. Cerebellar volume in patients with dementia. Braz J Psychiatry. (2011) 33:122–9. doi: 10.1590/S1516-44462011000200006
19. Caramelli P, Teixeira AL, Buchpiguel CA, Lee HW, Livramento JA, Fernandez LL, et al. Diagnosis of Alzheimer's disease in Brazil: supplementary exams. Dement Neuropsychol. (2011) 5:167–77. doi: 10.1590/S1980-57642011DN05030004
20. Avila R, Ribeiz S, Duran FL, Arrais JP, Moscoso MA, Bezerra DM, et al. Effect of temporal lobe structure volume on memory in elderly depressed patients. Neurobiol Aging. (2011) 32:1857–67. doi: 10.1016/j.neurobiolaging.2009.11.004
21. de Oliveira MS, Balthazar ML, D'Abreu A, Yasuda CL, Damasceno BP, Cendes F, et al. MR imaging texture analysis of the corpus callosum and thalamus in amnestic mild cognitive impairment and mild Alzheimer disease. AJNR Am J Neuroradiol. (2011) 32:60–6. doi: 10.3174/ajnr.A2232
22. Balthazar MLF, Yasuda CL, Lopes TM, Pereira FRS, Damasceno BP, Cendes F. Neural correlates of lexical-semantic memory: a voxel-based morphometry study in mild AD, aMCI and normal aging. Dement Neuropsychol. (2011) 5:69–77. doi: 10.1590/S1980-57642011DN05020003
23. Ferreira LK, Busatto GF. Neuroimaging in Alzheimer's disease: current role in clinical practice and potential future applications. Clinics (São Paulo). (2011) 66(Suppl .1):19–24. doi: 10.1590/S1807-59322011001300003
24. Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. Neurostructural predictors of Alzheimer's disease: a meta-analysis of VBM studies. Neurobiol Aging. (2011) 32:1733–41. doi: 10.1016/j.neurobiolaging.2009.11.008
25. de Souza RK, Martins RT, da Rocha SF, Sato HK, Kowacs PA. Never too old. Lancet. (2011) 378:1676. doi: 10.1016/S0140-6736(11)61225-7
26. Caixeta L, Caixeta M. Primary progressive aphasia beginning with a psychiatric disorder. Clinics (São Paulo). (2011) 66:1505–8. doi: 10.1590/S1807-59322011000800035
27. Oliveira FP, Costa JC, Marroni SP, Silva AM, Barreiro SH, Maeda FK, et al. Primary progressive aphasia patients evaluated using diffusion tensor imaging and voxel based volumetry-preliminary results. Arq Neuropsiquiatr. (2011) 69:446–51. doi: 10.1590/S0004-282X2011000400007
28. de Toledo Ferraz Alves TC, Scazufca M, Squarzoni P, de Souza Duran FL, Tamashiro-Duran JH, Vallada HP, et al. Subtle gray matter changes in temporo-parietal cortex associated with cardiovascular risk factors. J Alzheimers Dis. (2011) 27:575–89. doi: 10.3233/JAD-2011-110827
29. Vasconcelos LeG, Jackowski AP, Oliveira MO, Flor YM, Bueno OF, Brucki SM. Voxel-based morphometry findings in Alzheimer's disease: neuropsychiatric symptoms and disability correlations - preliminary results. Clinics (São Paulo). (2011) 66:1045–50. doi: 10.1590/S1807-59322011000600021
30. Tiel C, Sudo FK, Alves CEO, Alves GS, Ericeira-Valente L, Moreira DM, et al. Behavioral and psychological symptoms and hippocampal atrophy in subcortical ischaemic vascular disease. Dement Neuropsychol. (2012) 6:175–9. doi: 10.1590/S1980-57642012DN06030011
31. Lanna ME, Alves CE, Sudo FK, Alves G, Valente L, Moreira DM, et al. Cognitive disconnective syndrome by single strategic strokes in vascular dementia. J Neurol Sci. (2012) 322:176–83. doi: 10.1016/j.jns.2012.08.004
32. Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev. (2012) 36:1163–78. doi: 10.1016/j.neubiorev.2012.01.007
33. Alves GS, O'Dwyer L, Jurcoane A, Oertel-Knöchel V, Knöchel C, Prvulovic D, et al. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS ONE. (2012) 7:e52859. doi: 10.1371/journal.pone.0052859
34. Alves GS, Sudo FK, Alves CEO, Ericeira-Valente L, Moreira DM, Engelhardt E, et al. Diffusion tensor imaging studies in vascular disease: a review of the literature. Dement Neuropsychol. (2012) 6:158–63. doi: 10.1590/S1980-57642012DN06030008
35. Sudo FK, Alves CEO, Alves GS, Ericeira-Valente L, Tiel C, Moreira DM, et al. Dysexecutive syndrome and cerebrovascular disease in non-amnestic mild cognitive impairment: a systematic review of the literature. Dement Neuropsychol. (2012) 6:145–51. doi: 10.1590/S1980-57642012DN06030006
36. Borgio JG, Baldaçara L, Moraes WoS, Lacerda AL, Montaño MB, Jackowski AP, et al. Hippocampal volume and CDR-SB can predict conversion to dementia in MCI patients. Arq Neuropsiquiatr. (2012) 70:839–42. doi: 10.1590/S0004-282X2012001100003
37. Squarzoni P, Tamashiro-Duran J, Souza Duran FL, Santos LC, Vallada HP, Menezes PR, et al. Relationship between regional brain volumes and cognitive performance in the healthy aging: an MRI study using voxel-based morphometry. J Alzheimers Dis. (2012) 31:45–58. doi: 10.3233/JAD-2012-111124
38. Pedro T, Weiler M, Yasuda CL, D'Abreu A, Damasceno BP, Cendes F, et al. Volumetric brain changes in thalamus, corpus callosum and medial temporal structures: mild Alzheimer's disease compared with amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. (2012) 34:149–55. doi: 10.1159/000342118
39. Foss MP, Diniz PR, Formigheri P, Salmon CE, Speciali JG, Santos AC. Magnetic resonance imaging and neuropsychological testing in the spectrum of normal aging. Clinics (São Paulo). (2013) 68:1197–205. doi: 10.6061/clinics/2013(09)04
40. Menezes TL, Andrade-Valença LP, Valença MM. Magnetic resonance imaging study cannot individually distinguish individuals with mild cognitive impairment, mild Alzheimer's disease, and normal aging. Arq Neuropsiquiatr. (2013) 71:207–12. doi: 10.1590/0004-282X20130003
41. Radanovic M, Pereira FR, Stella F, Aprahamian I, Ferreira LK, Forlenza OV, et al. White matter abnormalities associated with Alzheimer's disease and mild cognitive impairment: a critical review of MRI studies. Expert Rev Neurother. (2013) 13:483–93. doi: 10.1586/ern.13.45
42. Sudo FK, Alves CE, Alves GS, Ericeira-Valente L, Tiel C, Moreira DM, et al. White matter hyperintensities, executive function and global cognitive performance in vascular mild cognitive impairment. Arq Neuropsiquiatr. (2013) 71:431–6. doi: 10.1590/0004-282X20130057
43. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. (2014) 13:614–29. doi: 10.1016/S1474-4422(14)70090-0
44. Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. (2014) 137:3047–60. doi: 10.1093/brain/awu248
45. Teipel SJ, Flatz W, Ackl N, Grothe M, Kilimann I, Bokde AL, et al. Brain atrophy in primary progressive aphasia involves the cholinergic basal forebrain and Ayala's nucleus. Psychiatry Res. (2014) 221:187–94. doi: 10.1016/j.pscychresns.2013.10.003
46. Weiler M, Fukuda A, Massabki LH, Lopes TM, Franco AR, Damasceno BP, et al. Default mode, executive function, and language functional connectivity networks are compromised in mild Alzheimer's disease. Curr Alzheimer Res. (2014) 11:274–82. doi: 10.2174/1567205011666140131114716
47. Andrade de Oliveira A, Carthery-Goulart MT, Oliveira Júnior PP, Carrettiero DC, Sato JR. Defining multivariate normative rules for healthy aging using neuroimaging and machine learning: an application to Alzheimer's disease. J Alzheimers Dis. (2015) 43:201–12. doi: 10.3233/JAD-140189
48. Weiler M, Teixeira CV, Nogueira MH, de Campos BM, Damasceno BP, Cendes F, et al. Differences and the relationship in default mode network intrinsic activity and functional connectivity in mild Alzheimer's disease and amnestic mild cognitive impairment. Brain Connect. (2014) 4:567–74. doi: 10.1089/brain.2014.0234
49. Rondina JM, Squarzoni P, Souza-Duran FL, Tamashiro-Duran JH, Scazufca M, Menezes PR, et al. Framingham coronary heart disease risk score can be predicted from structural brain images in elderly subjects. Front Aging Neurosci. (2014) 6:300. doi: 10.3389/fnagi.2014.00300
50. Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer's disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. (2014) 35:1237–46. doi: 10.1002/hbm.22248
51. Prezzi ED, Vasconcellos LF, Marussi VH. Overlapping MRI findings in progressive supranuclear palsy - corticobasal syndrome. Arq Neuropsiquiatr. (2014) 72:569–70. doi: 10.1590/0004-282X20140065
52. Weiler M, de Campos BM, Nogueira MH, Pereira Damasceno B, Cendes F, Balthazar ML. Structural connectivity of the default mode network and cognition in Alzheimer?s disease. Psychiatry Res. (2014) 223:15–22. doi: 10.1016/j.pscychresns.2014.04.008
53. Kilimann I, Grothe M, Heinsen H, Alho EJ, Grinberg L, Amaro E, et al. Subregional basal forebrain atrophy in Alzheimer's disease: a multicenter study. J Alzheimers Dis. (2014) 40:687–700. doi: 10.3233/JAD-132345
54. Ferreira LK, Tamashiro-Duran JH, Squarzoni P, Duran FL, Alves TC, Buchpiguel CA, et al. The link between cardiovascular risk, Alzheimer's disease, and mild cognitive impairment: support from recent functional neuroimaging studies. Braz J Psychiatry. (2014) 36:344–57. doi: 10.1590/1516-4446-2013-1275
55. Vasconcelos LeG, Jackowski AP, Oliveira MO, Flor YM, Souza AA, Bueno OF. The thickness of posterior cortical areas is related to executive dysfunction in Alzheimer's disease. Clinics (São Paulo). (2014) 69:28–37. doi: 10.6061/clinics/2014(01)05
56. Tovar-Moll F, de Oliveira-Souza R, Bramati IE, Zahn R, Cavanagh A, Tierney M, et al. White matter tract damage in the behavioral variant of frontotemporal and corticobasal dementia syndromes. PLoS ONE. (2014) 9:e102656. doi: 10.1371/journal.pone.0102656
57. Balthazar ML, de Campos BM, Franco AR, Damasceno BP, Cendes F. Whole cortical and default mode network mean functional connectivity as potential biomarkers for mild Alzheimer's disease. Psychiatry Res. (2014) 221:37–42. doi: 10.1016/j.pscychresns.2013.10.010
58. de Oliveira FF, de Barros LA, Bertolucci PH. A patient with agrammatic primary progressive aphasia developing frontotemporal dementia. Acta Neurol Belg. (2015) 115:763–6. doi: 10.1007/s13760-015-0446-8
59. Yokoyama JS, Lee AK, Takada LT, Busovaca E, Bonham LW, Chao SZ, et al. Apolipoprotein ε4 is associated with lower brain volume in cognitively normal Chinese but not white older adults. PLoS ONE. (2015) 10:e0118338. doi: 10.1371/journal.pone.0118338
60. Prado LGR, Bicalho ICS, Magalhães D, Caramelli P, Teixeira AL, de Souza LC. C9ORF72 and the FTD-ALS spectrum: a systematic review of neuroimaging studies. Dement Neuropsychol. (2015) 9:413–21. doi: 10.1590/1980-57642015DN94000413
61. Caixeta L, Vieira RT, Paes F, Carta MG, Nardi AE, Arias-Carrión O, et al. Comparative study of subcortical atrophy in patients with frontotemporal dementia and dementia with extrapyramidal signs. Clin Pract Epidemiol Ment Health. (2015) 11:125–9. doi: 10.2174/1745017901511010125
62. Forner SA, Takada LT, Bettcher BM, Lobach IV, Tartaglia MC, Torres-Chae C, et al. Comparing CSF biomarkers and brain MRI in the diagnosis of sporadic Creutzfeldt-Jakob disease. Neurol Clin Pract. (2015) 5:116–25. doi: 10.1212/CPJ.0000000000000111
63. Hayata TT, Bergo FP, Rezende TJ, Damasceno A, Damasceno BP, Cendes F, et al. Cortical correlates of affective syndrome in dementia due to Alzheimer's disease. Arq Neuropsiquiatr. (2015) 73:553–60. doi: 10.1590/0004-282X20150068
64. da Rocha AJ, Nunes RH, Maia ACM. Dementia in motor neuron disease: reviewing the role of MRI in diagnosis. Dement Neuropsychol. (2015) 9:369–79. doi: 10.1590/1980-57642015DN94000369
65. Balardin JB, Batistuzzo MC, Martin MaG, Sato JR, Smid J, Porto C, et al. Differences in prefrontal cortex activation and deactivation during strategic episodic verbal memory encoding in mild cognitive impairment. Front Aging Neurosci. (2015) 7:147. doi: 10.3389/fnagi.2015.00147
66. Weiler M, Agosta F, Canu E, Copetti M, Magnani G, Marcone A, et al. Following the spreading of brain structural changes in Alzheimer's disease: a longitudinal, multimodal MRI study. J Alzheimers Dis. (2015) 47:995–1007. doi: 10.3233/JAD-150196
67. Coutinho G, Drummond C, de Oliveira-Souza R, Moll J, Tovar-Moll F, Mattos P. Immediate story recall in elderly individuals with memory complaints: how much does it contribute to memory assessment? Int Psychogeriatr. (2015) 27:1679–86. doi: 10.1017/S1041610215000307
68. Alves GS, Oertel Knöchel V, Knöchel C, Carvalho AF, Pantel J, Engelhardt E, et al. Integrating retrogenesis theory to Alzheimer's disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity. Biomed Res Int. (2015) 2015:291658. doi: 10.1155/2015/291658
69. Promteangtrong C, Kolber M, Ramchandra P, Moghbel M, Houshmand S, Schöll M, et al. Multimodality imaging approach in Alzheimer disease. Part I: structural MRI, functional MRI, diffusion tensor imaging and magnetization transfer imaging. Dement Neuropsychol. (2015) 9:318–29. doi: 10.1590/1980-57642015DN94000318
70. Promteangtrong C, Kolber M, Ramchandra P, Moghbel M, Houshmand S, Schöll M, et al. Multimodality imaging approaches in Alzheimer's disease. Part II: 1H MR spectroscopy, FDG PET and Amyloid PET. Dement Neuropsychol. (2015) 9:330–42. doi: 10.1590/1980-57642015DN94000330
71. Haziot MEJ, Barbosa Junior SP, Vidal JE, de Oliveira FTM, de Oliveira ACP. Neuroimaging of HIV-associated neurocognitive disorders. Dement Neuropsychol. (2015) 9:380–4. doi: 10.1590/1980-57642015DN94000380
72. Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, et al. Occupational complexity and cognitive reserve in a middle-aged Cohort at risk for Alzheimer's disease. Arch Clin Neuropsychol. (2015) 30:634–42. doi: 10.1093/arclin/acv041
73. Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry. (2015) 20:594–601. doi: 10.1038/mp.2014.76
74. Agosta F, Weiler M, Filippi M. Propagation of pathology through brain networks in neurodegenerative diseases: from molecules to clinical phenotypes. CNS Neurosci Ther. (2015) 21:754–67. doi: 10.1111/cns.12410
75. Hamelin L, Bertoux M, Bottlaender M, Corne H, Lagarde J, Hahn V, et al. Sulcal morphology as a new imaging marker for the diagnosis of early onset Alzheimer's disease. Neurobiol Aging. (2015) 36:2932–9. doi: 10.1016/j.neurobiolaging.2015.04.019
76. Grothe MJ, Heinsen H, Amaro E, Grinberg LT, Teipel SJ. Cognitive correlates of basal forebrain atrophy and associated cortical hypometabolism in mild cognitive impairment. Cereb Cortex. (2016) 26:2411–26. doi: 10.1093/cercor/bhv062
77. Leuzy A, Zimmer ER, Dubois J, Pruessner J, Cooperman C, Soucy JP, et al. In vivo characterization of metabotropic glutamate receptor type 5 abnormalities in behavioral variant FTD. Brain Struct Funct. (2016) 221:1387–402. doi: 10.1007/s00429-014-0978-3
78. Resende EPF, Costa-Silva L, Carmona KC, Machado TH, Machado JCB, Guimarães HC, et al. Ischemic cerebrovascular burden evaluated by magnetic resonance imaging in an elderly Brazilian community: the Pietà study. eNeurologicalSci. (2016) 5:30–4. doi: 10.1016/j.ensci.2016.11.011
79. Corrêa DG, Zimmermann N, Tukamoto G, Doring T, Ventura N, Leite SC, et al. Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging. (2016) 44:1262–9. doi: 10.1002/jmri.25263
80. McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. (2016) 14:129. doi: 10.1186/s12916-016-0676-5
81. Corrêa DG, Zimmermann N, Netto TM, Tukamoto G, Ventura N, de Castro Bellini Leite S, et al. Regional cerebral gray matter volume in HIV-positive patients with executive function deficits. J Neuroimaging. (2016) 26:450–7. doi: 10.1111/jon.12327
82. Teixeira CV, Rezende TJ, Weiler M, Nogueira MH, Campos BM, Pegoraro LF, et al. Relation between aerobic fitness and brain structures in amnestic mild cognitive impairment elderly. Age (Dordr). (2016) 38:51. doi: 10.1007/s11357-016-9912-3
83. Weiler M, Northoff G, Damasceno BP, Balthazar MLF. Self, cortical midline structures and the resting state: implications for Alzheimer's disease. Neurosci Biobehav Rev. (2016) 68:245–55. doi: 10.1016/j.neubiorev.2016.05.028
84. Wang Y, Necus J, Kaiser M, Mota B. Universality in human cortical folding in health and disease. Proc Natl Acad Sci U S A. (2016) 113:12820–5. doi: 10.1073/pnas.1610175113
85. Ribeiro LG, Busatto G. Voxel-based morphometry in Alzheimers disease and mild cognitive impairment: systematic review of studies addressing the frontal lobe. Dement Neuropsychol. (2016) 10:104–12. doi: 10.1590/S1980-5764-2016DN1002006
86. Alves GS, de Carvalho LA, Sudo FK, Briand L, Laks J, Engelhardt E. A panel of clinical and neuropathological features of cerebrovascular disease through the novel neuroimaging methods. Dement Neuropsychol. (2017) 11:343–55. doi: 10.1590/1980-57642016dn11-040003
87. Pascoal TA, Mathotaarachchi S, Mohades S, Benedet AL, Chung CO, Shin M, et al. Amyloid-β and hyperphosphorylated tau synergy drives metabolic decline in preclinical Alzheimer's disease. Mol Psychiatry. (2017) 22:306–11. doi: 10.1038/mp.2016.37
88. Lajoie I, Nugent S, Debacker C, Dyson K, Tancredi FB, Badhwar A, et al. Application of calibrated fMRI in Alzheimer's disease. Neuroimage Clin. (2017) 15:348–58. doi: 10.1016/j.nicl.2017.05.009
89. Vasconcellos LF, Pereira JS, Adachi M, Greca D, Cruz M, Malak AL, et al. Correlation of MRI visual scales with neuropsychological profile in mild cognitive impairment of Parkinson's disease. Parkinsons Dis. (2017) 2017:7380102. doi: 10.1155/2017/7380102
90. Tascone LDS, Payne ME, MacFall J, Azevedo D, de Castro CC, Steffens DC, et al. Cortical brain volume abnormalities associated with few or multiple neuropsychiatric symptoms in Alzheimer's disease. PLoS ONE. (2017) 12:e0177169. doi: 10.1371/journal.pone.0177169
91. Ebadi A, Dalboni da Rocha JL, Nagaraju DB, Tovar-Moll F, Bramati I, Coutinho G, et al. Ensemble classification of Alzheimer's disease and mild cognitive impairment based on complex graph measures from diffusion tensor images. Front Neurosci. (2017) 11:56. doi: 10.3389/fnins.2017.00056
92. De Souza RKM, Josviak ND, Batistela MS, Santos PSF, Landemberger MC, Ramina R. First case of V180I rare mutation in a Brazilian patient with Creutzfeldt-Jakob disease. Prion. (2017) 11:465–8. doi: 10.1080/19336896.2017.1397869
93. Shigaeff N, Amaro E, Franco FGM, Jacinto AF, Chiochetta G, Cendoroglo MS, et al. Functional magnetic resonance imaging response as an early biomarker of cognitive decline in elderly patients with metabolic syndrome. Arch Gerontol Geriatr. (2017) 73:1–7. doi: 10.1016/j.archger.2017.07.002
94. Squarzoni P, Tamashiro-Duran JH, Duran FLS, Leite CC, Wajngarten M, Scazufca M, et al. High frequency of silent brain infarcts associated with cognitive deficits in an economically disadvantaged population. Clinics (São Paulo). (2017) 72:474–80. doi: 10.6061/clinics/2017(08)04
95. Fragoso DC, Gonçalves Filho AL, Pacheco FT, Barros BR, Aguiar Littig I, Nunes RH, et al. Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics. (2017) 37:234–57. doi: 10.1148/rg.2017160075
96. Radanovic M, Stella F, Silva LG, Talib LL, Forlenza OV. Increased CSF levels of total Tau in patients with subcortical cerebrovascular pathology and cognitive impairment. Dement Neuropsychol. (2017) 11:419–25. doi: 10.1590/1980-57642016dn11-040012
97. Resende EPF, Tovar-Moll FF, Ferreira FM, Bramati I, de Souza LC, Carmona KC, et al. Integrity of white matter structure is related to episodic memory performance in the low-educated elderly. Arq Neuropsiquiatr. (2017) 75:778–84. doi: 10.1590/0004-282x20170158
98. Weiler M, de Campos BM, Teixeira CVL, Casseb RF, Carletti-Cassani AFMK, Vicentini JE, et al. Intranetwork and internetwork connectivity in patients with Alzheimer disease and the association with cerebrospinal fluid biomarker levels. J Psychiatry Neurosci. (2017) 42:366–77. doi: 10.1503/jpn.160190
99. Rabelo AG, Teixeira CV, Magalhães TN, Carletti-Cassani AFM, Amato Filho AC, Joaquim HP, et al. Is cerebral microbleed prevalence relevant as a biomarker in amnestic mild cognitive impairment and mild Alzheimer's disease? Neuroradiol J. (2017) 30:477–85. doi: 10.1177/1971400917720465
100. Corrêa DG, Zimmermann N, Ventura N, Tukamoto G, Doring T, Leite SC, et al. Longitudinal evaluation of resting-state connectivity, white matter integrity and cortical thickness in stable HIV infection: preliminary results. Neuroradiol J. (2017) 30:535–45. doi: 10.1177/1971400917739273
101. Ramos Bernardes da Silva Filho S, Oliveira Barbosa JH, Rondinoni C, Dos Santos AC, Garrido Salmon CE, da Costa Lima NK, et al. Neuro-degeneration profile of Alzheimer's patients: a brain morphometry study. Neuroimage Clin. (2017) 15:15–24. doi: 10.1016/j.nicl.2017.04.001
102. Swardfager W, Yu D, Ramirez J, Cogo-Moreira H, Szilagyi G, Holmes MF, et al. Peripheral inflammatory markers indicate microstructural damage within periventricular white matter hyperintensities in Alzheimer's disease: a preliminary report. Alzheimers Dement (Amst). (2017) 7:56–60. doi: 10.1016/j.dadm.2016.12.011
103. Swardfager W, Yu D, Scola G, Cogo-Moreira H, Chan P, Zou Y, et al. Peripheral lipid oxidative stress markers are related to vascular risk factors and subcortical small vessel disease. Neurobiol Aging. (2017) 59:91–7. doi: 10.1016/j.neurobiolaging.2017.06.029
104. Ferreira LK, Rondina JM, Kubo R, Ono CR, Leite CC, Smid J, et al. Support vector machine-based classification of neuroimages in Alzheimer's disease: direct comparison of FDG-PET, rCBF-SPECT and MRI data acquired from the same individuals. Braz J Psychiatry. (2018) 40:181–91. doi: 10.1590/1516-4446-2016-2083
105. Maia da Silva MN, Millington RS, Bridge H, James-Galton M, Plant GT. Visual dysfunction in posterior cortical atrophy. Front Neurol. (2017) 8:389. doi: 10.3389/fneur.2017.00389
106. Smagula SF, Karim HT, Rangarajan A, Santos FP, Wood SC, Santini T, et al. Association of hippocampal substructure resting-state functional connectivity with memory performance in older adults. Am J Geriatr Psychiatry. (2018) 26:690–9. doi: 10.1016/j.jagp.2018.03.003
107. Branco LMT, de Rezende TJR, Roversi CO, Zanao T, Casseb RF, de Campos BM, et al. Brain signature of mild stages of cognitive and behavioral impairment in amyotrophic lateral sclerosis. Psychiatry Res Neuroimaging. (2018) 272:58–64. doi: 10.1016/j.pscychresns.2017.11.010
108. Simon SS, Hampstead BM, Nucci MP, Duran FLS, Fonseca LM, Martin MDGM, et al. Cognitive and brain activity changes after mnemonic strategy training in amnestic mild cognitive impairment: evidence from a randomized controlled trial. Front Aging Neurosci. (2018) 10:342. doi: 10.3389/fnagi.2018.00342
109. Teixeira CVL, Ribeiro de Rezende TJ, Weiler M, Magalhães TNC, Carletti-Cassani AFMK, Silva TQAC, et al. Cognitive and structural cerebral changes in amnestic mild cognitive impairment due to Alzheimer's disease after multicomponent training. Alzheimers Dement (N Y). (2018) 4:473–80. doi: 10.1016/j.trci.2018.02.003
110. Weiler M, Casseb RF, de Campos BM, de Ligo Teixeira CV, Carletti-Cassani AFMK, Vicentini JE, et al. Cognitive reserve relates to functional network efficiency in Alzheimer's disease. Front Aging Neurosci. (2018) 10:255. doi: 10.3389/fnagi.2018.00255
111. Bertrand E, Azar M, Rizvi B, Brickman AM, Huey ED, Habeck C, et al. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology. (2018) 32:700–10. doi: 10.1037/neu0000458
112. Ventura N, Douw L, Correa DG, Netto TM, Cabral RF, Lopes FCR, et al. Increased posterior cingulate cortex efficiency may predict cognitive impairment in asymptomatic HIV patients. Neuroradiol J. (2018) 31:372–8. doi: 10.1177/1971400918782327
113. Neale N, Padilla C, Fonseca LM, Holland T, Zaman S. Neuroimaging and other modalities to assess Alzheimer's disease in Down syndrome. Neuroimage Clin. (2018) 17:263–71. doi: 10.1016/j.nicl.2017.10.022
114. Miotto EC, Batista AX, Simon SS, Hampstead BM. Neurophysiologic and cognitive changes arising from cognitive training interventions in persons with mild cognitive impairment: a systematic review. Neural Plast. (2018) 2018:7301530. doi: 10.1155/2018/7301530
115. Axelrud LK, Santoro ML, Pine DS, Talarico F, Gadelha A, Manfro GG, et al. Polygenic risk score for Alzheimer's disease: implications for memory performance and hippocampal volumes in early life. Am J Psychiatry. (2018) 175:555–63. doi: 10.1176/appi.ajp.2017.17050529
116. Resende EPF, Rosen HJ, Chiang K, Staffaroni AM, Allen I, Grinberg LT, et al. Primary school education may be sufficient to moderate a memory-hippocampal relationship. Front Aging Neurosci. (2018) 10:381. doi: 10.3389/fnagi.2018.00381
117. Martins LT, Teixeira IA, Laks J, Marinho V. Recognizing late onset frontotemporal dementia with the DAPHNE scale: a case report. Dement Neuropsychol. (2018) 12:75–9. doi: 10.1590/1980-57642018dn12-010011
118. Jaswal G, Swardfager W, Gao FQ, Nestor SM, Ganda A, Cogo-Moreira H, et al. Reduced substantia innominata volume mediates contributions of microvascular and macrovascular disease to cognitive deficits in Alzheimer's disease. Neurobiol Aging. (2018) 66:23–31. doi: 10.1016/j.neurobiolaging.2018.01.025
119. Rondina JM, Ferreira LK, de Souza Duran FL, Kubo R, Ono CR, Leite CC, et al. Selecting the most relevant brain regions to discriminate Alzheimer's disease patients from healthy controls using multiple kernel learning: a comparison across functional and structural imaging modalities and atlases. Neuroimage Clin. (2018) 17:628–41. doi: 10.1016/j.nicl.2017.10.026
120. Magalhães TNC, Weiler M, Teixeira CVL, Hayata T, Moraes AS, Boldrini VO, et al. Systemic inflammation and multimodal biomarkers in amnestic mild cognitive impairment and Alzheimer's disease. Mol Neurobiol. (2018) 55:5689–97. doi: 10.1007/s12035-017-0795-9
121. Swardfager W, Cogo-Moreira H, Masellis M, Ramirez J, Herrmann N, Edwards JD, et al. The effect of white matter hyperintensities on verbal memory: mediation by temporal lobe atrophy. Neurology. (2018) 90:e673–e82. doi: 10.1212/WNL.0000000000004983
122. Resende EPF, Tovar-Moll FF, Ferreira FM, Bramati I, de Souza LC, Carmona KC, et al. White matter microstructure in illiterate and low-literate elderly Brazilians: preliminary findings. Cogn Behav Neurol. (2018) 31:193–200. doi: 10.1097/WNN.0000000000000173
123. Foss MP, Diniz PRB, da Roza DL, Gefen T, Maher AC, Formigheri P, et al. Anatomic and neuropsychological findings in low-educated cognitively intact elderly from a Brazilian cohort. Dement Neuropsychol. (2019) 13:378–85. doi: 10.1590/1980-57642018dn13-040003
124. Axelrud LK, Sato JR, Santoro ML, Talarico F, Pine DS, Rohde LA, et al. Genetic risk for Alzheimer's disease and functional brain connectivity in children and adolescents. Neurobiol Aging. (2019) 82:10–7. doi: 10.1016/j.neurobiolaging.2019.06.011
125. Wang Y, Necus J, Rodriguez LP, Taylor PN, Mota B. Human cortical folding across regions within individual brains follows universal scaling law. Commun Biol. (2019) 2:191. doi: 10.1038/s42003-019-0421-7
126. Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. (2019) 142:2558–71. doi: 10.1093/brain/awz193
127. Staffaroni AM, Ljubenkov PA, Kornak J, Cobigo Y, Datta S, Marx G, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. (2019) 142:443–59. doi: 10.1093/brain/awy319
128. Drummond C, Coutinho G, Monteiro MC, Assuncao N, Teldeschi A, de Souza AS, et al. Narrative impairment, white matter damage and CSF biomarkers in the Alzheimer's disease spectrum. Aging (Albany NY). (2019) 11:9188–208. doi: 10.18632/aging.102391
129. Oliveira LM, Nitrini R, Román GC. Normal-pressure hydrocephalus: a critical review. Dement Neuropsychol. (2019) 13:133–43. doi: 10.1590/1980-57642018dn13-020001
130. Schilling LP, Pascoal TA, Zimmer ER, Mathotaarachchi S, Shin M, de Mello Rieder CR, et al. Regional Amyloid-β load and white matter abnormalities contribute to hypometabolism in Alzheimer's dementia. Mol Neurobiol. (2019) 56:4916–24. doi: 10.1007/s12035-018-1405-1
131. Batista AX, Bazán PR, Conforto AB, Martins MDGM, Hoshino M, Simon SS, et al. Resting state functional connectivity and neural correlates of face-name encoding in patients with ischemic vascular lesions with and without the involvement of the left inferior frontal gyrus. Cortex. (2019) 113:15–28. doi: 10.1016/j.cortex.2018.11.016
132. Therriault J, Wang S, Mathotaarachchi S, Pascoal TA, Parent M, Beaudry T, et al. Rostral-caudal hippocampal functional convergence is reduced across the Alzheimer's disease spectrum. Mol Neurobiol. (2019) 56:8336–44. doi: 10.1007/s12035-019-01671-0
133. Ferrari BL, Neto GCC, Nucci MP, Mamani JB, Lacerda SS, Felício AC, et al. The accuracy of hippocampal volumetry and glucose metabolism for the diagnosis of patients with suspected Alzheimer's disease, using automatic quantitative clinical tools. Medicine (Baltimore). (2019) 98:e17824. doi: 10.1097/MD.0000000000017824
134. Yamashita AY, Falcão AX, Leite NJ, Initiative AsDN. The residual center of mass: an image descriptor for the diagnosis of Alzheimer disease. Neuroinformatics. (2019) 17:307–21. doi: 10.1007/s12021-018-9390-0
135. De Carvalho Neto EG, Gomes MF, De Oliveira M, Guete MIN, Santos IP, Monteiro MD, et al. The worst is yet to come: probable sporadic Creutzfeldt-Jakob disease in a well-controlled HIV patient. Prion. (2019) 13:156–9. doi: 10.1080/19336896.2019.1648985
136. Gonçalves SAB, Caramelli P, Mariano LI, Guimarães HC, Gambogi LB, Resende EPF, et al. Apathy in frontotemporal dementia is related to medial prefrontal atrophy and is independent of executive dysfunction. Brain Res. (2020) 1737:146799. doi: 10.1016/j.brainres.2020.146799
137. Martins-Filho RK, Zotin MC, Rodrigues G, Pontes-Neto O. Biomarkers related to endothelial dysfunction and vascular cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. (2020) 49:365–74. doi: 10.1159/000510053
138. Blevins BL, Vinters HV, Love S, Wilcock DM, Grinberg LT, Schneider JA, et al. Brain arteriolosclerosis. Acta Neuropathol. (2021) 141:1–−24. doi: 10.1007/s00401-020-02235-6
139. Rossini PM, Di Iorio R, Vecchio F, Anfossi M, Babiloni C, Bozzali M, et al. Early diagnosis of Alzheimer's disease: the role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin Neurophysiol. (2020) 131:1287–310. doi: 10.1016/j.clinph.2020.03.003
140. Dalboni da Rocha JL, Bramati I, Coutinho G, Tovar Moll F, Sitaram R. Fractional anisotropy changes in parahippocampal cingulum due to Alzheimer's disease. Sci Rep. (2020) 10:2660. doi: 10.1038/s41598-020-59327-2
141. Busatto Filho G, Duran FLS, Squarzoni P, Coutinho AMN, Rosa PGP, Torralbo L, et al. Hippocampal subregional volume changes in elders classified using positron emission tomography-based Alzheimer's biomarkers of β-amyloid deposition and neurodegeneration. J Neurosci Res. (2021) 99:481–501. doi: 10.1002/jnr.24739
142. Dalboni da Rocha JL, Coutinho G, Bramati I, Moll FT, Sitaram R. Multilevel diffusion tensor imaging classification technique for characterizing neurobehavioral disorders. Brain Imaging Behav. (2020) 14:641–52. doi: 10.1007/s11682-018-0002-2
143. Freitas CS, Pinheiro MGM, Fonte EJD, Hazin AN, Smid J, Barbosa BJAP. Posterior cortical ribboning in the Heidenhain variant of Creutzfeldt-Jakob disease. Arq Neuropsiquiatr. (2020) 78:241. doi: 10.1590/0004-282x20190176
144. Ducharme S, Dols A, Laforce R, Devenney E, Kumfor F, van den Stock J, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. (2020) 143:1632–50. doi: 10.1093/brain/awaa018
145. Ehrenberg AJ, Khatun A, Coomans E, Betts MJ, Capraro F, Thijssen EH, et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimers Res Ther. (2020) 12:56. doi: 10.1186/s13195-020-00601-w
146. Simon SS, Hampstead BM, Nucci MP, Duran FLS, Fonseca LM, Martin MDGM, et al. Training gains and transfer effects after mnemonic strategy training in mild cognitive impairment: a fMRI study. Int J Psychophysiol. (2020) 154:15–26. doi: 10.1016/j.ijpsycho.2019.03.014
147. Moura RR, Coelho AV, Balbino VeQ, Crovella S, Brandão LA. Meta-analysis of Brazilian genetic admixture and comparison with other Latin America countries. Am J Hum Biol. (2015) 27:674–80. doi: 10.1002/ajhb.22714
148. Fam J, Mahendran R, Kua EH. Dementia care in low and middle-income countries. Curr Opin Psychiatry. (2019) 32:461–4. doi: 10.1097/YCO.0000000000000523
149. Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. (2019) 76:264–73. doi: 10.1001/jamaneurol.2018.4249
150. Wong LCK, Wong MYZ, Tan CS, Vrooman H, Venketasubramanian N, Cheng CY, et al. Interethnic differences in neuroimaging markers and cognition in Asians, a population-based study. Sci Rep. (2020) 10:2655. doi: 10.1038/s41598-020-59618-8
151. Tamashiro-Duran JH, Squarzoni P, de Souza Duran FL, Curiati PK, Vallada HP, Buchpiguel CA, et al. Cardiovascular risk in cognitively preserved elderlies is associated with glucose hypometabolism in the posterior cingulate cortex and precuneus regardless of brain atrophy and apolipoprotein gene variations. Age (Dordr). (2013) 35:777–92. doi: 10.1007/s11357-012-9413-y
152. Scazufca M, Menezes PR, Vallada HP, Crepaldi AL, Pastor-Valero M, Coutinho LM, et al. High prevalence of dementia among older adults from poor socioeconomic backgrounds in São Paulo, Brazil. Int Psychogeriatr. (2008) 20:394–405. doi: 10.1017/S1041610207005625
153. Ortega LFV, Aprahamian I, Borges MK, Cação JC, Yassuda MS. Screening for Alzheimer's disease in low-educated or illiterate older adults in Brazil: a systematic review. Arq Neuropsiquiatr. (2019) 77:279–88. doi: 10.1590/0004-282x20190024
154. Rzezak P, Squarzoni P, Duran FL, de Toledo Ferraz Alves T, Tamashiro-Duran J, Bottino CM, et al. Relationship between brain age-related reduction in gray matter and educational attainment. PLoS ONE. (2015) 10:e0140945. doi: 10.1371/journal.pone.0140945
155. Lotufo PA, Goulart AC, Passos VMA, Satake FM, Souza MFM, França EB, et al. Cerebrovascular disease in Brazil from 1990 to 2015: global burden of disease 2015. Rev Bras Epidemiol. (2017) 20(Suppl.01):129–41. doi: 10.1590/1980-5497201700050011
156. Cortes-Canteli M, Iadecola C. Alzheimer's disease and vascular aging: JACC focus seminar. J Am Coll Cardiol. (2020) 75:942–51. doi: 10.1016/j.jacc.2019.10.062
157. Grinberg LT, Nitrini R, Suemoto CK, Lucena Ferretti-Rebustini RE, Leite RE, Farfel JM, et al. Prevalence of dementia subtypes in a developing country: a clinicopathological study. Clinics (São Paulo). (2013) 68:1140–5. doi: 10.6061/clinics/2013(08)13
158. Daroff RB, Jankovic J, Mazziotta JC, Pomeroy SL, Bradley WG. Bradley's Neurology in Clinical Practice. 7th ed. London, NY: Elsevier (2016). 2 volumes.
159. Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. (2020) 27:18. doi: 10.1186/s12929-019-0609-7
160. Cross D, Thomson S, Sinclair A. Research in Brazil: A Report for CAPES by Clarivate Analytics. Clarivate Analytics (2018).
161. Russo MJ, Gustafson D, Vázquez S, Surace E, Guinjoan S, Allegri RF, et al. Creation of the Argentina-Alzheimer's disease neuroimaging initiative. Alzheimers Dement. (2014) 10(Suppl.1):S84–7. doi: 10.1016/j.jalz.2013.09.015
Keywords: Alzheimer's disease, Brazil, dementia, mild cognitive impaiment, MRI, neuroimaging, scientometric analysis
Citation: Rizzi L, Aventurato ÍK and Balthazar MLF (2021) Neuroimaging Research on Dementia in Brazil in the Last Decade: Scientometric Analysis, Challenges, and Peculiarities. Front. Neurol. 12:640525. doi: 10.3389/fneur.2021.640525
Received: 11 December 2020; Accepted: 18 February 2021;
Published: 15 March 2021.
Edited by:
Agustin Ibanez, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Sonia Maria Dozzi Brucki, University of São Paulo, BrazilJiu Chen, Nanjing Medical University, China
Copyright © 2021 Rizzi, Aventurato and Balthazar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcio L. F. Balthazar, bWJhbHRoQHVuaWNhbXAuYnI=
†These authors have contributed equally to this work and share first authorship
 Liara Rizzi
Liara Rizzi Ítalo Karmann Aventurato
Ítalo Karmann Aventurato Marcio L. F. Balthazar
Marcio L. F. Balthazar