
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 24 February 2021
Sec. Neuroinfectious Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.633574
This article is part of the Research TopicCOVID-19 in CNS and PNS: Basic and Clinical Focus on the Mechanisms of Infection and New Tools for the Therapeutic ApproachView all 26 articles
 Carla Mucignat-Caretta1*
Carla Mucignat-Caretta1* Patrizia Bisiacchi2,3
Patrizia Bisiacchi2,3 Gian Luigi Marcazzan4
Gian Luigi Marcazzan4 Arianna Calistri1
Arianna Calistri1 Cristina Parolin1
Cristina Parolin1 Angelo Antonini3,5*
Angelo Antonini3,5*Background/Objective: During the COVID-19 pandemic, smell and taste disorders emerged as key non-respiratory symptoms. Due to widespread presence of the disease and to difficult objective testing of positive persons, the use of short surveys became mandatory. Most of the existing resources are focused on smell, very few on taste or trigeminal chemosensation called chemesthesis. However, it is possible that the three submodalities are affected differently by COVID-19.
Methods: We prepared a short survey (TaSCA) that can be administered at the telephone or through online resources to explore chemosensation. It is composed of 11 items on olfaction, taste, and chemesthesis, in order to discriminate the three modalities. We avoided abstract terms, and the use of semiquantitative scales because older patients may be less engaged. Statistical handling included descriptive statistics, Pearson's chi-squared test and cluster analysis.
Results: The survey was completed by 83 persons (60 females and 23 males), which reported diagnosis of COVID-19 by clinical (n = 7) or molecular (n = 18) means, the others being non-COVID subjects. Cluster analysis depicted the existence of two groups, one containing mostly asymptomatic and one mostly symptomatic subjects. All swab-positive persons fell within this second group. Only one item, related to trigeminal temperature perception, did not discriminate between the two groups.
Conclusions: These preliminary results indicate that TaSCA may be used to easily track chemosensory symptoms related to COVID-19 in an agile way, giving a picture of three different chemosensory modalities.
After the first weeks of SARS-CoV2 spreading (1) and the worldwide dissemination of COVID-19 pandemic, it became clear that the virus is able to affect different organs, while giving the most dismal outcomes in the case of respiratory tract infection (2). In addition to severe respiratory symptoms, rather non-specific signs were reported as initial manifestation of infection, like fever, myalgia, and headache1. However, other symptoms emerged as associated to the infection, including the loss of smell termed anosmia (3–5), whose sudden onset appeared as a typical feature of the COVID-19 disease (6, 7). Actually, other viruses may affect the ability to smell, however in these cases the loss of olfactory function is smoother and associated to various degree of nasal symptoms, like running nose (8), while in the case of COVID-19, the loss of smell may appear also in the absence of any other symptom, often in a sudden and dramatic way. The consequences of olfactory loss, whatever the cause, may be life-threatening, by reducing the awareness of potentially dangerous stimuli like gas or rotten food (9).
While the loss of smell is the most apparent sign of chemosensory involvement and can be regarded as a predictor of COVID-19 (10–14) also taste loss (ageusia) may be present, and in some cases the trigeminal chemical sense (15) called chemesthesis is involved (14, 16).
During the initial phase of the pandemic, we followed some mildly symptomatic COVID-19 patients experiencing symptoms related to the chemical senses. However, most of the existing survey at that time were focused on olfaction, and none put together olfaction, taste and chemesthesis. We observed that patients could not easily discriminate between the three different chemosensory modalities, which are sensitive to different stimuli and use a variety of transduction pathways and central connections to reach specific sensory areas in the brain. Hence, we felt compelled to create an agile tool to collect information on these three chemosensory modalities, that could be used either online or as direct or telephone interview, to allow the widest collection of data and reach even persons in remote areas.
The need for a tool easy to use and manage, that allows patients to respond and follow the evolution of their symptoms, prompted us to create a very short online survey on the three chemosensory modalities, focusing on items which are part of everyday life for most people. We referred to sensory experiences related to specific objects, instead of using more generic terms (like “smell”) and tried to discriminate between different odorant sources, in case the subject has no relevant experience: for example, one person in isolation may not have a direct contact with perfumes, yet may still retrieve some soap to smell. We also avoided the use of semi-structured scales, since these may be difficult to be adopted by older persons. Taste-Smell-Chemesthesis Agile (TaSCA) survey is presented here along with the responses collected online from April 30 to the end of May 2020, from asymptomatic, non-affected persons and COVID-19 positive patients. The aim of the present work is to show that this tool may discriminate between persons showing chemosensory systems involvement or not, and may serve as a proof-of-principle for the use of TaSCA in conditions in which direct testing is prevented.
The survey was created to collect data for an observational, prospective single center study aimed at determining medium/long term consequences of COVID-19 on neurological status (NEUROCOVID, Ethical Committee Prot. 056881). Data herein presented were collected online in May 2020, during the first COVID-19 outbreak, by soliciting patients and non-affected persons to participate. No exclusion criteria were set, since we aimed at establishing differences between groups of patients and healthy subjects and explore the viability of TaSCA as a tool for COVID-19 patients.
The questionnaire is composed of three sheets implemented in Google Forms. One introductory sheet is entitled “COVID-19 and taste and smell disturbances” and shows an introductory statement. It presents one mandatory question, on the willingness to participate, and 3 more questions (name or nickname, gender, and age). The following sheet is the actual survey. The subject should answer to 12 questions. The first 11 questions take in consideration the sensory experience in the last days, with respect to the usual sensitivity. One of four responses is possible: (1) No change in the last period (I sense as always), (2) Moderate change (I sense less than usual),3 Loss of function (I don't sense those items), (3) Don't know/don't remember, in the case the person did not had the chance to sense the item in the last period, because of physical constraints. The questions explore smell (questions 1, 3, 5), nasal and oral chemesthesis (questions 2, 4, 10, 11), and taste (questions 6, 7, 8, 9). The last question refers to the presence of symptoms related to COVID-19, with the possibility to add some notes. The third and last sheet is salutation and thanks. The questionnaire in the original language is available at the link:
The printout is presented in the Supplementary Material 1, while the English translation is shown in Figure 1.
Data were analyzed using IBM SPSS Statistics v26 (RRID:SCR_019096) to produce descriptive statistics, Pearson's chi-squared analysis and cluster analysis. Descriptive statistics were calculated for each item.
As a first step, we asked whether the items were able to indicate a difference among persons experiencing a change in sensitivity and COVID-19 status. Hence, the 4 types of responses were coded as 0 (no change in sensitivity, answer 1) or 1 (there was a change in the last period, answers 2 and 3). Concerning answer 4, we manually coded it as 0 if all the other responses indicated no change.
The hierarchical agglomerative algorithm according to Ward's method was applied for clustering (17), since this warrants robust results (18), using squared Euclidean distance for assessing similarity and Pearson's chi-square to test for difference between the clusters for each item. Significance level was set at p < 0.0045 after Bonferroni correction for multiple comparisons.
Eighty-three persons completed the survey: 60 females and 23 males. 56 were asymptomatic, 7 had a COVID-19 clinical diagnosis based on symptoms (because during the first pandemic, access to naso-pharyngeal swab was restricted to patients with severe symptoms), 18 had a SARS-CoV2 positive nasopharyngeal swab and 2 self-reported sudden olfactory loss but when the swab test become available, it turned out to be negative. Age ranged from 19 to 73 years old (mean ± SD: 35.98 ± 16.61). All subjects presented valid data: percentages of response for each item are presented in Table 1.
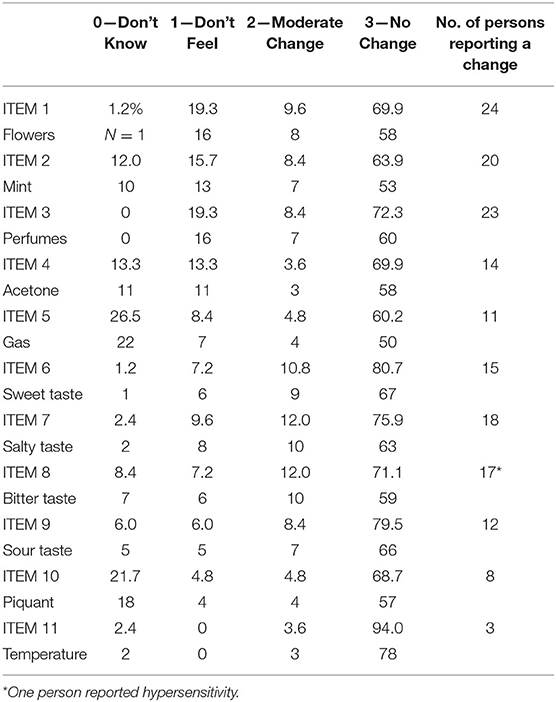
Table 1. Percentage (%) of response and number (N) of persons reporting a modification in their sensitivity.
In the open question, 42 subjects (50.6%) reported no symptoms related to COVID-19, 27 (32.5%) declared some symptoms and 14 (16.9) answered “I don't know.”
Several items had 5 cases or less for the responses 0-1-2 (Table 1): 6 for response 0, 5 for response 1, and 4 for response 2. Due to the low frequencies, the responses were categorized in 2 classes namely, 0: No change compared to previous sensitivity, 1: Change compared to previous sensitivity (see Table 1, last column).
Cluster analysis showed that two main groups emerged from data, one composed of 53 subjects (63.8%) and the other of 30 subjects (36.2%). The relative dendrogram is shown in Figure 2.
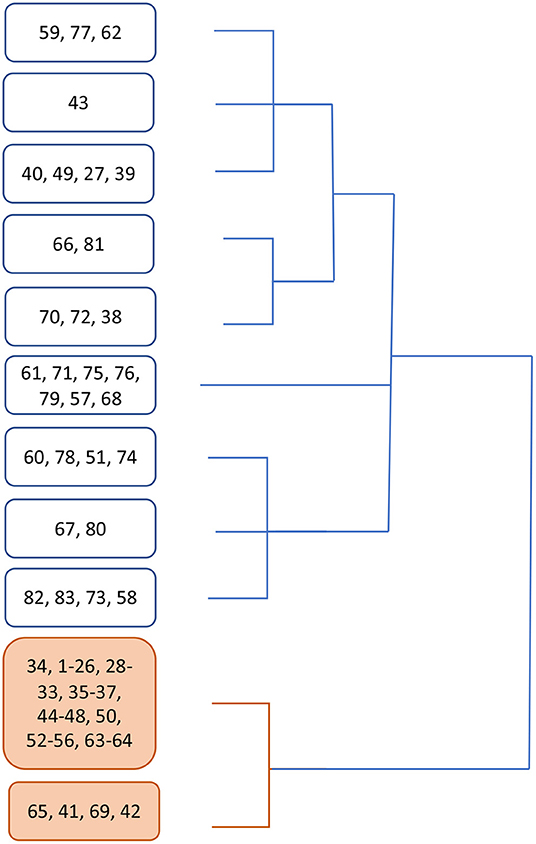
Figure 2. Dendrogram showing clustering emerged from cluster analysis. In white boxes, the branch containing mostly symptomatic patients, in orange the branch containing no swab-positive subject.
Apparently, group 1 mostly collects asymptomatic subjects (92.5%), while 73% persons in group 2 received a diagnosis of COVID-19 (Supplementary Material 2).
Supplementary Material 3 reports the distribution of subjects in the two groups emerged from the cluster analysis with respect to the absence or presence of modification in sensitivity: for items 1 to 10, the percentage of subjects reporting a change in sensitivity is significantly different in the two groups. Only for item 11, related to temperature perception, the reported change in sensitivity is not significantly different between the two groups (Supplementary Material 3).
Supplementary Material 4 reports the raw data for the cluster analysis.
The mean responses in the two groups are shown in Figure 3.
Concerning the last item, on the presence of COVID-19-related symptoms, 3 responses were analyzed: (1) Yes, (2) No, (3) I don't know. The two groups emerged from cluster analysis significantly differed between each other (Pearsons' chi-squared = 42.344, df = 2, p < 0.0001). In group 1, 71.7% (38 subjects) reported no symptoms, 7.5% (n = 4) reported symptoms and 20.8% (n = 11) did not know, while in group 2 13.3% (n = 4) did not report symptoms, 76.7% reported symptoms (n = 23), and 10% (n = 3) did not know.
Often regarded as an ancillary sense, olfaction has a great role in our everyday life for its involvement in lifeguarding processes (e.g., avoiding dangerous chemicals) as well in food evaluation.
The COVID-19 pandemic brought to the attention of clinicians an astonishing number of persons infected with mild symptoms, the most intriguing being a sudden and complete loss of olfaction (6), which may last for a variable period of time, often accompanied by taste and chemesthesis impairment (14). While anecdotical self-reports are intriguing, to pose a diagnosis and follow the course of the disease, it is necessary to develop instruments that allow a reliable quantification of the functional impairment.
In the case of chemical senses, two main objective tests have been developed and used over the years in the clinic, namely the Sniffin' Sticks (19) and the University of Pennsylvania Smell Inventory Test (UPSIT) (20), while others are being developed. Their use is mandatory to have an objective evaluation of the impairment, since the subjective report is often misleading (21). However, they both require the direct testing of the patient, which may be difficult or impossible in the case of COVID-19 infected persons. On the contrary, collecting data from patients may be of paramount importance to follow the disease. Sadly, the utility of self-reporting about chemical sensitivity has been repeatedly questioned (22).
Many questionnaires are available to test olfactory function and some of them are widely used (23–25). However, in the case of COVID-19 disease some caution should be warranted. First, most of the currently available surveys are focused on either smell or taste and almost none take into consideration the trigeminal chemical sensitivity. However, one of the most intriguing feature of COVID-19-related chemical senses impairment is the involvement of multiple chemosensory modalities in the disease, as well as their sudden loss of function. Hence, the development of surveys specifically targeting all three chemical sensitivity is long needed. Another feature of COVID-19 disease is the wide range of age of affected persons, yet the diagnostic tools should be easily accessible to most of them: hence, we avoided the use of visual-analog scales, which may be more sensitive than other descriptors but less flexible in terms of accessibility.
Other tools have been developed, including the 40-items GCCR multi-lingual online questionnaire (14), however this requires the access to the web and a certain degree of confidence in using online surveys, which may prevent older persons to access it. Moreover, given the worldwide diffusion of the pandemic, it may be useful to create agile instruments that are easily administered using different tools, including self-administration, direct interviews or over the phone, besides online presentation. Lastly, while most available tools may be complete and hence rather long, we focused the questions on the three chemical modalities to have a glimpse of their relative degree of impairment. By keeping the questions tied to real objects that could be sensed and avoiding abstract terms, we tried to grasp the sensory experience in a more faithful way.
Present data show that mostly asymptomatic patients cluster together, while most patients experiencing chemosensory impairment, with particular involvement of olfaction, cluster together, suggesting that TaSCA survey may be a valuable tool in case of impossibility to administer more objective tests. It may also help in collecting critical information on the objective sensitivity in a fast and easy way.
The present work is limited to the initial collections of a case-series to test whether it could discriminate between symptomatic and non-symptomatic patients. Since it relied on the voluntary participation of subjects, we did not attempt to refine sampling, to stay closer to the real-life use: hence, the sex balance is skewed toward females, probably because females are more compliant and willing to take part in preliminary testing. Similarly, we did not take any action on age balance, leaving this for future investigations.
Some initial speculations are possible on the present data. Whether items concerning olfaction are the most discriminative indexes, compared to taste and trigeminal will be explored in future work. Also, with a larger sample it will be possible to fully assess the psychometric and statistical properties of the questionnaire, including for example case-control approaches and cutoff point determination. Interestingly, item 11 on thermal sensitivity does not appear to discriminate between the 2 groups that emerged from cluster analysis. This suggests that the trigeminus nerve may be differentially affected in its different sensory components. Chemical sensitivity in trigeminal afferents involves receptors which are also temperature-gated (26, 27), but cold receptors also exist (28–30), and functional specializations have been reported for trigeminal receptors (31). It is worth to include item 11 because it may discriminate among persons experiencing true chemosensory deficit from deceitful answers.
While obtained in a limited number of subjects, these data show that TaSCA is a short survey available in the same form online, on paper or for oral interview that may be used to screen chemosensory deficits in COVID-19 patients. It remains to be determined its possible use in other conditions where these sensory systems may be involved.
In COVID-19 patients which are still positive, isolated at home or when direct objective testing is not feasible or recommendable, TaSCA could be easily administered. It is fast, yet complete in exploring all three chemosensory modalities and could be used also with patients still presenting annoying symptoms, which may prevent them from extensive sessions of online or live testing.
Possibly, it could be useful in the future for repeated self-checking with substances commonly available in most houses, given the necessity of long-term monitoring for possible adverse outcomes (32).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Comitato Etico per la Sperimentazione Clinica della Provincia di Padova (affiliation: Padova province). The patients/participants provided their written informed consent to participate in this study.
CM-C conceived the questionnaire. CM-C and PB analyzed the data. GM prepared the online material. CM-C, GM, AC, and CP participated in data collection. AA provided financial support. All authors drafted the manuscript.
This work was funded by Fondazione Cassa di Risparmio di Padova e Rovigo (CARIPARO) for a COVID-19 related call.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Lucia Ronconi for assistance with cluster analysis. We thank all the persons that agreed to participate during the lockdown period.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.633574/full#supplementary-material
1. ^https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed November 23, 2020).
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
3. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. (2020) 277:2251–61. doi: 10.1007/s00405-020-05965-1
4. Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. (2020) 10:944-950. doi: 10.1002/alr.22587
5. Hornuss D, Lange B, Schröter N. Anosmia in COVID-19 patients. Clin Microbiol Infect. (2020) 26:1426–7. doi: 10.1016/j.cmi.2020.05.017
6. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. a novel syndrome? Rhinology. (2020) 58:299–301. doi: 10.4193/Rhin20.114
7. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. (2020) 323:2512–4. doi: 10.1001/jama.2020.8391
8. Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am. (2004) 37:1159–66. doi: 10.1016/j.otc.2004.06.007
9. Rebholz H, Braun RJ, Ladage D, Knoll W, Kleber C, Hassel AW. Loss of olfactory function-early indicator for Covid-19, other viral infections and neurodegenerative disorders. Front Neurol. (2020) 11:569333. doi: 10.3389/fneur.2020.569333
10. Rocke J, Hopkins C, Philpott C, Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: a systematic review and meta-analysis. Clin Otolaryngol. (2020) 1:13620. doi: 10.1111/coa.13620
11. Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. (2020) 26:1037–40. doi: 10.1038/s41591-020-0916-2
12. Seden N, Yigit E, Yigit Ö, Kaygisiz I. Objective evaluation of odor loss in COVID-19 and other suspected cases. Am J Otolaryngol. (2020) 42:102761. doi: 10.1016/j.amjoto.2020.102761
13. Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, et al. More than smell – COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. (2020) 45:609–22. doi: 10.1093/chemse/bjaa041
14. Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses. (2020) 25:bjaa081. doi: 10.1093/chemse/bjaa081
15. Viana F. Chemosensory properties of the trigeminal system. ACS Chem Neurosci. (2011) 2:38–50. doi: 10.1021/cn100102c
16. Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. (2020) 107:219–33. doi: 10.1016/j.neuron.2020.06.032
17. Ward JHJ. Hierarchical grouping to optimize an objective function. J Am Stat Ass. (1963) 58:236–44.
18. Milligan GW. A review of Monte Carlo tests of cluster analysis. Multivar Behav Res. (1981) 16:379–407.
19. Hummel T, Sekinger B, Wolf SR. ‘Sniffin' Sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. (1997) 22:39–52.
20. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania smell identification test: A rapid quantitative olfactory function test for the clinic. Laryngoscope. (1984) 94:176–8.
21. Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinology. (2016) 56:1–30. doi: 10.4193/Rhin16.248
22. Lötsch J, Hummel T. Clinical usefulness of self-rated olfactory performance-a data science-based assessment of 6000 patients. Chem Senses. (2019) 44:357–64. doi: 10.1093/chemse/bjz029
23. Zou LQ, Linden L, Cuevas M, Metasch ML, Welge-Lüssen A, Hähner A, et al. Self-reported mini olfactory questionnaire (Self-MOQ): a simple and useful measurement for the screening of olfactory dysfunction. Laryngoscope. (2020) 130:E786–E90. doi: 10.1002/lary.28419
24. Mullol J, Alobid I, Mariño-Sánchez F, Quintó L, de Haro J, Bernal-Sprekelsen M, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. (2012) 2:e001256. doi: 10.1136/bmjopen-2012-001256
25. Langstaff L, Pradhan N, Clark A, Boak D, Salam M, Hummel T, et al. Validation of the olfactory disorders questionnaire for English- speaking patients with olfactory disorders. Clin Otolaryngol. (2019) 44:715–28. doi: 10.1111/coa.13351
26. Silver WL, Clapp TR, Stone LM, Kinnamon SC. TRPV1 receptors and nasal trigeminal chemesthesis. Chem Senses. (2006) 31:807–12. doi: 10.1093/chemse/bjl022
27. Simon SA, Gutierrez R. Chapter 7: TRP channels at the periphery of the taste and trigeminal systems. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton, FL: CRC Press; Taylor & Francis; (2017).
28. Babes A. Ion channels involved in cold detection in mammals: TRP and non-TRP mechanisms. Biophys Rev. (2009) 1:193–200. doi: 10.1007/s12551-009-0020-9
29. Kichko TI, Neuhuber W, Kobal G, Reeh PW. The roles of TRPV1, TRPA1 and TRPM8 channels in chemical and thermal sensitivity of the mouse oral mucosa. Eur J Neurosci. (2018) 47:201–10. doi: 10.1111/ejn.13799
30. Iftinca M, Altier C. The cool things to know about TRPM8!. Channels. (2020) 14:413–20. doi: 10.1080/19336950.2020.1841419
31. Lemon CH, Kang Y, Li J. Separate functions for responses to oral temperature in thermo-gustatory and trigeminal neurons. Chem Senses. (2016) 41:457–71. doi: 10.1093/chemse/bjw02
Keywords: COVID-19, olfaction, taste, chemesthesis, trigeminus, self-assessment, chemical senses, recovery
Citation: Mucignat-Caretta C, Bisiacchi P, Marcazzan GL, Calistri A, Parolin C and Antonini A (2021) TaSCA, an Agile Survey on Chemosensory Impairments for Self-Monitoring of COVID-19 Patients: A Pilot Study. Front. Neurol. 12:633574. doi: 10.3389/fneur.2021.633574
Received: 25 November 2020; Accepted: 02 February 2021;
Published: 24 February 2021.
Edited by:
Ulises Gomez-Pinedo, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos, SpainReviewed by:
Florian Ph. S. Fischmeister, University of Graz, AustriaCopyright © 2021 Mucignat-Caretta, Bisiacchi, Marcazzan, Calistri, Parolin and Antonini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Mucignat-Caretta, Y2FybGEubXVjaWduYXRAdW5pcGQuaXQ=; Angelo Antonini, YW5nZWxvLmFudG9uaW5pQHVuaXBkLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.