
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 09 April 2021
Sec. Neuroepidemiology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.627824
This article is part of the Research TopicPopulation Neuroscience of Development and AgingView all 14 articles
 Jonathan A. Abuga1,2*
Jonathan A. Abuga1,2* Symon M. Kariuki1,3,4
Symon M. Kariuki1,3,4 Samson M. Kinyanjui1,3,5
Samson M. Kinyanjui1,3,5 Michael Boele van Hensbroek2
Michael Boele van Hensbroek2 Charles R. Newton1,3,4
Charles R. Newton1,3,4Background: Neurological impairment (NI) and disability are associated with reduced life expectancy, but the risk and magnitude of premature mortality in children vary considerably across study settings. We conducted a systematic review to estimate the magnitude of premature mortality following childhood-onset NI worldwide and to summarize known risk factors and causes of death.
Methods: We searched various databases for published studies from their inception up to 31st October 2020. We included all cohort studies that assessed the overall risk of mortality in individuals with childhood-onset epilepsy, intellectual disability (ID), and deficits in hearing, vision and motor functions. Comparative measures of mortality such as the standardized mortality ratio (SMR), risk factors and causes were synthesized quantitatively under each domain of impairment. This review is registered on the PROSPERO database (registration number CRD42019119239).
Results: The search identified 2,159 studies, of which 24 studies were included in the final synthesis. Twenty-two (91.7%) studies originated from high-income countries (HICs). The median SMR was higher for epilepsy compared with ID (7.1 [range 3.1–22.4] vs. 2.9 [range 2.0–11.6]). In epilepsy, mortality was highest among younger age groups, comorbid neurological disorders, generalized seizures (at univariable levels), untreatable epilepsy, soon after diagnosis and among cases with structural/metabolic types, but there were no differences by sex. Most deaths (87.5%) were caused by non-epilepsy-related causes. For ID, mortality was highest in younger age groups and girls had a higher risk compared to the general population. Important risk factors for premature mortality were severe-to-profound severity, congenital disorders e.g., Down Syndrome, comorbid neurological disorders and adverse pregnancy and perinatal events. Respiratory infections and comorbid neurological disorders were the leading causes of death in ID. Mortality is infrequently examined in impairments of vision, hearing and motor functions.
Summary: The risk of premature mortality is elevated in individuals with childhood-onset NI, particularly in epilepsy and lower in ID, with a need for more studies for vision, hearing, and motor impairments. Survival in NI could be improved through interventions targeting modifiable risk factors and underlying causes.
Neurological impairments (NI) are a group of disorders resulting from damage to or dysfunction of the central nervous system (1–3). The most prevalent domains of NI include epilepsy, cognitive, sensorineural, and motor impairments (4, 5). The burden of NI varies greatly between and within regions and countries, which is attributed to epidemiological and demographic transitions (6–10). For example, improved child survival and persistence or emergence of risk factors for NI have increased the burden in older children and adolescents in LMICs (11–13).
Current evidence suggests an increased risk of premature mortality or reduced life expectancy among individuals with NI and disability (14–19). For instance, the risk of premature mortality is 2–3 times higher among people with epilepsy compared with the general population (16, 19); the risk is highest in LMICs (16) and childhood-onset seizures (19). The risk of mortality is also higher in structural/metabolic, untreated and intractable epilepsy. The causes of death in epilepsy include: (i) sudden unexplained death in epilepsy (SUDEP); (ii) accidents/burns; or (iii) acute/chronic infectious or non-infectious disease (16, 19), but it is unclear if mortality for other domains of NI is related to these or other different causes. Cohort studies are logistically intensive to conduct, which can influence the extent to which mortality is examined following NI across the world, particularly in LMICs.
We conducted a systematic review to: (i) estimate the magnitude of premature mortality in individuals with childhood-onset NI; (ii) summarize known risk factors; and (iii) describe causes of premature mortality among individuals who died. This evidence is required to inform medical and community-based interventions that might improve the survival and quality of life for individuals with NI and disability.
We used the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (20) and the Centre for Reviews and Dissemination (CRD) recommendations for undertaking reviews in healthcare (21) for the searches, identification, appraisal of eligible studies, synthesis, and reporting of findings in this review. A protocol is registered in the international prospective register of systematic reviews (PROSPERO-registration number CRD42019119239) (22).
A search was conducted in the PubMed, EMBASE, and Scopus databases for cohort studies from database inception up to 31st October 2020 using terms in three groups: (i) neurologic impairments, cognitive impairments or intellectual disability, motor impairments, visual impairments, hearing impairments and epilepsy; (ii) mortality, death or survival; (iii) risk factors, predictors or causes of death (see Table 1 for details of the search strategy in PubMed).
Two reviewers reviewed the retrieved citations in a two-stage process. In the first stage, the first reviewer (JA) reviewed all titles and available abstracts to identify relevant studies. The second reviewer (SK) independently reviewed 30% of the titles and abstracts; both reviewers compared their lists and resolved disagreements by consensus. In the second stage, both reviewers assessed whether the identified articles met the inclusion criteria.
We included: (i) original cohort studies of mortality following children with NI in five domains (epilepsy and impairments in cognitive, hearing, vision, and motor functions); (ii) studies with a childhood-onset or diagnosis of impairment (between the ages 0–19 years); (iii) studies reporting all-cause mortality as the primary outcome; and (iv) studies with an appropriate comparison group such as matched controls or the general population. We excluded studies with adulthood-onset of NI (≥20 years), studies of mental and psychiatric problems, studies without an appropriate comparison group, those reporting the same data in different papers, reviews, editorials and studies reported in other languages that could not easily be translated into English.
An assessment was done on whether the definitions of NI in each study were in alignment with the International Classification of Diseases (ICD), the Diagnostic and Statistical Manual for Mental Disorders (DSM), or both with the versions dependent on the year of study. Childhood-onset epilepsy was defined according to the International League Against Epilepsy (ILAE) as the presence of two or more unprovoked seizures occurring within 12 months identified before the age of 18 years (23). Individuals with cognitive impairment, hereafter referred to as intellectual disability (ID), refer to those who had IQ scores <70 or z-scores < −3 based on age-appropriate neuropsychological tests and age-inappropriate adaptive skills with a childhood-onset (24). Historically, cognitive impairment has been conceptualized as structural or functional limitations based on the medical model (2, 25); however, recent definitions have used the term ID to depict the misfit between contextual demands and the person's capabilities (3, 26). Motor impairments referred to limitations in muscle control, movement, or mobility, or complete absence of motor functioning based on valid criteria such as the Gross Motor Function Classification System (GMFCS) (27). Hearing impairment was defined as hearing loss >25–30 dB in the best hearing hear (28) and vision impairment as a deficit in sight presenting with visual acuity worse than 6/12 (29). We extracted data on study setting, population characteristics, cohort sizes, duration of follow-up, comparative measures of mortality risk such as mortality rate ratios (MRR), hazard ratios (HR) and standardized mortality ratios (SMR), and risk factors and causes of death as reported in the individual studies.
Guidelines from the Joanna Briggs Institute's (JBI) critical appraisal checklist for cohort studies were used (30) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (31) to appraise the methodological quality of the included studies. Our emphasis on quality was focused on: (i) reliability/sensitivity of NI diagnosis and case ascertainment; (ii) sensitivity/reliability of mortality case and cause of death ascertainment; (iii) representativeness of the study population; (iv) risk of bias e.g., selection bias; (v) follow-up duration; and (vi) use of appropriate analytic methods. We grouped each report into an aggregate score of four classes: class 1 studies represent those studies with an overall score between 75 and 100%; class 2 studies scored between 50 and 74%; class 3 studies scored between 25 and 49% and class 4 studied scored below 25% representing the weakest evidence.
We estimated the overall risk of premature mortality separately for each domain of NI because of heterogeneity in the diagnosis of each, and that each impairment has a unique underlying process and prognosis. Summary measures such as the median and range were used for descriptive analysis of SMR, MRR, and HR reported in the included studies. Similarly, we summarized the measures of effect on mortality for each risk factor and cause-specific proportionate mortality per domain of NI. Overall estimates of mortality from primary studies could not be combined in a meta-analysis because there was very high heterogeneity within and between the included studies (32).
The results of the systematic search are in Figure 1. A total of 24 studies met the inclusion criteria of which 9 (37.5%) were on epilepsy, 10 (41.7%) on ID, 3 (12.5%) on motor-related impairments or cerebral palsy (CP), 1 (4.2%) on vision impairment and 1 on multiple domains of NI (Supplementary Table 1). Fourteen studies (58.3%) reported findings from population-based cohorts and 10 (41.7%) from clinical cohorts. Of the 24 studies, 5 (20.8%) were prospective in design while the rest (79.2%) identified study participants retrospectively. Europe provided 11 (45.8%) studies, North America 7 (31.8%) studies, Australia 4 (18.2%) studies, Asia one (4.5%) study, and Africa one study (Supplementary Table 1).
Most studies (63%) were classified as class 1 or excellent quality (Supplementary Table 1) and the median quality score for all studies was 91% (range [55–100]). The median quality score was similar for population-based studies and clinical cohort studies (91% [55–100] vs. 87% [64–100]; p = 0.76). The median quality score for retrospective cohort studies was also comparable with the median score for prospective studies (91% [55–100] vs. 100% [82–100], p = 0.21).
The median SMR was 7.1 (range 3.1–22.4) for children with epilepsy (Table 2), and none of these studies originated from LMICs. The median SMR for clinical cohort studies (33–35) was 7.5 (range 7.0–22.4) and 6.8 (range 3.1–9.0) for population-based studies (36, 37, 39, 41). One study (38) reported a MRR of 14.9 (95% CI 13.9–16.1) and the other study (40) a hazard ratio of 3.8 (95% CI 3.1–4.7).
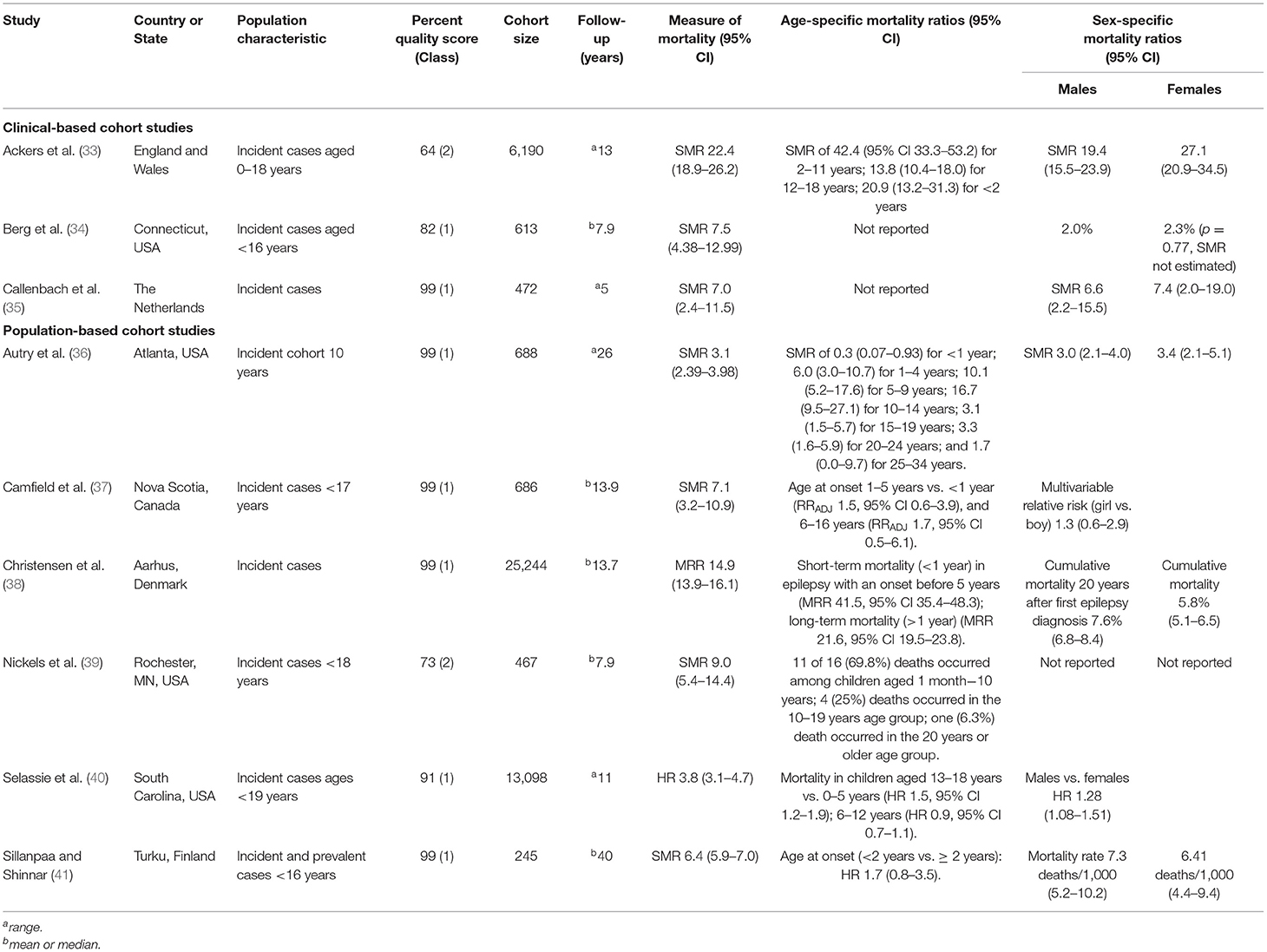
Table 2. General study characteristics, overall risk, and mortality by age and sex in children with epilepsy.
Four studies reported a significantly higher risk of mortality in younger children (33, 36, 38, 39), and mortality was highest among teenagers only in one study (40). Age was not associated with mortality in two reports (37, 41), and the age-mortality association was not reported in two studies (Table 2). The risk of mortality comparing boys and girls with epilepsy was similar in most studies (Table 2). Only one study (40) reported a significantly higher risk among boys (HR 1.3, [95% CI 1.1–1.5]).
Most deaths (68.8%) occurred in the first 10 years after epilepsy diagnosis, and mortality declined significantly in the subsequent decades but remained higher compared with the general populations (36, 38, 39). Mortality was higher in structural/metabolic epilepsy compared with epilepsy of genetic or unknown etiology in three studies (34, 35, 41). The risk of mortality was also very high in epilepsy with comorbid brain disorders, some of which were possible causes of epilepsy (Supplementary Table 2).
Generalized seizures were associated with an increased risk of mortality at the univariable level, but not at the multivariate level (41). Mortality was increased in epilepsy syndromes such as Lennox-Gastaut syndrome and infantile spasms in one report (36). Most reports included in this review, however, did not report the effect of seizure type on mortality (Supplementary Table 2).
Mortality was also higher in: (i) epilepsy patients using more than 2 antiepileptic drugs (AEDs) (33, 39); (ii) patients without a 5 year terminal remission after treatment with AEDs (41); (iii) patients from rural settings (40); and (iv) patients with Medicare health insurance plan compared with private/commercial insurance in one US-based study (40).
Most deaths (median percentage 87.5% [range 74.9–90.6]) were caused by non-epilepsy-related causes such as underlying neurological disorders or respiratory problems (Table 3); but infections, tumors, cardiovascular disorders, and injuries were also reported in other studies (36, 40). Status epilepticus and sudden unexplained death in epilepsy (SUDEP) were the most common causes of epilepsy-related mortality (Table 3).
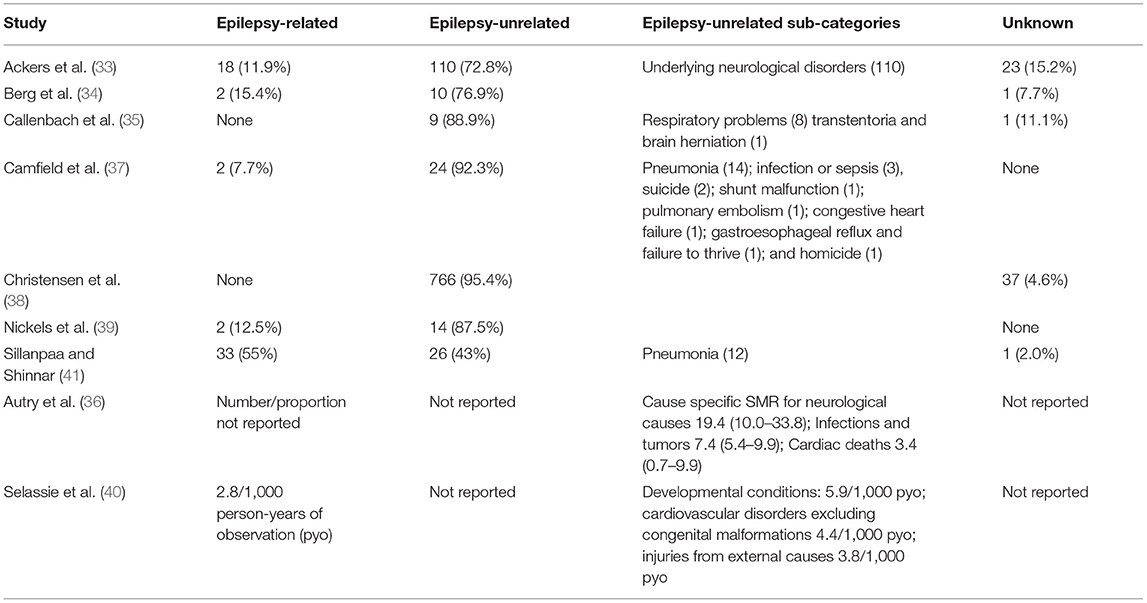
Table 3. Proportionate mortality or cause-specific mortality rates/ratios for epilepsy and non-epilepsy related causes.
The median SMR was 2.9 (range 2.0–11.6) for ID; however, one study reported a HR of 6.1 (95% CI 5.3–7.0) after 25 years of follow-up, and two studies reported crude mortality ratios of 1.8 and 1.7, respectively (Table 4). There was no difference in the overall risk of mortality between studies classifying ID using the International Classification of Disease Ninth Revision (ICD-9) or earlier versions and the Diagnostic and Statistical Manual of Mental Disorders Fourth Revision (DSM-IV) or earlier versions compared with studies classified using the ICD-10 or DSM-5. Studies of ID mainly utilized data from population-wide or state-wide disability service providers linked retrospectively with registries of mortality. Information about the definition of ID and sources of mortality data for each report are provided in Supplementary Table 3.
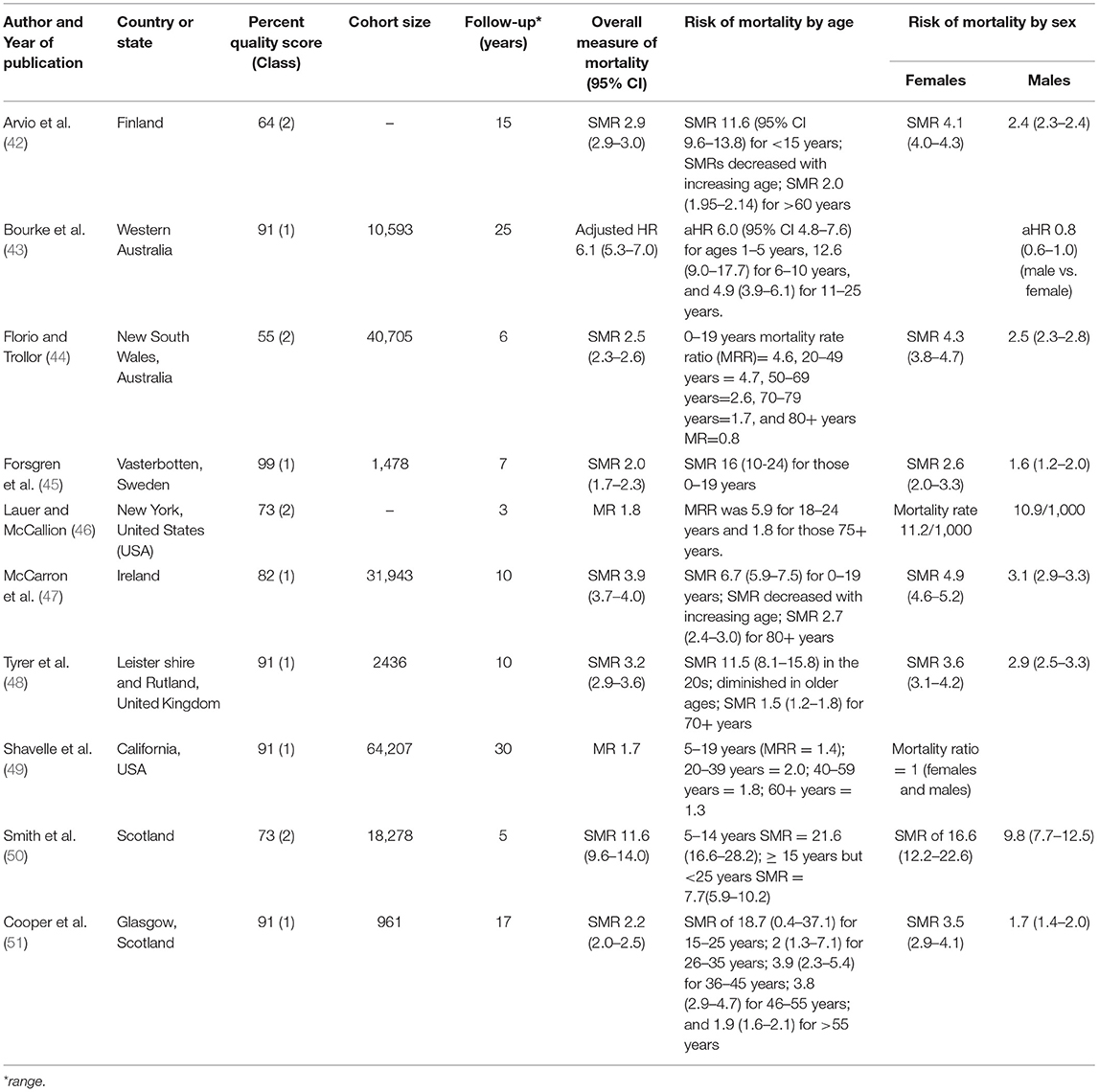
Table 4. General study characteristics, overall risk, and sociodemographic risk factors for mortality in people with intellectual disability.
Mortality was highest in younger age-groups in all studies (Table 4). The median SMR for the ages 0–19 years was 13.8 (range 6.7–21.6). Most studies reported a monotonic decline of mortality ratios with increasing age, and mortality was slightly higher than the respective general populations in older age groups (60+ years), suggesting a healthy survivor effect (Table 4). The median SMR was 4.1 (range 2.6–16.6) for females and 2.5 (range 1.6–9.8) for males. Mortality was similarly high in males and females in a study excluding ID cases with significant physical impairment or comorbid/underlying degenerative conditions (49).
The risk of premature mortality was consistently higher in severe or profound ID compared with mild or moderate ID (Table 5). Three studies, however, did not report mortality by the severity of ID (44, 46, 50). Mortality in ID was significantly increased by genetic disorders (Down syndrome and Fragile X), maternal alcohol use, low-birth weight and postnatal injury, and the presence and number of comorbid neurological conditions such as epilepsy and cerebral palsy (43, 45, 48, 51) (Table 5).
Respiratory infections (34%), accidents (18%), and epilepsy (10.7%) were the most common causes of death in ID in one study from Western Australia (43); while, the leading causes of death in a Swedish study were congenital malformations (SMR 46.3 [32.9–65.0]), neurological diseases (SMR 9.7 [5.5–17.0]), mental disorders (SMR 4.0 [1.9–8.4]), and respiratory diseases (SMR 3.3 [2.0–5.5]) (45). Diseases of the respiratory system (21.8%), the circulatory system (19.1%) and the nervous system (13.0%) were the most common causes of death in the Scottish study (51). Another Scottish study (50) identified diseases of the nervous system (33%), congenital malformations, deformations and chromosomal anomalies (22%), and nutritional, metabolic and endocrinal diseases (8%) as the most frequent causes of death. Six out of 10 (60%) studies did not report the causes of death in ID.
We identified 3 studies of CP (52–54) which assessed the effect of motor impairment on the risk of mortality in children. The risk of mortality in CP was highest between the ages of 2–15 years compared to the general population rates (SMRs>25) and declined steadily to 2–3 times higher than the population rates by the age of 40 years (52, 54). The study from Bangladesh did not estimate the SMR but crude mortality rates (19.5 per 1,000 person-years) and mortality was highest in the youngest age group (<5 years).
Lack of independent ambulation was the strongest predictor of mortality (adjusted HR 6.1, [3.3–11.8]) in the Australian study (54). However, motor impairment was a weaker predictor of premature mortality (MRR 1.4 [1.1–1.7]) than ID (MRR 2.1, [1.9–2.4]) in the Western Australian study (52). The latter study further reported that the overall disability score was a better predictor of mortality than motor impairment and ID, separately. While mortality was elevated among those with hearing impairment (adjusted HR 2.9 [1.2–6.7]) and swallowing difficulties (adjusted HR 2.3 [1.0–4.9]) in the study from Bangladesh (53), the risk of severe motor impairment (GMFCS levels III-V) on mortality did not reach statistical significance (adjusted HR 2.4 [0.7–8.4]).
CP as an underlying cause of death was accountable for 79% of deaths in the Western Australian study with 59% of the fatalities directly resulting from respiratory problems (52). Similarly, respiratory causes were the most common direct causes of death in the Australian study (54). Meningitis (31.0%) and pneumonia (27.6%) were the leading causes of death in the study from Bangladesh (53) and most children who died were either severely malnourished or had feeding problems.
The study from Malmöhus, Sweden (55) reported an odds ratio for mortality of 60.11 (95% CI 35.2–97.9) in visually impaired children and adolescents compared with an age- and sex-matched sample from the population. Most of the visually impaired children had additional impairments such as CP and ID, and respiratory diseases were the most common cause of death in this study.
The study from Kenya (14) investigated the long-term risk of premature mortality in children aged 6–9 years with NI in 5 domains (epilepsy and impairments in cognitive, hearing, vision and motor functions) compared with an age-matched sample from the general population. The overall risk of mortality was >3 times higher among those with any impairment compared to the general population (SMR 3.2 [1.7–5.5]). Developmental delay (adjusted HR 18.9, [2.2–160.4]) and severe malnutrition (20.9, [3.14–139.11]) increased the risk of mortality, and infections such as HIV and accidents were the most common causes of death.
The studies in this systematic review reported that the measures of mortality were significantly greater in children with NI compared with the general population. The estimates were greatest for CP (SMR > 25) and lowest for ID (SMR = 2.9), with few studies reporting mortality outcomes for visual impairment, and no data for hearing impairment. Clinical cohort studies had higher estimates than population-based cohort studies, probability of severity bias in the former. These estimates should be interpreted carefully since the methodology and follow-up periods differed across the studies, complicating the combining of estimates across studies. The risk of mortality following NI depends on younger age, the severity of the primary impairment and the number and severity of comorbid disorders. Most causes of the mortality including infections, cardiovascular diseases, and tumors were unrelated to NI.
Mortality was significantly higher in children with epilepsy compared with the reference population similar to a previous review of mortality in pediatric epilepsy (56), and so were the factors underlying mortality in NI (16, 19, 57). Early-onset epilepsy occurs in a period of vulnerability to adversities such as infections and severe epileptic encephalopathy, which can increase the risk of mortality (58). Structural epilepsy complicated by head injuries and infections of the brain has poor outcomes e.g., mortality, particularly in LMICs (16). Mortality risks are highest in intractable epilepsy with reduced responsiveness to AEDs (19). Mortality was generally similar in both males and females, although few studies reported high rates in males, probably related to their vulnerability to accidents from injuries and violence (40). Mortality risk reduces in subsequent decades (59); implying that those surviving may have responded better to AEDs or had the fewest risk factors for mortality. There are other context-specific factors for increased mortality such as residence and type of insurance which are related to social, economic and cultural disadvantages that influence access to AED treatment.
Lastly, our findings concur with previous studies that most deaths are caused by underlying comorbid brain disorders and respiratory infections and not by seizures or epilepsy (57, 60). Comorbidity conditions occur more frequently in those with severe epilepsy, who are more likely to die (61). Reducing mortality requires the management of both epilepsy and other comorbid conditions. Risk is elevated in generalized tonic seizures, which are often reported by many who die from SUDEP (62, 63), a condition that is not accurately documented in LMICs because coroner's autopsy reports are unavailable. Like SUDEP, status epilepticus was an infrequently identified cause of mortality compared to non-epilepsy causes, yet it occurs in 25–30% of people with epilepsy (64) and is known to increase mortality in children (16, 65). In many LMICs, status epilepticus is a complication of endemic infections that should be additionally prevented or managed (66) and may be due to poor access to AEDs, inappropriate treatment and delayed initiation of treatment (67). Most reported causes of death were non-epilepsy related e.g., cardiovascular problems, and respiratory problems, and so a comprehensive care and public health plan for children with epilepsy that includes other medical conditions is advised, especially in settings where these are endemic.
This review corroborates findings from a previous review (18) that the overall risk of mortality in people with childhood-onset ID is higher than the general population. Mortality risk was, however, lower than that for epilepsy, which might imply better care for ID patients in HICs, use of cohorts of milder ID, or misreported due to stigma. Underreporting of deaths due to stigma related to ID intellectual disability not only underestimates the prevalence in general but also reduces the SMRs or relative risk for deaths related to ID if these deaths are classified under the general population. There was no single study of ID from LMICs, where prevalence and associated mortality may be higher (68). A median SMR of 2.9 for ID is probably an underestimate because a majority of the studies utilized a retrospective design which may be subject to three types of bias: (i) over-representation of severe ID in clinic samples; (ii) loss to follow-up bias and; (iii) bias due to incomplete data linkage for follow up which might under-uncertain the risk of mortality artificially lowering the SMRs (69). The risk of mortality is highest in the youngest age-groups, decreasing steadily with age, which is well-appreciated in the literature (70–72). The SMRs are higher in females than males, probably consistent with inequality in access to care (18). The risk of mortality is higher in severe-profound ID, which expectedly would be associated with significant functional limitation and disability.
Genetic causes of ID were important risk factors for mortality, and Fragile X syndrome was not exceptional in this review, with a remarkable reduction in life expectancy compared with general populations. It is known that neuropsychiatric problems like epilepsy are very common in ID (73–75) and can increase mortality. Pregnancy and perinatal factors were important risk factors suggesting they will not only cause ID but will worsen its prognosis including premature mortality. Noteworthy was maternal drug abuse and mortality in ID which can be explained by poor parenting of the affected children (76). Children with ID are susceptible to accidents that can be fatal and safety measures and close supervision are encouraged. Respiratory infections are important causes of morbidity and mortality which should be prevented and managed to improve outcomes in children with ID.
Cerebral palsy (CP) had the greatest risk of mortality, which is well-recognized in other studies (77–79). Motor impairments and ID were the strongest predictors of premature mortality in CP in the studies included in this review; both comorbidities are debilitating complications of CP. Two previous studies (80, 81), concur that additional neurological comorbidity worsens the disability score significantly reducing the chances of survival among children with CP. Disability scores, therefore, offer a better prediction of survival compared with motor impairment and/or ID, separately. Rehabilitative therapies to manage physical impairment while optimizing the mobility of children with CP may improve the quality of life and reduce the risk of mortality (82, 83). Respiratory infections are the most common direct causes of death perhaps because CP impairs breathing and respiratory hygiene. Studies from LMICs settings highlight the significance of preventing other infections such as HIV, pneumonia, and meningitis as well as severe malnutrition to improve CP outcomes.
Mortality is higher among visually impaired children having comorbid and severe neurological disorders, but the study did not provide SMRs, affecting comparisons with the general population. Only cause-specific estimates of mortality would separate the effects of loss of sight on mortality from the comorbid disorders, but these data were not available for this review. The risk of mortality for visual impairment alone was not significantly higher in the Kenyan study, where children with five domains of NI were followed for over 14 years to determine mortality, which was due to small numbers. There was no single study on hearing impairments and associated all-cause mortality, which could be due to publication bias (4) or because this impairment is often overlooked to follow up of mortality outcomes. In this review, children with CP had hearing impairments which increased the risk of premature mortality (53), suggesting the need to give attention to morbidity and disability of deaf children.
The distribution of the studies identified by our searches was sparse, with most from HICs such as Europe and North America with fewest in Asia and Africa and none from South America. This may affect the generalizability of these estimates across continents or other countries, where specific studies are needed. The follow-up periods were variable, whereby studies with shorter follow-ups may underestimate the true burden of premature mortality. Follow-up times were reported differently in the primary studies, for instance, most studies neither reported the mean nor the median follow-up duration in years. This differential reporting of the follow-up duration hindered the estimation of weighted median SMRs. There is often incomplete documentation and certification of deaths due to a lack of functional vital registration systems, which affects case ascertainments yielding lower estimates of mortality. Because the grading process involved qualitative judgment by the reviewers, the interpretation of study methods and the application of criteria might be inconsistent and unreliable. The study populations from which we obtained the data vary greatly in terms of population characteristics, health, and social systems. Clinical-based estimates may overrepresent severe forms of NI that have additional risk for mortality; most LMICs have limited resources to do follow-up studies of mortality, often doing these studies in high-risk zones that are not representative of other low-risk areas. Despite these limitations, our review obtained critical evidence that might increase the survival of children with childhood-onset NI and disability.
The risk of premature mortality is elevated in children with NI, or adults with childhood-onset NI, it being higher in CP and epilepsy, and lower in ID. There are few SMR studies for visual, hearing, and motor impairments. We recommend future population-based follow-up studies for multiple domains of NI in children, especially for those with visual, hearing, and motor impairments, and in LMICs where there is a dearth of evidence. Few studies in this review originated from LMICs, yet these countries have a concentration of risk factors and the highest burden of childhood NI and disability. The similarity of risk factors and causes of death across the five domains of NI provides an opportunity for integration of preventive, curative and rehabilitative services. Integrated interventions targeting modifiable risk factors, for instance, improving access to AEDs and prompt treatment of childhood epilepsy as well as caregiver/parental training, child supervision, and prevention of respiratory infections for children with ID, immunization, and improved nutrition in LMICs, are required to improve survival and quality of life among the affected children and families. It worth advising the families that premature death among children cannot be preventable in the presence of genetic causes for NI conditions that are untreatable and progressive such as mucopolysaccharidosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JA: protocol development, searches, identification, appraisal of eligible studies, data synthesis, and writing the first draft of the manuscript. SKa: protocol development, searches, identification, and subsequent review of drafts. SKi: protocol development and review of subsequent drafts. MB: protocol development and review of subsequent drafts. CN: protocol development and review of subsequent drafts. All authors: have read and approved the final manuscript.
JA is a Ph.D. fellow funded by the DELTAS (Developing Excellence in Leadership, Training, and Science) Africa Initiative. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant 107754/Z/15/Z- DELTAS Africa Sub-Saharan Africa Consortium for Advanced Biostatistics (SSACAB) programme] and The Initiative To Develop African Research Leaders [DEL-15-003] and the UK government. SKa was supported by MQ: Transforming Mental Health (MQF17-18 Kariuki). The views expressed in this review are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. The authors had full access to the study data and had the final responsibility for the decision to submit this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge René Spijker-an information specialist at the Cochrane Netherlands, and Mary Bitta-a researcher at the KEMRI-Wellcome Research Programme, for their invaluable ideas in developing the search strategy for this systematic review.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.627824/full#supplementary-material
1. Mung'ala-Odera V, Newton CR. Identifying children with neurological impairment and disability in resource-poor countries. Child Care Health Dev. (2007) 33:249–56. doi: 10.1111/j.1365-2214.2006.00714.x
2. WHO. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of disease, Published in Accordance With Resolution WHA29.35 of the Twenty-ninth World Health ASSEMBLY, May 1976. World Health Organization 1980. Available online at: https://apps.who.int/iris/handle/10665/41003
3. WHO. International Classification of Functioning, Health and Disability (ICF). (2001). Available online at: https://www.who.int/classifications/icf/en/
4. Bitta M, Kariuki S, Abubakar A, Newton C. Burden of neurodevelopmental disorders in low and middle-income countries: a systematic review and meta-analysis. Wellcome Open Res. (2017) 2:121. doi: 10.12688/wellcomeopenres.13540.1
5. Mung'ala-Odera V, Meehan R, Njuguna P, Mturi N, Alcock KJ, Newton CR. Prevalence and risk factors of neurological disability and impairment in children living in rural Kenya. Int J Epidemiol. (2006) 35:683–8. doi: 10.1093/ije/dyl023
6. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study (2010). Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61689-4
7. Newton CR. Global burden of pediatric neurological disorders. Semin Pediatr Neurol. (2018) 27:10–5. doi: 10.1016/j.spen.2018.03.002
8. Olusanya BO, Davis AC, Wertlieb D, Boo N-Y, Nair MKC, Halpern R, et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study (2016). Lancet Global Health. (2018) 6:e1100–21. doi: 10.1016/S2214-109X(18)30309-7
9. Olusanya BO, de Vries PJ. Nurturing care for children with developmental disabilities: a moral imperative for sub-Saharan Africa. Lancet Child Adolesc Health. (2018) 2:772–4. doi: 10.1016/S2352-4642(18)30281-5
10. Wang H, Liddell C, Coates M, Mooney M, Levitz C, Schumacher A, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:957–79. doi: 10.1016/S0140-6736(14)60497-9
11. Kassebaum N, Kyu HH, Zoeckler L, Olsen HE, Thomas K, Pinho C, et al. Child and Adolescent health from 1990 to 2015: findings from the global burden of diseases, injuries, and risk factors 2015 study. JAMA Pediatr. (2017) 171:573–92. doi: 10.1001/jamapediatrics.2017.0250
12. Newton C. Neurodevelopmental disorders in low- and middle-income countries. Dev Med Child Neurol. (2012) 54:1072. doi: 10.1111/j.1469-8749.2012.04384.x
13. Scherzer AL, Chhagan M, Kauchali S, Susser E. Global perspective on early diagnosis and intervention for children with developmental delays and disabilities. Dev Med Child Neurol. (2012) 54:1079–84. doi: 10.1111/j.1469-8749.2012.04348.x
14. Abuga JA, Kariuki SM, Kinyanjui SM, Boele Van Hensbroek M, Newton CRJC. Premature mortality in children aged 6–9 years with neurological impairments in rural Kenya: a cohort study. Lancet Global Health. (2019) 7:e1728–35. doi: 10.1016/S2214-109X(19)30425-5
15. Charlson FJ, Baxter AJ, Dua T, Degenhardt L, Whiteford HA, Vos T. Excess mortality from mental, neurological, and substance use disorders in the Global Burden of Disease study 2010. In: Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora MEE, editors. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed. Washington, DC: International Bank for Reconstruction and Development/the World Bank (2016). p. 4.
16. Levira F, Thurman DJ, Sander JW, Hauser WA, Hesdorffer DC, Masanja H, et al. Premature mortality of epilepsy in low- and middle-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. (2017) 58:6–16. doi: 10.1111/epi.13603
17. Ngugi AK, Bottomley C, Fegan G, Chengo E, Odhiambo R, Bauni E, et al. Premature mortality in active convulsive epilepsy in rural Kenya: causes and associated factors. Neurology. (2014) 82:582–9. doi: 10.1212/WNL.0000000000000123
18. O'Leary L, Cooper SA, Hughes-McCormack L. Early death and causes of death of people with intellectual disabilities: a systematic review. J Appl Res Intellect Disabil. (2018) 31:325–42. doi: 10.1111/jar.12417
19. Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR, et al. The burden of premature mortality of epilepsy in high-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. (2017) 58:17–26. doi: 10.1111/epi.13604
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
21. Tacconelli E. Systematic reviews: CRD's guidance for undertaking reviews in health care. Lancet Infect Dis. (2010) 10:226. doi: 10.1016/S1473-3099(10)70065-7
22. NHS. PROSPERO: International Prospective Register of Systematic Reviews York, UK YO10 5DD. Centre for Reviews and Dissemination, University of York (2018). Available online at: https://www.crd.york.ac.uk/prospero/ (accessed July 31, 2018).
23. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
24. APA. Diagnostic and Statistical Manual of Mental Health Disorders. Washington, DC: American Psychological Association (2013).
25. Wehmeyer ML, Shogren K, Angel Verdugo M, Nota L, Soresi S, Lee S-H, et al. Cognitive impairment and intellectual disability. Special education international perspectives: biopsychosocial, cultural, and disability aspects. Adv Spec Educ. (2014) 27:55–89. doi: 10.1108/S0270-4013_2014_0000027002
26. WHO. WHO Global Disability action Plan 2014-2021: Better Health for All People With Disability. Geneva: World Health Organization, 2015 (2015).
27. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. (1997) 39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x
28. WHO. Deafness and Hearing Loss. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss
29. WHO. Blindness and Vision Impairment. (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed September 24, 2019).
30. Sandeep Moola ZM, Tufanaru C, Aromataris E, Sears K, Sfetc R, Currie M, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris EMZ, editor. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute (2017). Available online at: https://wiki.jbi.global/display/MANUAL/Chapter+7%3A+Systematic+reviews+of+etiology+and+risk
31. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. (2007) 4:e297. doi: 10.1371/journal.pmed.0040297
32. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
33. Ackers R, Besag FM, Hughes E, Squier W, Murray ML, Wong IC. Mortality rates and causes of death in children with epilepsy prescribed antiepileptic drugs: a retrospective cohort study using the UK General Practice Research Database. Drug Saf. (2011) 34:403–13. doi: 10.2165/11588480-000000000-00000
34. Berg AT, Shinnar S, Testa FM, Levy SR, Smith SN, Beckerman B. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med. (2004) 158:1147–52. doi: 10.1001/archpedi.158.12.1147
35. Callenbach PM, Westendorp RG, Geerts AT, Arts WF, Peeters EA, van Donselaar CA, et al. Mortality risk in children with epilepsy: the Dutch study of epilepsy in childhood. Pediatrics. (2001) 107:1259–63. doi: 10.1542/peds.107.6.1259
36. Autry AR, Trevathan E, Van Naarden Braun K, Yeargin-Allsopp M. Increased risk of death among children with Lennox-Gastaut syndrome and infantile spasms. J Child Neurol. (2010) 25:441–7. doi: 10.1177/0883073809348355
37. Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet. (2002) 359:1891–5. doi: 10.1016/S0140-6736(02)08779-2
38. Christensen J, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term mortality in children and young adults with epilepsy–a population-based cohort study. Epilepsy Res. (2015) 114:81–8. doi: 10.1016/j.eplepsyres.2015.05.001
39. Nickels KC, Grossardt BR, Wirrell EC. Epilepsy-related mortality is low in children: a 30-year population-based study in Olmsted County, MN. Epilepsia. (2012) 53:2164–71. doi: 10.1111/j.1528-1167.2012.03661.x
40. Selassie AW, Wilson DA, Wagner JL, Smith G, Wannamaker BB. Population-based comparative analysis of risk of death in children and adolescents with epilepsy and migraine. Epilepsia. (2015) 56:1957–65. doi: 10.1111/epi.13219
41. Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. (2010) 363:2522–9. doi: 10.1056/NEJMoa0911610
42. Arvio M, Salokivi T, Tiitinen A, Haataja L. Mortality in individuals with intellectual disabilities in Finland. Brain Behav. (2016) 6:e00431. doi: 10.1002/brb3.431
43. Bourke J, Nembhard WN, Wong K, Leonard H. Twenty-five year survival of children with intellectual disability in Western Australia. J Pediatr. (2017) 188:232–9.e2. doi: 10.1016/j.jpeds.2017.06.008
44. Florio T, Trollor J. Mortality among a cohort of persons with an intellectual disability in New South Wales, Australia. J Appl Res Intellect Disabil. (2015) 28:383–93. doi: 10.1111/jar.12190
45. Forsgren L, Edvinsson SO, Nystrom L, Blomquist HK. Influence of epilepsy on mortality in mental retardation: an epidemiologic study. Epilepsia. (1996) 37:956–63. doi: 10.1111/j.1528-1157.1996.tb00533.x
46. Lauer E, McCallion P. Mortality of people with intellectual and developmental disabilities from select US state disability service systems and medical claims data. J Appl Res Intellect Disabil. (2015) 28:394–405. doi: 10.1111/jar.12191
47. McCarron M, Carroll R, Kelly C, McCallion P. Mortality rates in the general irish population compared to those with an intellectual disability from 2003 to 2012. J Appl Res Intellect Disabil. (2015) 28:406–13. doi: 10.1111/jar.12194
48. Tyrer F, Smith LK, McGrother CW. Mortality in adults with moderate to profound intellectual disability: a population-based study. J Intellect Disabil Res. (2007) 51(Pt. 7):520–7. doi: 10.1111/j.1365-2788.2006.00918.x
49. Shavelle RM, Sweeney LH, Brooks JC. Comparative mortality of persons with intellectual disability in California: an update (2000-2010). J Insurance Med. (2014) 44:158–63.
50. Smith GS, Fleming M, Kinnear D, Henderson A, Pell JP, Melville C, et al. Rates and causes of mortality among children and young people with and without intellectual disabilities in Scotland: a record linkage cohort study of 796 190 school children. BMJ Open. (2020) 10:e034077. doi: 10.1136/bmjopen-2019-034077
51. Cooper SA, Allan L, Greenlaw N, McSkimming P, Jasilek A, Henderson A, et al. Rates, causes, place and predictors of mortality in adults with intellectual disabilities with and without Down syndrome: cohort study with record linkage. BMJ Open. (2020) 10:e036465. doi: 10.1136/bmjopen-2019-036465
52. Blair E, Watson L, Badawi N, Stanley FJ. Life expectancy among people with cerebral palsy in Western Australia. Dev Med Child Neurol. (2001) 43:508–15. doi: 10.1017/S0012162201000949
53. Jahan I, Karim T, Das MC, Muhit M, McIntyre S, Smithers-Sheedy H, et al. Mortality in children with cerebral palsy in rural Bangladesh: a population-based surveillance study. Dev Med Child Neurol. (2019) 61:1336–43. doi: 10.1111/dmcn.14256
54. Reid SM, Carlin JB, Reddihough DS. Survival of individuals with cerebral palsy born in Victoria, Australia, between 1970 and (2004). Dev Med Child Neurol. (2012) 54:353–60. doi: 10.1111/j.1469-8749.2012.04218.x
55. Blohme J, Tornqvist K. Visually impaired Swedish children. The 1980 cohort study–aspects on mortality. Acta Ophthalmol Scand. (2000) 78:560–5. doi: 10.1034/j.1600-0420.2000.078005560.x
56. Appleton RE. Mortality in paediatric epilepsy. Arch Dis Child. (2003) 88:1091–4. doi: 10.1136/adc.88.12.1091
57. Mbizvo GK, Bennett K, Simpson CR, Duncan SE, Chin RFM. Epilepsy-related and other causes of mortality in people with epilepsy: a systematic review of systematic reviews. Epilepsy Res. (2019) 157:106192. doi: 10.1016/j.eplepsyres.2019.106192
58. Moseley BD, Wirrell EC, Wong-Kisiel LC, Nickels K. Early onset epilepsy is associated with increased mortality: a population-based study. Epilepsy Res. (2013) 105:410–4. doi: 10.1016/j.eplepsyres.2013.03.002
59. Neligan A, Bell GS, Shorvon SD, Sander JW. Temporal trends in the mortality of people with epilepsy: a review. Epilepsia. (2010) 51:2241–6. doi: 10.1111/j.1528-1167.2010.02711.x
60. Berg AT, Nickels K, Wirrell EC, Geerts AT, Callenbach PM, Arts WF, et al. Mortality risks in new-onset childhood epilepsy. Pediatrics. (2013) 132:124–31. doi: 10.1542/peds.2012-3998
61. Wilmshurst JM, Kakooza-Mwesige A, Newton CR. The challenges of managing children with epilepsy in Africa. Semin Pediatr Neurol. (2014) 21:36–41. doi: 10.1016/j.spen.2014.01.005
62. Tomson T, Walczak T, Sillanpaa M, Sander JW. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia. (2005) 46(Suppl. 11):54–61. doi: 10.1111/j.1528-1167.2005.00411.x
63. Whitney R, Donner EJ. Risk factors for Sudden Unexpected Death in Epilepsy (SUDEP) and their mitigation. Curr Treat Opt Neurol. (2019) 21:7. doi: 10.1007/s11940-019-0547-4
64. Kariuki SM, Kakooza-Mwesige A, Wagner RG, Chengo E, White S, Kamuyu G, et al. Prevalence and factors associated with convulsive status epilepticus in Africans with epilepsy. Neurology. (2015) 84:1838–45. doi: 10.1212/WNL.0000000000001542
65. Sadarangani M, Seaton C, Scott JA, Ogutu B, Edwards T, Prins A, et al. Incidence and outcome of convulsive status epilepticus in Kenyan children: a cohort study. Lancet Neurol. (2008) 7:145–50. doi: 10.1016/S1474-4422(07)70331-9
66. Kariuki SM, Matuja W, Akpalu A, Kakooza-Mwesige A, Chabi M, Wagner RG, et al. Clinical features, proximate causes, and consequences of active convulsive epilepsy in Africa. Epilepsia. (2014) 55:76–85. doi: 10.1111/epi.12392
67. Newton CR, Kariuki SM. Status epilepticus in sub-Saharan Africa: new findings. Epilepsia. (2013) 54(Suppl. 6):50–3. doi: 10.1111/epi.12277
68. Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. (2011) 32:419–36. doi: 10.1016/j.ridd.2010.12.018
69. Vena JE, Sultz HA, Carlo GL, Fiedler RC, Barnes RE. Sources of bias in retrospective cohort mortality studies: a note on treatment of subjects lost to follow-up. J Occup Med. (1987) 29:256–61.
70. Boyle CA, Decoufle P, Holmgreen P. Contribution of developmental disabilities to childhood mortality in the United States: a multiple-cause-of-death analysis. Paediatr Perinatal Epidemiol. (1994) 8:411–22. doi: 10.1111/j.1365-3016.1994.tb00480.x
71. Decoufle P, Autry A. Increased mortality in children and adolescents with developmental disabilities. Paediatr Perinatal Epidemiol. (2002) 16:375–82. doi: 10.1046/j.1365-3016.2002.00430.x
72. Landes SD. The intellectual disability mortality disadvantage: diminishing with age? Am J Intellect Dev Disabil. (2017) 122:192–207. doi: 10.1352/1944-7558-122.2.192
73. Einfeld SL, Ellis LA, Emerson E. Comorbidity of intellectual disability and mental disorder in children and adolescents: a systematic review. J Intellect Dev Disabil. (2011) 36:137–43. doi: 10.1080/13668250.2011.572548
74. Kinnear D, Morrison J, Allan L, Henderson A, Smiley E, Cooper SA. Prevalence of physical conditions and multimorbidity in a cohort of adults with intellectual disabilities with and without Down syndrome: cross-sectional study. BMJ Open. (2018) 8:e018292. doi: 10.1136/bmjopen-2017-018292
75. Matson JL, Cervantes PE. Comorbidity among persons with intellectual disabilities. Res Autism Spect Disord. (2013) 7:1318–22. doi: 10.1016/j.rasd.2013.07.018
76. Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. (2015) 40:61–87. doi: 10.1038/npp.2014.147
77. Crichton JU, Mackinnon M, White CP. The life-expectancy of persons with cerebral palsy. Dev Med Child Neurol. (1995) 37:567–76. doi: 10.1111/j.1469-8749.1995.tb12045.x
78. Hutton JL, Cooke T, Pharoah PO. Life expectancy in children with cerebral palsy. BMJ. (1994) 309:431–5. doi: 10.1136/bmj.309.6952.431
79. Strauss DJ, Shavelle RM, Anderson TW. Life expectancy of children with cerebral palsy. Pediatr Neurol. (1998) 18:143–9. doi: 10.1016/S0887-8994(97)00172-0
80. Hutton JL, Colver AF, Mackie PC. Effect of severity of disability on survival in north east England cerebral palsy cohort. Arch Dis Child. (2000) 83:468–74. doi: 10.1136/adc.83.6.468
81. Hutton JL, Pharoah PO. Effects of cognitive, motor, and sensory disabilities on survival in cerebral palsy. Arch Dis Child. (2002) 86:84–9. doi: 10.1136/adc.86.2.84
Keywords: neurological, neurodevelopmental, disability, impairment, mortality, children
Citation: Abuga JA, Kariuki SM, Kinyanjui SM, Boele van Hensbroek M and Newton CR (2021) Premature Mortality, Risk Factors, and Causes of Death Following Childhood-Onset Neurological Impairments: A Systematic Review. Front. Neurol. 12:627824. doi: 10.3389/fneur.2021.627824
Received: 10 November 2020; Accepted: 11 March 2021;
Published: 09 April 2021.
Edited by:
Tomáš Paus, University of Toronto, CanadaReviewed by:
Paola Costa-Mallen, Bastyr University, United StatesCopyright © 2021 Abuga, Kariuki, Kinyanjui, Boele van Hensbroek and Newton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan A. Abuga, amFidWdhQGtlbXJpLXdlbGxjb21lLm9yZw==; YWJ1Z2FqbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.