- 1Division of Pediatrics Pulmonology, China Medical University, Children's Hospital, Taichung, Taiwan
- 2Department of Biomedical Imaging and Radiological Science, College of Medicine, China Medical University, Taichung, Taiwan
- 3School of Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan
- 4Department of Chinese Medicine, China Medical University Hospital, Taichung, Taiwan
- 5Department of Pediatrics, School of Medicine, Chung Shan Medical University Hospital and Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 6Division of Pediatrics Neurology, China Medical University, Children's Hospital, Taichung, Taiwan
- 7College of Chinese Medicine, Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
- 8Department of Medical Research, China Medical University Hospital, Taichung, Taiwan
- 9Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
- 10College of Medicine, China Medical University, Taichung, Taiwan
Background: Kawasaki disease is a common vasculitis of childhood in East Asia. The complications following Kawasaki disease mostly included cardiovascular sequelae; non-cardiac complications have been reported but less studied. This study investigated potential epilepsy following Kawasaki disease in Taiwanese children.
Objectives: Through National Health Insurance Research Database, we retrospectively analyzed the data of children aged <18 years with clinically diagnosed Kawasaki disease from January 1, 2000 to December 31, 2012 in Taiwan. These patients were followed up to estimate the incidence of epilepsy in the Kawasaki cohort in comparison with that in the non-Kawasaki cohort in Taiwan.
Results: A total of 8,463 and 33,872 patients in the Kawasaki and non-Kawasaki cohorts were included in the study, respectively. Of the total eligible study subjects, 61.1% were boys and 38.9% were girls; most patients with newly diagnosed Kawasaki disease were aged <5 years [88.1%]. Patients with Kawasaki disease showed a higher incidence rate [47.98 vs. 27.45 every 100,000 person years] and significantly higher risk [adjusted hazard ratio = 1.66, 95% confidence interval = 1.13–2.44] of epilepsy than those without the disease. Additionally, female sex [adjusted hazard ratio = 2.30, 95% confidence interval = 1.31–4.04] and age <5 years [adjusted hazard ratio = 1.82, 95% confidence interval = 1.22–2.72] showed a significantly higher risk of epilepsy in the Kawasaki cohort.
Conclusion: Results revealed a higher incidence rate and significant risk of epilepsy in Taiwanese children with Kawasaki disease than in those without the disease. Therefore, children diagnosed with Kawasaki disease are recommended follow-up as they have a high risk of epilepsy and seizure disorders.
Background
Kawasaki disease [KWD], also known as mucocutaneous lymph node syndrome, has long been considered a common childhood vasculitis disorder with various cardiovascular complications, most commonly involving medium-sized muscular arteries (1). Additionally, non-cardiac complications followed by KWD has aroused interest during the past few decades (2). Despite neurological complications involving the central nervous system (CNS) not being uncommon in KWD, the extent of their influence on the brains of children and the effect of KWD on the development of various neurodevelopmental disorders (NDD) remains unclear (3). Although neurological complications such as seizures, hemiplegia, facial palsy, cerebral vasculitis, myositis, and aseptic meningitis have been occasionally reported in conjunction with KWD, the long-term sequelae such as epilepsy have never been reported (4–7).
In precedence to the present study, our research team conducted a study in 2019 that included 612 children with KWD. The results of a long-term follow-up indicated that these children had an increased risk of developing a neurodevelopmental disorder (NDD) with KWD. Although not all of the children developed an NDD, the prevalence was statistically significant for epilepsy and Tourette syndrome (8). Here, we conducted a wide-ranging investigation in the child population by using Taiwan National Health Insurance Research Database [NHIRD] to determine whether an association exists between KWD and childhood epilepsy.
Methods
Data Source
National Health Insurance program [Taiwan NHI] was established in 1995 and enrolled >99.9% of the population in Taiwan. NHIRD was established by Taiwan NHI and consisted of information on outpatients, hospitalization, medical treatment or operation, and other medical services of patients' each hospital visit. To conduct this study analysis, we used a population-based inpatient file based on NHIRD. The identification number was encrypted before the database was released to protect the privacy of each study subject. Diagnoses in Taiwan NHI are coded according to the International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM]. The Research Ethics Committee of China Medical University and Hospital in Taiwan approved the study [CMUH-104-REC2-115-R4].
Study Population
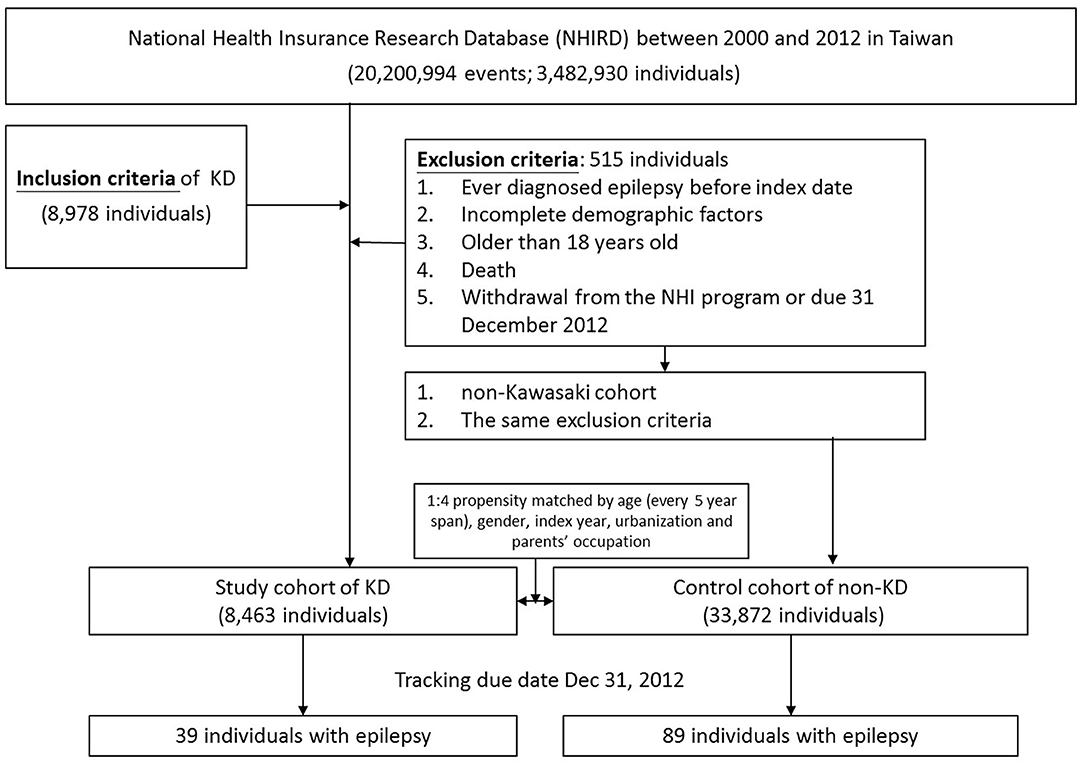
To clarify the association between KWD [ICD-9-CM: 446.1] and epilepsy [ICD-9-CM: 345, A225], we identified two cohorts: Kawasaki and non-Kawasaki cohorts. Patients who were newly diagnosed as having KWD from 2000 to 2012 were classified into the Kawasaki cohort, and the diagnosis date was set as the index date; patients without any KWD diagnosis were classified into the non-Kawasaki cohort. Patients ever diagnosed as having epilepsy before the index date, with incomplete demographic factors, >18 years old, who died, or who withdrew from the NHI program or due date to December 31, 2012 were excluded from this study. Each patient in the Kawasaki cohort was 1:4 propensity matched based on age [every 5-year span], sex, index year, urbanization, and parent occupation (Figure 1). To avoid wrongly diagnosing acute seizures as epilepsy during the KWD course, the gain of diagnosis with epilepsy was separated from the admission course of KWD for at least 1 month.
Statistical Analysis
We used two sample t-tests for age and chi-square test for sex, urbanization, and parent occupation to compare the difference between Kawasaki and non-Kawasaki cohorts. The Kaplan–Meier method was used to demonstrate the cumulative incidence of epilepsy and cerebrovascular diseases between two cohorts, and the log rank test was applied to estimate the difference between the two curves.
To calculate epilepsy risk between Kawasaki and the comparison cohorts, we used a Cox proportional-hazard model and presented hazard ratios, adjusted hazard ratios [aHRs], and 95% confidence intervals [CIs] before and after adjusting demographic factors. The significance criterion was set at <0.05 for two-side testing of p-value. All statistical analyses were performed using SAS statistical software, version 9.4 [SAS Institute Inc., Cary, NC, USA]; the cumulative incidence curve was plotted using R software.
Results
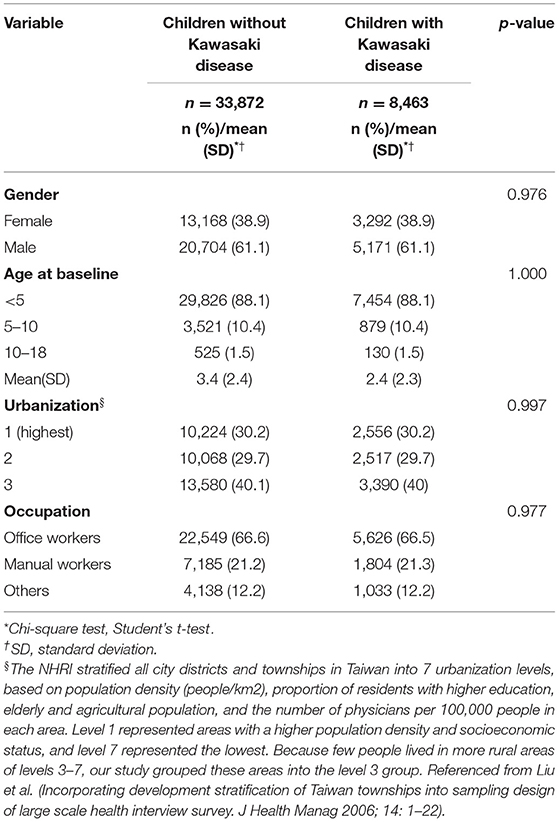
We eventually enrolled 8,463 and 33,872 patients in the Kawasaki and non-Kawasaki cohorts. There were no statistically significant differences in urbanization level, geographic region, head injury, parents' occupation (all p > 0.05) (Table 1), respectively. Of the total eligible study subjects, 61.1% were boys and the other 38.9% were girls; most patients were newly diagnosed as having KWD at age ≤5 years [88.1%], and the mean age was 2.4 years. Table 2 presents the number, incidence rate, and epilepsy risk in the Kawasaki and non-Kawasaki cohorts. Patients with KWD showed a higher incidence rate [47.98 vs. 27.45 every 100,000 person years] and significantly higher risk [aHR = 1.66, 95% CI = 1.13–2.44] of epilepsy. Other potential risk factors such as sex, increasing age, and urbanization had a non-significant effect on epilepsy risk. To confirm the association between KWD and epilepsy, a multivariable stratified analysis was performed. Female sex [aHR = 2.30, 95% CI = 1.31–4.04] and age <5 years [aHR = 1.82, 95% CI = 1.22–2.72] showed a significantly higher risk of epilepsy in the Kawasaki cohort (Table 3). After stratification, patients with a follow-up period of 5 years after KWD diagnosis had a significantly higher risk of epilepsy [aHR = 1.82, 95% CI = 1.17–2.82] than those with a follow-up period of >5 years (Table 4). Patients with KWD had a significantly higher cumulative incidence of epilepsy [p = 0.0032] than did those without KWD (Figure 2).
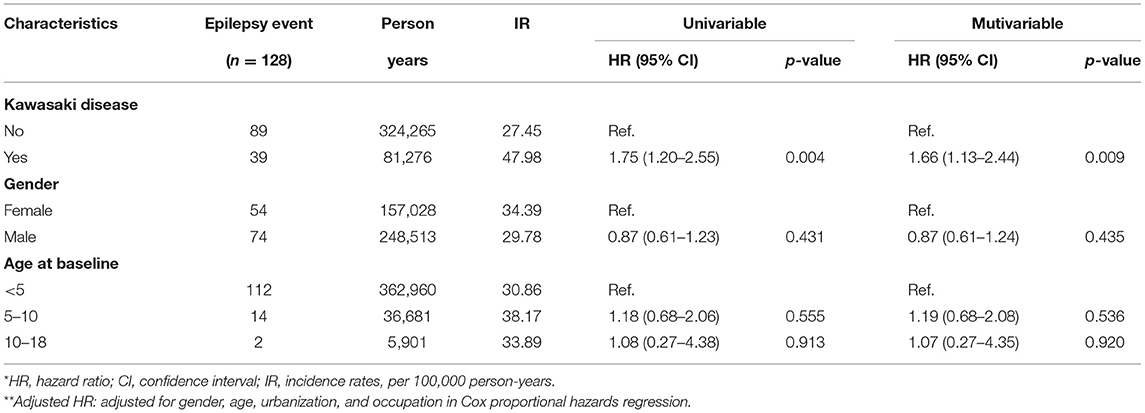
Table 2. Cox model measured hazard ratio and 95% confidence intervals of epilepsy associated with and without Kawasaki disease.
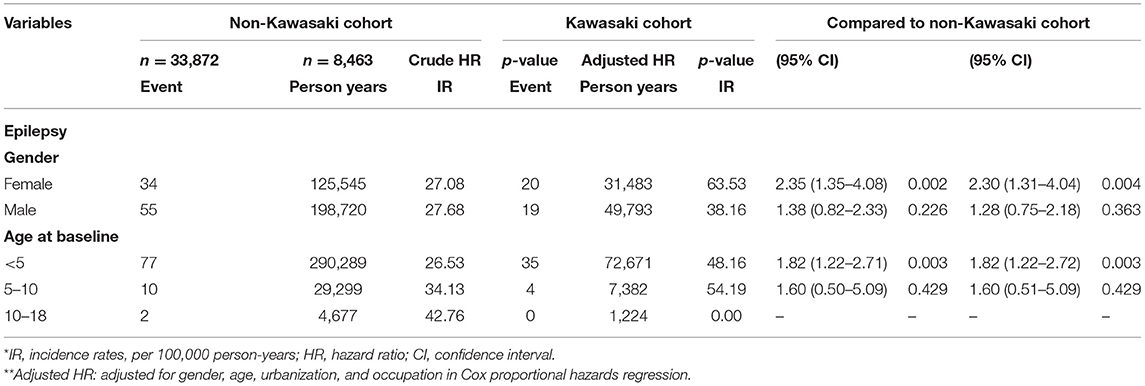
Table 3. Incidence rates, hazard ratio and confidence intervals of epilepsy in different stratification.
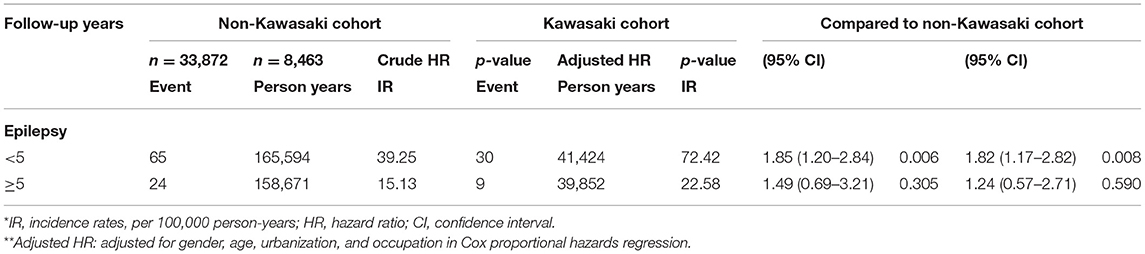
Table 4. Incidence rates, hazard ratio, and confidence intervals of epilepsy in different follow-up stratification.
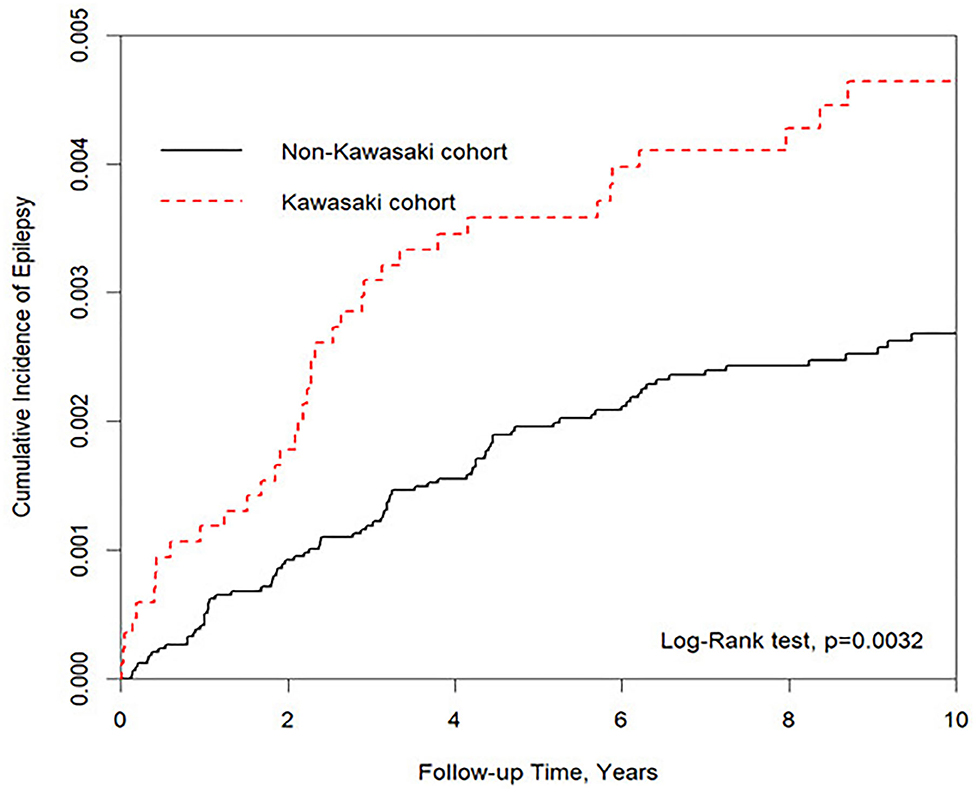
Figure 2. Kaplan–Meier for cumulative incidence of epilepsy under aged stratified by Kawasaki disease with log-rank test.
Discussion
As early as 1977, Nakane et al. reported a case of mucocutaneous lymph node syndrome complicated by acute occlusion of the right middle cerebral artery and hemiconvulsion–hemiplegia–epilepsy syndrome. Since then, numerous neurological involvements associated with KWD have been discovered (9). These include headache, convulsions, somnolence, irritability, meningoencephalit5is, ptosis, sensorineural hearing loss, and facial palsy (4, 6, 7). Even so, most of them subsided completely following the successful management of KWD without any clinically apparent signs of CNS injury. However, few studies have examined whether acute KWD is followed by subtle but clinically apparent changes in the brain of children, which might lead to the long-term development of NDDs (4–7). Therefore, the present study adopted a larger nationwide database to trace the incidence of epilepsy in Taiwanese children with KWD. In the present study, we demonstrated that children with KWD experience epilepsy later in life. The trend was independent of sex, age at KWD diagnosis, and urbanization.
Additionally, our results revealed that children aged <5 years with KWD had a higher risk of epilepsy. We hypothesize that from birth to age 5 years, a child's brain develops more than at any other time in life (10), and KWD, a vasculitis that occurs most often in early childhood, may interfere with brain development and probably subtly alter the structure of human brain, causing epilepsy (11). Although no reports refer to alterations of the brain structure of children following KWD, and our cohort observation study failed to provide evidence of such alterations, we presumed the involvement of a mechanism and pathogenesis comparable to those of central nervous system vasculitis, in which brain vessel wall inflammation can result in intraluminal stenosis and platelet adhesion to the vessel wall, eventually leading to a substantial brain parenchymal lesion and the development of seizure disorders (12, 13). We also determined that epileptogenesis largely occurred within 5 years of KWD diagnosis. Although no existing reports explain it, we speculated that such would be the case, with time for secondary injury processes (such as neuroinflammation) following KWD to occur (14, 15). A similar phenomenon occurs in epileptogenesis after traumatic brain injury (16). However, more evidence derived from neuroimaging studies and animal brain models is necessary to validate our hypothesis.
This study revealed that female patients with KWD had a higher risk of epilepsy than did male patients, but the reason underlying these finding remains unclear. One argument is that women have stronger immune responses to infection and inflammation than men do (17). In addition, sex hormones play a crucial role in immune system activities (18). Women undergo endocrinological changes two to three times in their lifetime [puberty, childbearing, breastfeeding, and menopause], and each hormone's transition results in a significant effect on the immune system, rendering female patients with an underlying autoimmune disease susceptible to associated complications (19, 20). This theory was studied in systemic lupus erythematosus—a well-known autoimmune disease (21). However, because the present study's patients were young children (due to the nature of KWD), the aforementioned assumption regarding endocrinological changes, which is suitable for people who are adolescents or older, may not be applicable here. Perhaps hormones present only in boys (e.g., testosterone) played a role in our results. Because testosterone has been proven to play roles in the inhibition of vascular inflammation, neuroinflammation, and aneurysm formation (22–26), its possible role in the inhibition of brain inflammation associated with KWD would be another area worth studying.
Nonetheless, previous studies have suggested that early brain insult might alter the brains of boys and girls differently but lead to the development of NDDs later in life (27). For example, autism spectrum disorder and attention-deficit/hyperactivity disorder are more likely to be diagnosed in boys (28, 29). A study in the rat model, using lipopolysaccharide as a stimulator of the innate immune system, examined blood–brain barrier dysfunction under inflammatory conditions. It was found that following lipopolysaccharide stimulation, females had higher levels of cytokines and chemokines in the blood and brain compared with males. Hence, although females have a stronger capability to resist infections, females may have an exacerbated inflammatory response compared with males when presented with the same dose of an immune stimulus (30). Further research is required to confirm whether the same theory can be applied in female patients with epilepsy following KWD.
In addition to epilepsy, some long-term behavioral problems, including somatic problems and withdrawal trait, have been noted in children following KWD (Table 5) (8, 31–37), but the conclusions are conflicting (37). Separately, the role of brain-reactive autoantibodies has been studied in brain development and cognitive impairment from many different autoimmune conditions and autoimmune encephalitis (38–41), Likewise, some autoantibodies were studied in relation to KWD, including antithyroid microsomal antibody, antiparietal cell antibody, antiliver kidney microsomal antibody, antiendothelial cell antibodies, antinuclear antibody, antineutrophil cytoplasmic antibody, antimitochondrial antibody, and antismooth muscle antibody. These autoantibodies likely develop in children with KWD during the acute stage and may persist for many years (42, 43). However, whether these autoantibodies increase the risk of autoimmune disease in the future is unclear, although some studies have explored the association between them (44, 45). Furthermore, epilepsy after KWD is another problem, and whether epilepsy after KWD shares a common mechanism as seizure occurring in the acute stage of vasculitis during KWD or any other autoimmune encephalitis remains unclear and intrigues us. If such is the case, two hypotheses may be explained. First, some autoantibodies triggered by KWD could persist in vivo for years, which in turn leads to some autoantibody-mediated diseases or systemic autoimmune diseases so that seizure disorders or epilepsy could only be sentinel disorders of these diseases (46, 47). Second, given that medium-sized vessel vasculitis is the bedrock of KWD, it is reasonable to infer that human brain, an organ rich in vessels, is subject to cryptogenic changes in structures, which contribute to epilepsy later in life (48–50). Although none has been proven, both are possible. Future studies are encouraged to investigate these assumptions.
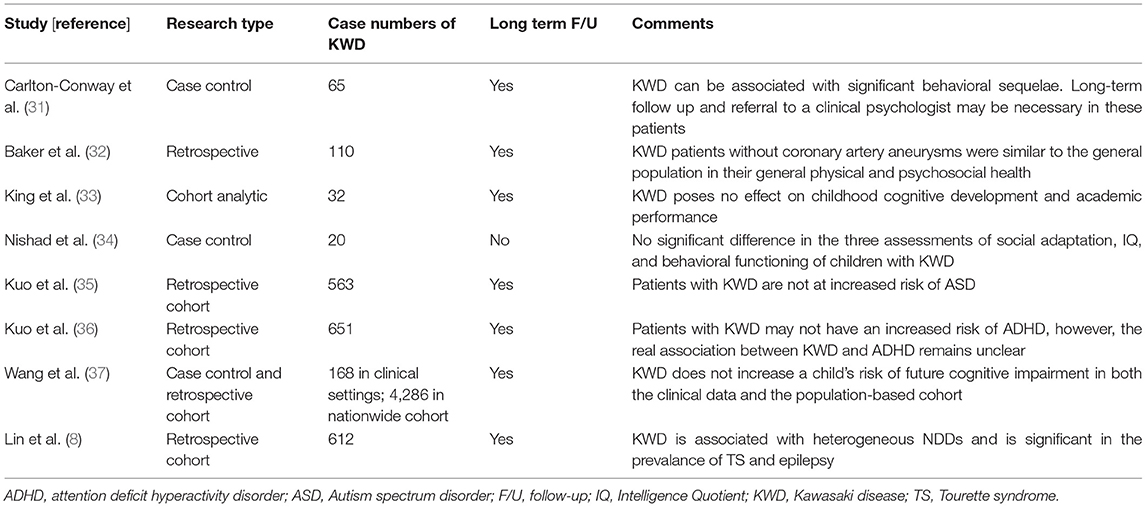
Table 5. Literature search and article analysis for neurodevelopmental disorders in children with KWD.
This study has some limitations. First, we were unable to preclude some confounding factors, including pharmacological treatment of children with KWD, KWD severities, and other genetic and environmental risk factors associated with KWD. Second, the diagnoses of epilepsy and KWD in this study were derived based on the ICD-9-CM code. Although it is a powerful tool for disease categorization, it lacks scientific validation. Third, although a retrospective cohort study is an effective approach to enrolling numerous individuals and discovering hidden medical phenomenon over a long time period and from a broad perspective, the approach cannot explore underlying mechanisms or pathogeneses of diseases. Hence, this study might serve as a pilot study attracting public attention and further research. The recommended methods are (1) randomized control trials to provide more rigorous conclusions with appropriate control of confounders such as the pharmacological treatment of KWD and KWD severity; and (2) animal model–based studies to clarify the pathogenesis of epilepsy following KWD.
Conclusions
The results of this study revealed that KWD in childhood might be a potential risk factor for childhood epilepsy. Therefore, the brain development of children diagnosed as having KWD should be carefully followed up using neuroimaging to monitor for the development of epilepsy, especially within the 5 years after KWD diagnosis. A massive randomized control trial and a detailed, structured animal cell model containing different cofounders are necessary in future.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The analysis was generated by the national health insurance database, which was safeguarded and was only accessed by a strict application process. Requests to access these datasets should be directed to Mei-Chen Lin, Y29vbGluZG1AZ21haWwuY29t.
Author Contributions
S-YH collected the data, analyzed the data, and prepared the initial draft of the manuscript. I-CL and C-HL took part in designing the study and wrote the final draft of the manuscript. The statistics for the study were compiled by J-NL, W-DL, M-CL, and I-CC, who also took part in the editing process and the revision of the tables. All of the authors read and approved of the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to express our appreciation to the Taiwan Ministry of Health and Welfare Clinical Trial Center [MOHW109-TDU-B-212-114004], MOST Clinical Trial Consortium for Stroke [MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002)], Tseng-Lien Lin Foundation, Taichung, Taiwan, and the China Medical University Hospital Medical Research Department [#DMR-108-199, #DMR-109-042, and #DMR-108-043] for giving assistance to this work.
References
1. Sundel RP. Kawasaki disease. Rheum Dis Clin North Am. (2015) 41:63–73. doi: 10.1016/j.rdc.2014.09.010
2. Jindal AK, Pilania RK, Prithvi A, Guleria S, Singh S. Kawasaki disease: characteristics, diagnosis, and unusual presentations. Expert Rev Clin Immunol. (2019) 15:1089–104. doi: 10.1080/1744666X.2019.1659726
3. Falcini F. Kawasaki disease. Curr Opin Rheumatol. (2006) 18:33–8. doi: 10.1097/01.bor.0000197998.50450.f6
4. Liu X, Zhou K, Hua Y, Wu M, Liu L, Shao S, et al. Neurological involvement in Kawasaki disease: a retrospective study. Pediatr Rheumatol Online J. (2020) 18:61. doi: 10.1186/s12969–020-00452–7
5. Martínez-Guzmán E, Gámez-González LB, Rivas-Larrauri F, Sorcia-Ramírez G, Yamazaki-Nakashimada M. Manifestaciones neurológicas en la enfermedad de Kawasaki atípica [Neurological manifestations in atypical Kawasaki disease]. Rev Alerg Mex. (2017) 64:376–80. doi: 10.29262/ram.v64i3.231
6. Zhang B, Hao Y, Zhang Y, Yang N, Li H, Liang J. Kawasaki disease manifesting as bilateral facial nerve palsy and meningitis: a case report and literature review. J Int Med Res. (2019) 47:4014–8. doi: 10.1177/0300060519854287
7. Hameed A, Alshara H, Schleussinger T. Ptosis as a complication of Kawasaki disease. BMJ Case Rep. (2017) 2017:bcr2017219687. doi: 10.1136/bcr-2017–219687
8. Lin CH, Lin WD, Chou IC, Lee IC, Hong SY. Heterogeneous neurodevelopmental disorders in children with Kawasaki disease: what is new today? BMC Pediatr. (2019) 19:406. doi: 10.1186/s12887–019-1786-y
9. Nakane A, Yajima K, Osawa M, Fukuyama Y, Kumagai K. A case of mucocutaneous lymph node syndrome complicated with acute occlusion of right middle cerebral artery and hemiconvulsions-hemiplegia-epilepsy syndrome (in Japanese). J Tokyo Wom Med Coli. (1977) 47:124–30.
10. Gale CR, O'Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. (2004) 127(Pt 2):321–9. doi: 10.1093/brain/awh034
11. Moharir M, Shroff M, Benseler SM. Childhood central nervous system vasculitis. Neuroimaging Clin N Am. (2013) 23:293–308. doi: 10.1016/j.nic.2012.12.008
12. Elbers J, Halliday W, Hawkins C, Hutchinson C, Benseler SM. Brain biopsy in children with primarain biopsy in children with primary small-vessel central nervous system vasculitis. Ann Neurol. (2010) 68:602–10. doi: 10.1002/ana.22075
13. Twilt M, Benseler SM. Central nervous system vasculitis in adults and children. Handb Clin Neurol. (2016) 133:283–300. doi: 10.1016/B978–0-444–63432-0.00016–5
14. D'Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. (2004) 17:731–5. doi: 10.1097/00019052–200412000-00014
15. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. (2018) 15:144. doi: 10.1186/s12974–018-1192–7
16. Webster KM, Sun M, Crack P, O'Brien TJ, Shultz SR, Semple BD. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation. (2017) 14:10. doi: 10.1186/s12974–016-0786–1
17. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
18. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. (2018) 9:2279. doi: 10.3389/fimmu.2018.02279
19. Desai MK, Brinton RD. Autoimmune disease in women: endocrine transition and risk across the lifespan. Front Endocrinol. (2019) 10:265. doi: 10.3389/fendo.2019.00265
20. Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. (2012) 11:A502–14. doi: 10.1016/j.autrev.2011.12.003
21. Pan Q, Chen X, Liao S, Chen X, Zhao C, Xu YZ, et al. Updated advances of linking psychosocial factors and sex hormones with systemic lupus erythematosus susceptibility and development. PeerJ. (2019) 7:e7179. doi: 10.7717/peerj.7179
22. Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc. (2018) 3:91–107. doi: 10.1210/js.2018–00186
23. Vodo S, Bechi N, Petroni A, Muscoli C, Aloisi AM. Testosterone-induced effects on lipids and inflammation. Mediators Inflamm. (2013) 2013:183041. doi: 10.1155/2013/183041
24. Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. (2019) 22:129–40. doi: 10.1080/13685538.2018.1482487
25. Son BK, Kojima T, Ogawa S, Akishita M. Testosterone inhibits aneurysm formation and vascular inflammation in male mice. J Endocrinol. (2019) 241:307–17. doi: 10.1530/JOE-18–0646
26. Kurth F, Luders E, Sicotte NL, Gaser C, Giesser BS, Swerdloff RS, et al. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. (2014) 4:454–60. doi: 10.1016/j.nicl.2014.03.001
27. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. (2018) 13:612–32. doi: 10.1177/1747493018778713
28. Grissom NM, Reyes TM. Let's call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology. (2019) 44:86–96. doi: 10.1038/s41386–018-0179–5
29. Singh R, Turner RC, Nguyen L, Motwani K, Swatek M, Lucke-Wold BP. Pediatric traumatic brain injury and autism: elucidating shared mechanisms. Behav Neurol. (2016) 2016:8781725. doi: 10.1155/2016/8781725
30. Erickson MA, Liang WS, Fernandez EG, Bullock KM, Thysell JA, Banks WA. Genetics and sex influence peripheral and central innate immune responses and blood-brain barrier integrity. PLoS ONE. (2018) 13:e0205769. doi: 10.1371/journal.pone.0205769
31. Carlton-Conway D, Ahluwalia R, Henry L, Michie C, Wood L, Tulloh R. Behaviour sequelae following acute Kawasaki disease. BMC Pediatr. (2005) 5:14. doi: 10.1186/1471–2431-5–14
32. Baker AL, Gauvreau K, Newburger JW, Sundel RP, Fulton DR, Jenkins KJ. Physical and psychosocial health in children who have had Kawasaki disease. Pediatrics. (2003) 111:579–83. doi: 10.1542/peds.111.3.579
33. King WJ, Schlieper A, Birdi N, Cappelli M, Korneluk Y, Rowe PC. The effect of Kawasaki disease on cognition and behavior. Arch Pediatr Adolesc Med. (2000) 154:463–8. doi: 10.1001/archpedi.154.5.463
34. Nishad P, Singh S, Sidhu M, Malhi P. Cognitive and behaviour assessment following Kawasaki disease–a study from North India. Rheumatol Int. (2010) 30:851–4. doi: 10.1007/s00296–009-1078–1
35. Kuo HC, Wu CM, Chang WP, Kuo CN, Yeter D, Lin CY, et al. Association between Kawasaki disease and autism: a population-based study in Taiwan. Int J Environ Res Public Health. (2014) 11:3705–16. doi: 10.3390/ijerph110403705
36. Kuo HC, Chang WC, Wang LJ, Li SC, Chang WP. Association of Attention deficit hyperactivity disorder and Kawasaki disease: a nationwide population-based cohort study. Epidemiol Psychiatr Sci. (2016) 25:573–80. doi: 10.1017/S2045796015000840
37. Wang LJ, Kuo HC. Cognitive development after kawasaki disease- clinical study and validation using a nationwide population-based cohort. Circ J. (2018) 82:517–23. doi: 10.1253/circj.CJ-17–0557
38. Mader S, Brimberg L, Diamond B. The role of brain-reactive autoantibodies in brain pathology and cognitive impairment. Front Immunol. (2017) 8:1101. doi: 10.3389/fimmu.2017.01101
39. Rubin DB, Batra A, Vaitkevicius H, Vodopivec I. Autoimmune neurologic disorders. Am J Med. (2018) 131:226–36. doi: 10.1016/j.amjmed.2017.10.033
40. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
41. Hermetter C, Fazekas F, Hochmeister S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol. (2018) 9:706. doi: 10.3389/fneur.2018.00706
42. Basha A, Rawat A, Jindal AK, Gupta A, Anand S, Garg R, et al. Autoantibody profile in children with Kawasaki disease on long-term follow-up: a prospective study from North India. Int J Rheum Dis. (2018) 21:2036–40. doi: 10.1111/1756–185X.13372
43. Grunebaum E, Blank M, Cohen S, Afek A, Kopolovic J, Meroni PL, et al. The role of anti-endothelial cell antibodies in Kawasaki disease - in vitro and in vivo studies. Clin Exp Immunol. (2002) 130:233–40. doi: 10.1046/j.1365–2249.2002.02000.x
44. Stagi S, Simonini G, Ricci L, de Martino M, Falcini F. Coeliac disease in patients with Kawasaki disease. Is there a link? Rheumatology. (2006) 45:847–50. doi: 10.1093/rheumatology/kel007
45. Sakurai Y. Autoimmune aspects of Kawasaki disease. J Investig Allergol Clin Immunol. (2019) 29:251–61. doi: 10.18176/jiaci.0300
46. Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. (2015) 125:2194–202. doi: 10.1172/JCI78084
47. Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. (2017) 8:603. doi: 10.3389/fimmu.2017.00603
48. Berlit P. Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord. (2010) 3:29–42. doi: 10.1177/1756285609347123
49. Garcia-Bermejo P, Patro SN, Ahmed AZ, Al Rumaihi G, Akhtar N, Kamran S, et al. Baseline occlusion angiographic appearance on mechanical thrombectomy suggests underlying etiology and outcome. Front Neurol. (2019) 10:499. doi: 10.3389/fneur.2019.00499
Keywords: Kawasaki disease, children, epilepsy, seizure, risk
Citation: Lin C-H, Lai J-N, Lee I-C, Chou I-C, Lin W-D, Lin M-C and Hong S-Y (2021) Association Between Kawasaki Disease and Childhood Epilepsy: A Nationwide Cohort Study in Taiwan. Front. Neurol. 12:627712. doi: 10.3389/fneur.2021.627712
Received: 17 November 2020; Accepted: 10 March 2021;
Published: 06 April 2021.
Edited by:
Kette D. Valente, Universidade de São Paulo, BrazilReviewed by:
Felippe Borlot, Alberta Children's Hospital, CanadaKuo-Liang Chiang, Kuang Tien General Hospital, Taiwan
Copyright © 2021 Lin, Lai, Lee, Chou, Lin, Lin and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syuan-Yu Hong, ZGF6aW5nZG9nQGhvdG1haWwuY29t
 Chien-Heng Lin
Chien-Heng Lin Jung-Nien Lai
Jung-Nien Lai Inn-Chi Lee5
Inn-Chi Lee5 I-Ching Chou
I-Ching Chou Mei-Chen Lin
Mei-Chen Lin Syuan-Yu Hong
Syuan-Yu Hong