- 1Evidence-Based Medicine Centre, School of Basic Medical Sciences of Lanzhou University, Lanzhou, China
- 2Evidence-Based Nursing Center, School of Nursing, Lanzhou University, Lanzhou, China
- 3The First Clinical Medical College, Lanzhou University, Lanzhou, China
- 4Zhongshan Ophthalmic Center, Sun Yat-Sen University, Guangzhou, China
- 5Department of Critical Care Medicine, Gansu Provincial Hospital, Lanzhou, China
- 6The Second Clinical Medical College, Lanzhou University, Lanzhou, China
Background: The population-based studies conducted thus far do not provide conclusive evidence of the link between diabetic retinopathy (DR) and stroke. The aim of the present systematic review was to determine whether DR is specifically associated with stroke.
Methods: MEDLINE, Embase, and Web of Science were systematically searched from their inception to July 31, 2020. All cohort studies that reported associations between the presence of DR and incident stroke were included. The pooled hazard ratios (HRs), pooled risk ratios (RRs), and 95% confidence intervals (CIs) were calculated.
Results: The meta-analysis included 19 cohort studies involving 81,452 diabetic patients. The pooled effect size of any DR related to stroke was 1.25 for HR (95% CI: 1.12–1.39; P < 0.0001) and 1.96 for RR (95% CI: 1.60–2.39; P < 0.0001). Subgroup analysis for the type of diabetes yielded pooled HR of 1.29 (95% CI: 1.10–1.50; P = 0.001) in patients with type 2 diabetes mellitus (T2DM). The pooled RR was 2.29 (95% CI: 1.77–2.96; P < 0.0001) in patients with T2DM. Two studies addressed the DR-related stroke among type 1 diabetes mellitus (T1DM) patients. One study found a significant association between DR and stroke (OR: 1.6; 95% CI: 1.1–2.3; P < 0.01), while the other did not identify an association between these two conditions (RR: 1.40; 95% CI: 0.62–2.18; P = 0.178).
Conclusions: The presence of DR is associated with an increased risk of stroke in diabetic patients. This correlation is robust in T2DM patients but uncertain in T1DM patients. Our findings indicate that DR is an important biomarker for the prediction of stroke. To further validate the role of DR in stroke-risk stratification, additional research is required on the association between the stage of DR and stroke risk, and more studies including T1DM patients are necessary.
Introduction
Diabetic retinopathy (DR) is a common and specific microvascular complication of diabetes. Although largely preventable, DR affects a third of diabetic patients (1) and is the leading cause of vision loss in working-age individuals (2). The evidence is growing that DR reflects systemic microcirculatory disease affecting not only the eye but also other vital organs (3). The presence of DR signifies an increased heightened risk of life-threatening systemic vascular complications (3). Retinopathy was proposed to represent a novel biomarker of the risk for vascular disease patients with diabetes due to its specificity and the possibility of a non-invasive assessment (4). Evidence from meta-analyses documented that both early and advanced stages of DR are linked to macrovascular complications and all-cause mortality of diabetic patients (5, 6). However, no meta-analysis has been performed to specifically determine the relationship between DR and the individual clinical manifestations of cardiovascular disease (CVD), and the existing population-based studies did not provide conclusive evidence of the link between DR and stroke (7).
Worldwide, stroke constitutes a major health and societal burden for patients and their families (8). Given limited therapeutic options, effective preventive strategies (9) and methods for early diagnosis (10) are needed. The clinical symptoms of stroke manifest late in the disease course, but the underlying subclinical pathological processes take place much earlier (10). The retina and the brain share similar embryological origin, anatomical features, and physiological properties. Therefore, the retina offers a unique and easily accessible “window” to study the correlates and consequences of subclinical pathological changes underlying stroke (11). Vascular lesions seen in the eyes of patients affected by DR, such as microaneurysms, exudates, hemorrhages, and cotton wool spots, may mirror similar pathological processes occurring in the cerebral microcirculation (3). Therefore, understanding the relationship between DR and stroke has important implications for DR screening, the management of risk factors for stroke in diabetic patients, and the prediction of stroke risk.
The aim of the present study was to determine whether DR is specifically associated with any stroke and with specific subtypes of stroke by conducting a comprehensive systematic review and meta-analysis. Additionally, the association between DR severity or DR lesions and stroke was also evaluated.
Methods
The study design and reporting of data are compliant with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (12) (Supplementary Table 1). The protocol for this systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO); the registration number is CRD42020202571.
Eligibility Criteria
The eligibility criteria related to study characteristics were as follows: (1) study design: population-based cohort studies or randomized controlled trials (RCTs) reporting an association between the presence of DR and incident stroke event; (2) participants: patients with type 1 or type 2 diabetes, regardless of age, race, and region; (3) exposure: DR was diagnosed by a reliable technology (e.g., retinal photography, fluorescein angiography) and the degree and/or severity was defined according to well-validated scales [e.g., Early Treatment Diabetic Retinopathy Study (ETDRS) adaptation of the modified Airlie House Classification of DR (13), International Clinical Diabetic Retinopathy Severity Scale (14)]; and (4) outcome: fatal or non-fatal stroke event. The stroke was defined as either a clinically diagnosed stroke or transient ischemic attack (with or without cerebral imaging).
The eligibility criteria related to the report characteristics were as follows: (1) publication language: English and (2) publication status: abstracts of studies were excluded.
Data Sources and Searches
Two reviewers (K-YH and M-YJ) independently searched the MEDLINE (via PubMed), Embase, and Web of Science databases from their inception to July 31, 2020. The following terms were used: “stroke,” “cerebrovascular accident,” “apoplexy,” “hemiplegia,” “paresis,” “transient ischemic attack,” “cerebral,” “cerebellar,” “brain,” “vertebrobasilar,” “subarachnoid,” “infarct,” “ischemia,” “thrombosis,” “emboli,” “hemorrhage,” “hematoma,” “bleed,” “diabetic retinopathy,” “NPDR,” and “PDR.” The search strategy is detailed in Supplementary Table 2. To identify potential additional studies, we also searched Google Scholar, relevant reviews, and the references cited in included studies.
Study Selection
All search results were exported to the EndNote X8 software for the removal of duplicates. Two reviewers (K-YH and M-YJ) independently screened titles and abstracts based on the eligibility criteria. Prior to the formal selection of studies, a random sample of 10% of records was independently evaluated by the two reviewers, and the final selection process did not commence until a satisfactory agreement (>90%) was achieved between them. Studies were subcategorized into three groups (included, excluded, and unsure) in this step. Then, two reviewers independently examined the full text of potentially eligible and unclear studies to reach the final decision of inclusion or exclusion. Any disagreements between the two reviewers were resolved by discussion or through consultation with a third author (BM).
Data Extraction
Two reviewers (K-YH and QZ) independently extracted relevant data using a standardized, predefined data collection form prepared using Microsoft Excel 2016. Extracted data were reviewed and cross-checked by the two reviewers prior to cleaning and analysis. Any disagreements were solved by discussion or through consultation with a third author (W-TZ). Before the final extraction, a pretest using a random sample of five included studies was carried out to revise the form, and its final version was consulted with clinicians.
The extracted information included the following: (1) general characteristics of the study: first author, country, year of publication, study design, number of study centers, the origin of the cohort, setting, sample size, and follow-up period; (2) general characteristics of the subjects: age, gender, diagnostic criteria of diabetes, diabetes type, duration of diabetes, diabetes treatment, hypertension, systolic blood pressure, diastolic blood pressure, total cholesterol level, triglycerides, high-density lipoprotein cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), previous stroke, atrial fibrillation, and current smoking; (3) details of the exposure: the prevalence of DR and the method of DR identification; (4) study outcomes: method of stroke event identification, stroke type, and stroke event incidence; (5) effect size: hazard ratio (HR), risk ratio (RR), odds ratio (OR), and 95% confidence intervals (CI), as well as their adjustment factors. When several adjusted values of the effect size were available in a study, the most adjusted estimate was extracted. For duplicated study populations, the one with the longest follow-up or with the largest sample size was selected. Corresponding authors were contacted when it was not possible to extract the necessary data from a published paper.
Assessment of the Risk of Bias
Two reviewers (K-YH and QZ) independently evaluated the risk of bias in the study using the Newcastle Ottawa Scale (NOS) (15), which consists of three parameters of quality: selection, comparability, and exposure assessment. Studies scoring >7 were considered to have a low risk of bias, scores of 5–7 indicated a moderate risk of bias, and scores of <5 indicated a high risk of bias. Any disagreement between two reviewers in the quality assessment was resolved by discussion or consultation with a third author (BM).
Statistical Analysis
The pooled risk estimate of HRs, RRs, and their 95% CIs extracted from the included studies or calculated from the extracted data were examined to summarize stroke event associated with DR. If stroke events were rare (incidence <5%), OR and RR were considered to be equivalent (16); otherwise, OR was converted to RR for data synthesis (16). Statistical heterogeneity was assessed by the forest plot and tested using the χ2 and I2 method. In the absence of statistical heterogeneity [P > 0.10 and I2 < 50% (17)] among the results, the meta-analysis was performed using the Mantel–Haenszel statistical method with the fixed-effect model. In the presence of statistical heterogeneity [P < 0.10 and I2 > 50% (17)] among the results, the Mantel–Haenszel statistical method with the random effect model was used for meta-analysis. We planned to conduct subgroup analyses using the following factors: type of diabetes [type 1 diabetes mellitus (T1DM) vs. type 2 diabetes mellitus (T2DM)], subtypes of stroke (ischemic vs. hemorrhagic), and subtypes of ischemic stroke (lacunar vs. cortical). When 10 or more studies were included (18) in each outcome, metaregression analyses were used to explore the source and size of heterogeneity in a study and to explain the impact of heterogeneity in the meta-analysis. If necessary, sensitivity analyses were performed by excluding studies with a high risk of bias and studies with a small sample size. Publication bias was assessed using funnel plots and Egger's regression tests when at least 10 studies were available (19). The level of statistical significance was set at P < 0.05 using two-sided tests. The Stata 12.0 software was used to perform data synthesis.
Results
Literature Search and Selection Results
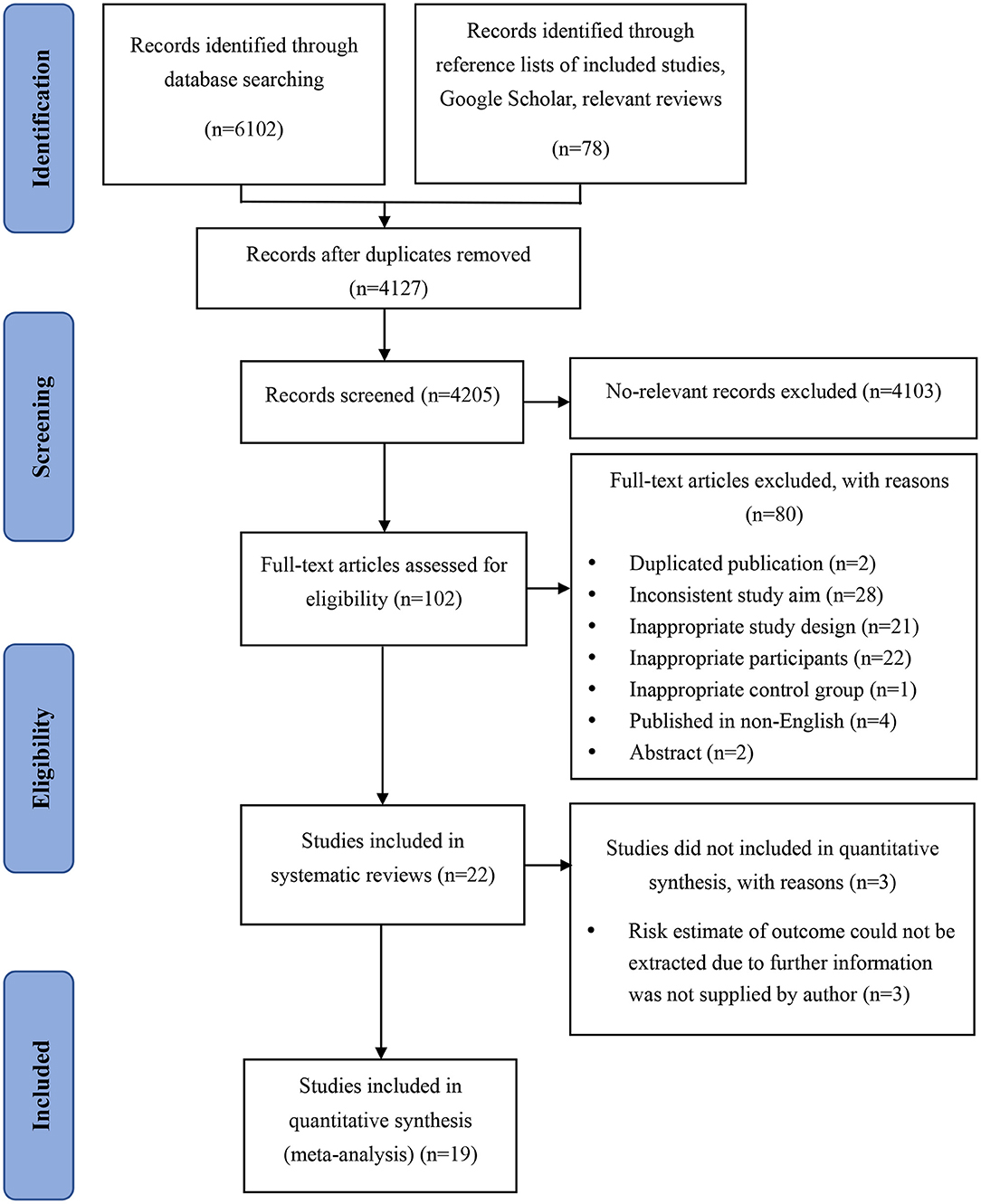
The search of electronic databases identified 4,127 studies after duplicates were removed. A supplementary search conducted on August 1, 2020, identified 78 additional potentially relevant studies. The study selection process is presented in Figure 1. After detailed assessment based on eligibility criteria, 22 studies were included in the review, of which 3 did not report risk estimate or stroke event incidence, necessitating to contact the authors to obtain these further data. Since no reply was received from the authors of two studies (20, 21) after three contact attempts, and the author of one study (22) replied that they did not have the resources at that time to rerun the models, these three publications have been excluded. Finally, 19 studies (23–41) were included in the present meta-analysis.
Characteristics of Studies and Participants
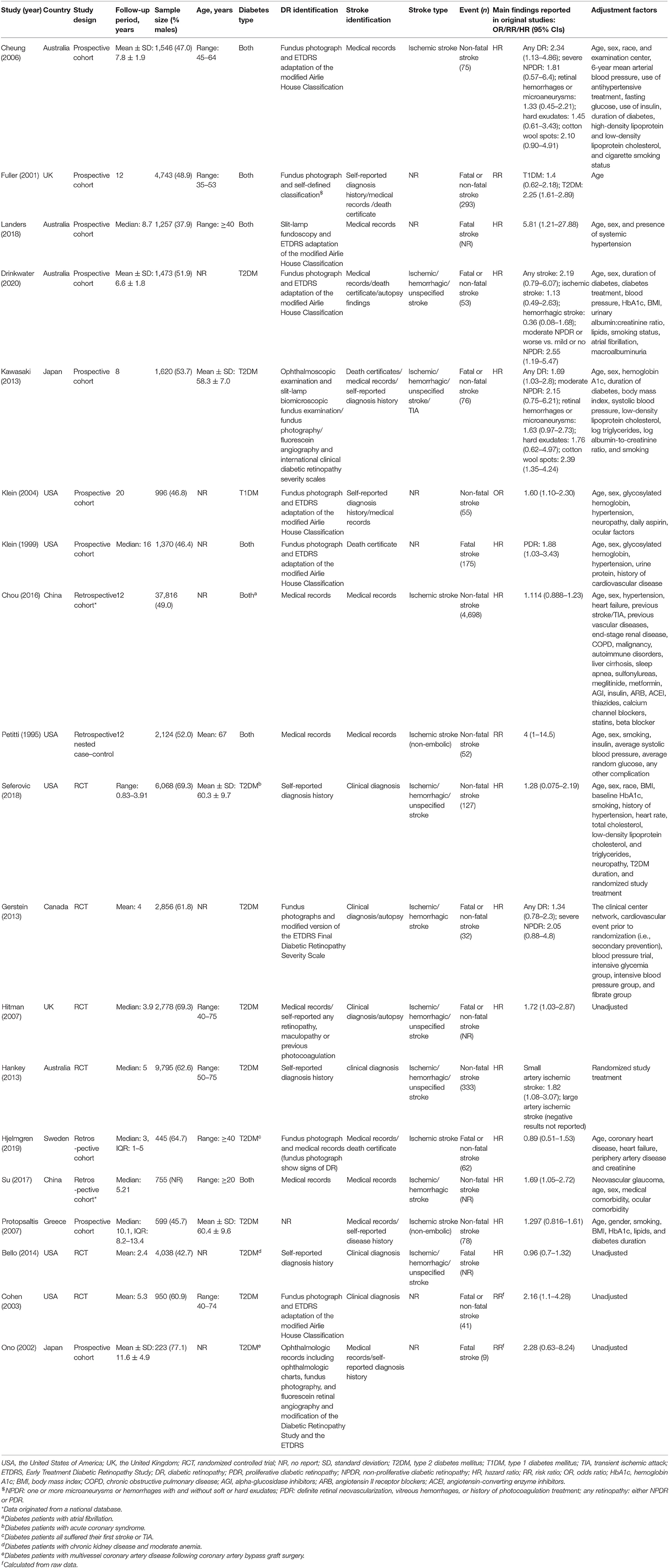
The characteristics of the 19 studies are shown in Table 1, and additional information is provided in Supplementary Table 4. In 12 studies (23–27, 29–31, 33, 38, 40, 41), the risk of bias was rated as low, and the remaining seven studies (28, 32, 34–37, 39) were rated as having a moderate risk of bias (Supplementary Table 5). Eleven publications (23–31, 36, 37) reported large population-based studies, two (38, 41) were hospital-based studies, and six originated from within a clinical trial (32–35, 39, 40). These studies were performed in the USA, UK, Australia, Japan, China, Canada, Sweden, and Greece.
The 19 studies included 81,452 diabetic patients. In the 13 studies that provided this information, the age of patients varied from 20 to 75 (34, 37). The gender of patients was extracted from 18 publications that provided this information. Among these studies, the fraction of males per studies varied from 37.9 to 77.1%. Ten studies (26, 27, 32–36, 38–41) included only T2DM patients, one study (28) included only T1DM patients, and the remaining eight studies (23–25, 29–31, 37) included patients with both types of diabetes.
The retinal assessment techniques were well-described and performed in most studies. Eight of them performed retinal photography (23, 24, 26, 28, 29, 33, 36, 40); one performed direct ophthalmoscopy (25); two performed retinal photography, direct ophthalmoscopy, or fluorescein angiography (27, 41); three relied on medical records (30, 31, 37); three utilized self-reported diagnostic history (32, 35, 39); and one used medical records, self-reported diagnosis, or treatment history (34). One study did not record the method of retinal assessment (38). Nine of the 19 studies used DR classification scales that had been well-validated either internally or externally (23, 25–29, 33, 40, 41).
The definition of stroke was similar in all studies. Thirteen of them defined “stroke” in the index paper or related publications. All these 13 studies (23, 26, 27, 30–39) assessed some measures of clinical stroke, and one also included transient ischemic attack (TIA) (27). Eight studies (26, 27, 32–35, 37, 39) included patients with any type of stroke, and two of these studies (26, 35) subtyped stroke into hemorrhagic or ischemic, with one further subtyping the ischemic stroke into large artery ischemic stroke and small artery ischemic stroke (35). The remaining five studies (23, 30, 31, 36, 38) only included ischemic stroke, and two of them (31, 38) only included non-embolic stroke.
The methods used for stroke identification varied widely. Four studies (32, 35, 39, 40) used examination by a specialist at the time of stroke; two studies (33, 34) relied on clinical assessment by study investigators, but not necessarily at the time of the stroke; and 13 studies (23–31, 36–38, 41) used the self-reported history of diagnosis, review of medical records, or death certificates. The incidence of stroke varied from 1.1 to 13.9% in 16 studies (23, 24, 26–36, 38, 40, 41) that provided this information. Among the 19 included studies, eight reported non-fatal stroke only (23, 28, 30–32, 35, 37, 38), four reported fatal stroke only (25, 29, 39, 41), and the remaining seven reported both fatal and non-fatal stroke (24, 26, 27, 33, 34, 36, 40).
Fourteen studies (23, 25–27, 29, 30, 32–39) reported HR as effect size. One study (28) reported OR as effect size. Two studies (24, 31) reported RR as effect size, and two (40, 41) reported only the incidence of stroke in the exposure and control groups, from which we calculated the crude RR for data synthesis. In 15 of the 19 studies (23–33, 35–38), the data were adjusted appropriately.
Association Between DR and Stroke Event
Any DR and Stroke Event
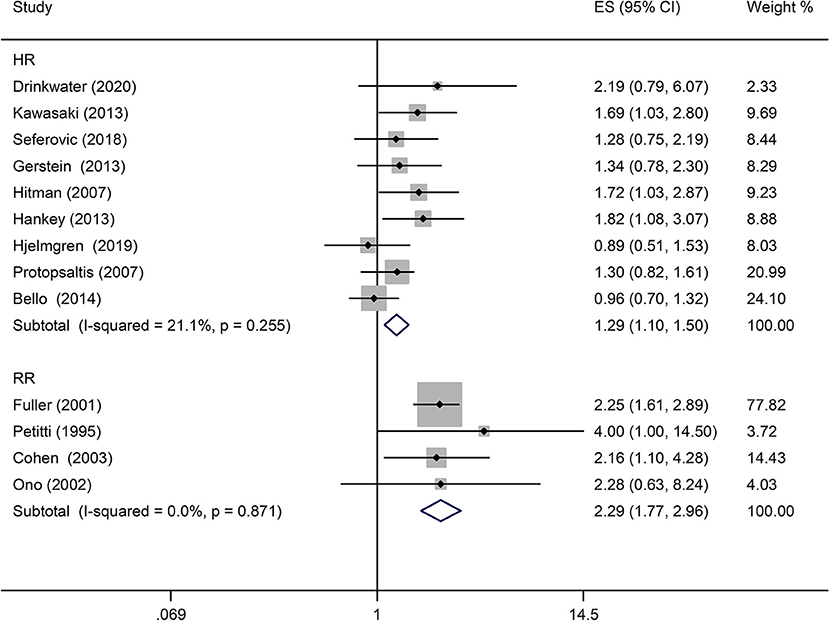
A comprehensive analysis involving HR as an effect measure included 13 studies (23, 25–27, 30, 32–39). The pooled HR of any DR was found to be significantly associated with that of stroke event (HR: 1.25; 95% CI: 1.12–1.39; P < 0.0001; Figure 2). Eleven of these studies (23, 25–27, 30, 32, 33, 35–38) reported appropriately adjusted data. The median duration of the follow-up for 71,046 patients in the 13 studies was 5.21 years (interquartile range: 3.9–8 years). Since heterogeneity was low (I2 = 40.8%, Q = 20.27), the fixed-effects model was employed. Comprehensive analysis with RR as an effect measure included five studies (24, 28, 31, 40, 41). The pooled RR of any DR was found to be significantly associated with that of stroke event (RR: 1.96; 95% CI: 1.60–2.39; P < 0.0001; Figure 2). Since heterogeneity was low (I2 = 0.0%, Q = 4.34), the fixed-effects model was employed. Three of these five studies (24, 28, 31) reported appropriately adjusted data. The median duration of follow-up for 9,036 patients in the five studies was 12.00 years (interquartile range: 11.6–12.0 years). Metaregression analysis was performed to explore the source and size of heterogeneity. Our regression analysis showed that study design, diabetes type, and stroke identification method were not the sources of heterogeneity (Supplementary Table 3). However, due to missing information, other factors that might cause heterogeneity, such as follow-up period, mean age, the fraction of males, and geographic regions, have not been evaluated. The funnel plots and the Egger's test (Supplementary Figures 1, 2) showed that a significant publication bias (P = 0.01) was present in the association of any DR with stroke event in diabetes patients. Subgroup analysis for the type of diabetes yielded pooled HR of 1.29 (95% CI: 1.10–1.50; P = 0.001; Figure 3) and pooled RR of 2.29 (95% CI: 1.77–2.96; P < 0.0001; Figure 3) among patients with T2DM. Since heterogeneity was low (HR: I2 = 21.1%, Q = 10.14; RR: I2 = 0.0%, Q = 4.34), the fixed-effects model was employed. Two studies (24, 28) addressed the DR-related stroke among T1DM patients. One study (28) found a significant association between DR and stroke (OR: 1.6; 95% CI: 1.1–2.3; P < 0.01; Table 1), while the other (24) did not find such an association (RR: 1.40; 95% CI: 0.62–2.18; P = 0.178; Table 1). Subgroup analysis for stroke type yielded pooled HR of 1.19 (95% CI: 1.04–1.36; P = 0.01; Figure 4) for ischemic stroke. Since heterogeneity was low (I2 = 36.0%, Q = 7.81), the fixed-effects model was employed. Two studies (26, 35) analyzed the hemorrhagic subtypes of stroke and found that DR was not significantly associated with hemorrhagic stroke [HR: 0.36; 95% CI: 0.08–1.65; P = 0.188; one study (35) did not provide negative results; Table 1]. Only one study (35) subtyped ischemic stroke into large artery ischemic stroke and small artery ischemic stroke and determined that DR was significantly associated with small artery ischemic stroke (HR: 1.82; 95% CI: 1.08–3.07; P = 0.03, Table 1) but not associated with large artery ischemic stroke (no negative results reported, Table 1).
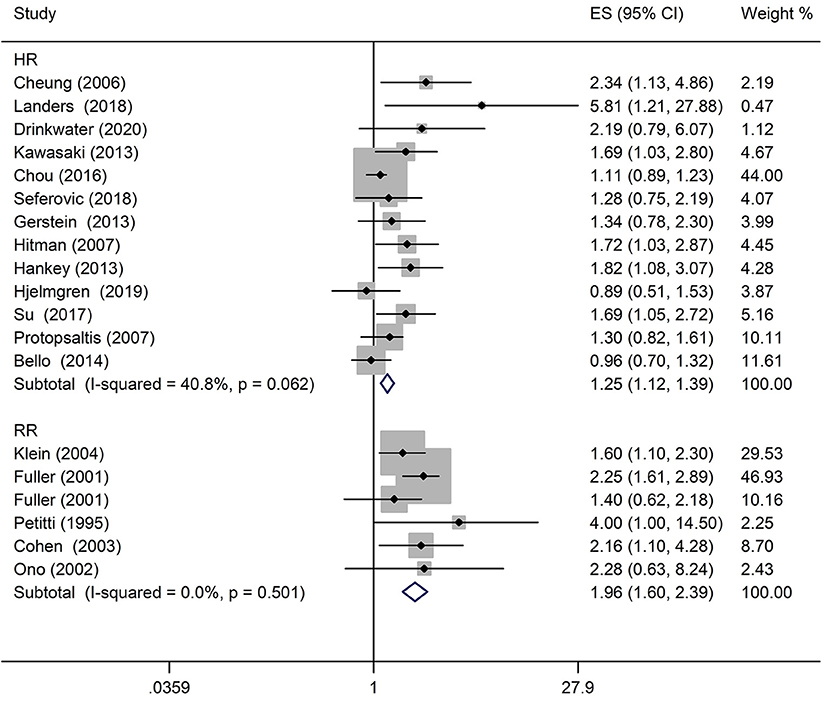
Figure 2. Pooled hazard ratio/risk ratio (HR/RR) for the association of any diabetic retinopathy (DR) with stroke event in patients with any type of diabetes.
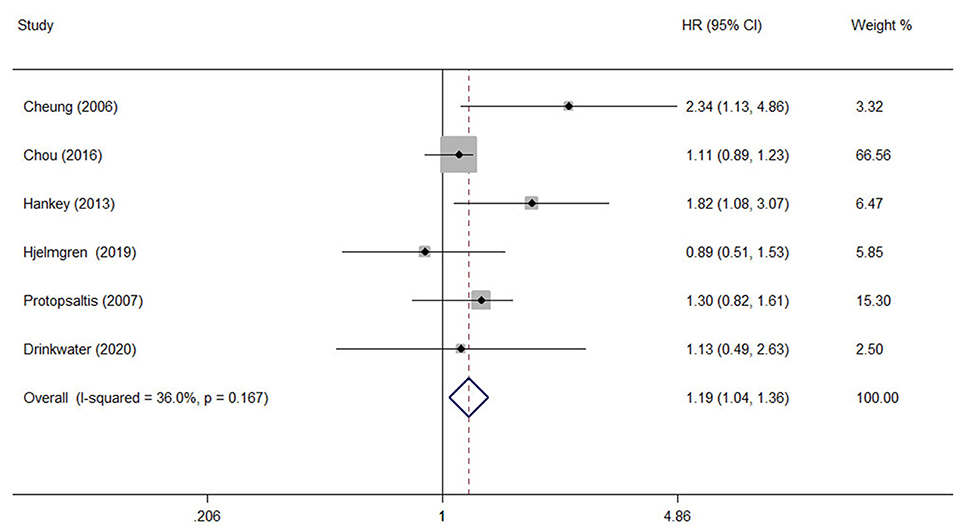
Figure 4. The pooled HR for the association of any DR with ischemic stroke type in diabetic patients.
DR Severity and Stroke Event
The analysis of the effect of DR severity on stroke event included five studies (23, 26, 27, 29, 33). The pooled analysis revealed that the HR for stroke events in moderate non-proliferative diabetic retinopathy (NPDR) or more severe DR was 2.08 (95% CI: 1.44–2.99, P < 0.0001; Figure 5) when compared with individuals with mild NPDR or no DR. All studies reported appropriately adjusted data. The median duration of follow-up for 8,865 patients in the five studies was 7.80 years (interquartile range: 6.6–8.0 years). Since heterogeneity was low (I2 = 0%, Q = 0.44), the fixed-effects model was employed.
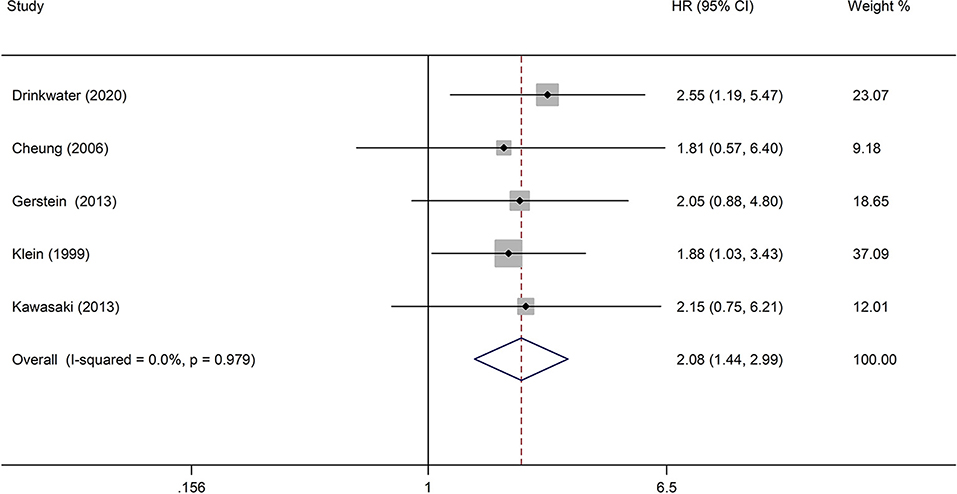
Figure 5. Pooled HR for the association of moderate NPDR or more severe DR with stroke event in diabetic patients.
DR Lesions and Stroke Event
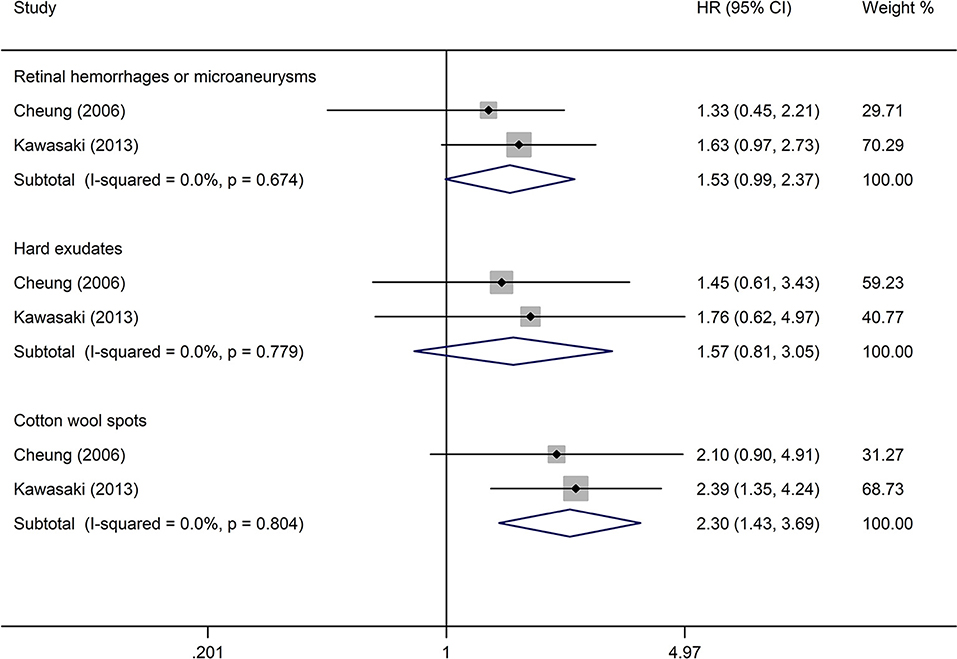
The analysis of the effect of DR lesions on stroke event included only two studies (23, 27). The pooled HR of DR lesions related to stroke event in retinal hemorrhages or microaneurysms, hard exudates, and cotton wool spots was, respectively, 1.53 (95% CI: 0.99–2.37; P = 0.053; Figure 6), 1.57 (95% CI: 0.81–3.05; P = 0.184; Figure 6), and 2.30 (95% CI: 1.43–3.69; P = 0.001; Figure 6). All studies reported appropriately adjusted data. The median duration of follow-up for 3,166 patients in the two studies was 7.9 years (interquartile range: 7.8–8.0 years). Since heterogeneity was low (retinal hemorrhages or microaneurysms: I2 = 0%, Q = 0.18; hard exudates: I2 = 0%, Q = 0.08; cotton wool spots: I2 = 0%, Q = 0.06), the fixed-effects model was employed.
Discussion
The performed meta-analysis, which included 81,452 participants across 19 cohort studies, demonstrated that the presence of any DR was associated with an increased risk for stroke event (fatal and non-fatal) in diabetic patients. This evidence was robust in T2DM patients but inconclusive in T1DM patients. When stroke subtypes were considered, robust evidence was found that any DR was significantly associated with ischemic stroke. Only one study considered the subtypes of ischemic stroke and found that any DR was associated with small artery ischemic stroke but not with large artery ischemic stroke. The association between DR and hemorrhagic stroke was not observed, but this finding was based on two studies only. Furthermore, we found a significant trend for the increased risk of stroke with increasing the stage of DR and severity of DR lesions.
The link between DR and stroke in diabetic patients can be explained by the fact that diabetes-related changes in the retinal microvasculature mirror those in the cerebral microvasculature (3, 7). Microvascular dysfunction is a widespread phenomenon in individuals with diabetes, including the effects on the brain (3, 7). The microvasculature of the retina and brain is closely linked, offering a unique opportunity to study microvascular changes in the brain because the retinal structures can be directly visualized (11), while direct evaluation of the brain microvasculature is impossible with current neuroimaging techniques. Another potential mechanism linking DR with stroke might be the common risk factors for DR and stroke, such as elevated hemoglobin A1c (HbA1c), hyperglycemia, hypertension, and dyslipidemia (42, 43). In the subgroup analysis, we observed a strong association between any DR and stroke in T2DM, but this association was uncertain in T1DM. This could be explained by shared risk factors for DR and stroke, such as poor glycemic control, high blood pressure, and dyslipidemia, which are more often present in T2DM than T1DM due to the relatively older age (44). However, the underlying mechanism of the difference between the effects of T2DM and T1DM needs to be further clarified. To better understand the pathophysiology of DR-related stroke, we conducted two subgroup analyses for subtypes of stroke and subtypes of ischemic stroke. The result suggested that DR was significantly associated with ischemic stroke. One study showed that DR was a predictor of small artery ischemic stroke but not large artery ischemic stroke. Moreover, two studies found DR was not associated with hemorrhagic stroke. These findings are consistent with the role of cerebral microvascular dysfunction in diabetes (7).
The detailed analysis of the association between DR and stroke documented that the risk of stroke was 2.08 times higher in patients with moderate NPDR or more severe DR than in subjects without DR, which was significantly higher than the risk of stroke in patients with any DR (HR 1.25, 95% CI: 1.12–1.39). This finding supports indirectly the possibility of an association between the severity of DR and higher risk of stroke. Another identified dose–response effect was represented by the association of a higher risk of stroke with increased severity of DR lesions. The meta-analysis based on two studies suggested that the presence of cotton wool spots was significantly associated with stroke, but the presence of retinal hemorrhages or microaneurysms, as well as the presence of hard exudates, was not significantly associated with stroke. This finding further confirmed that the increased level of DR is related to a higher risk of stroke to a certain degree because the classification of DR was based on the severity of DR lesions. Furthermore, the strong association between the presence of cotton wool spots and stroke further confirms the possibility that similar pathologic changes are present in the cerebral and retinal microcirculation in DR patients. Pathologically, cotton wool spots in the retina constitute a focal retinal capillary obstruction (45), and the ischemic change in the retina observed as cotton wool spots may reflect similar pathologic changes in the cerebral microcirculation, which can trigger an ischemic stroke.
The performed meta-analysis has shown that DR was significantly associated with stroke in diabetic patients. DR is an important biomarker predicting stroke, and retinal imaging techniques offer an excellent way to study the effects of microvascular dysfunction in diabetes on small cerebral vessels. The obtained data point to the need for a better stroke monitoring and follow-up in patients with DR. Additionally, they highlight the strength of using DR as a biomarker to predict stroke in a clinical setting. First, DR is characterized by long-term stability (27), and complete natural resolution after the initial diagnosis is unlikely. Second, as the marker of concomitant cerebral microangiopathy (3, 7), DR can be directly assessed by non-invasive visualization using retinal imaging techniques. The evaluation of DR can identify a specific subgroup of patients who could benefit from a more intensive and expensive brain imaging (11). Finally, given that assessment of DR is performed by ophthalmologists, sharing this information and using it proactively in stroke risk management will benefit both clinicians and patients by allowing better prediction of stroke with minimum additional effort and cost.
This is the first systematic review assessing the relationship between DR and stroke in a large number of diabetic patients. Our results provide the best current evidence for the association between DR and stroke and contribute to the debate on whether or not DR predicts the incidence of stroke. We found that the studies with contradicting results have the following common characteristics: the use of retrospective study design (30, 36) or the RCT post-analysis data without appropriate adjustments (33, 39), a small sample size (38, 41), an inaccurate method for DR identification (32), and heterogeneity of the included population (e.g., duration or type of diabetes, comorbidity), which may introduce bias to the studies' results and affect the internal validity of researches. This systematic review has certain limitations. First, most of the included studies identified stroke based on self-reported diagnosis history, review of medical records, or death certificates, rather than relying on an assessment by an expert done at the time of the stroke and supported by the presence of clinical features and appropriately timed brain imaging. Although being deemed acceptable for assessing the prevalence of stroke in epidemiological studies, this simplified approach reduces the accuracy of diagnosis (46, 47) and may be inadequate for detailed studies of pathophysiology. Second, studies differed in their inclusion/exclusion criteria, follow-up period, DR identification method, geographic regions, and adjusted factors. All these differences may account for the observed heterogeneity, which may reduce the reliability of our analysis. Third, we found publication bias based on funnel plot inspection and Egger's regression test results. Although we performed a comprehensive systematic search, studies published in non-English languages were excluded due to restricted resources. These limitations should be considered when interpreting the results.
Additionally, this systematic review highlighted several gaps in the knowledge to be filled by future research. First, our study did not provide robust evidence of the association between DR severity and stroke since only a small number of studies carefully characterized DR level and lesions. Therefore, further studies are required to analyze in more detail the association between these two complications and stroke by the use of reliable methods for retinal assessment and the determination of DR classification. These developments would contribute significantly to the validation of the effect of DR on stroke-risk stratification. Second, only two studies addressed the DR-related stroke among T1DM patients, and their conclusions were inconsistent. Typically, T1DM patients have a higher level of HbA1c because of the lack of insulin. A previous study showed that high HbA1c values accounted for up to 11% of the risk of developing DR (48), and a higher prevalence of DR has been estimated in T1DM patients than in T2DM patients (49). We believe that future investigations should pay more attention to the DR-related stroke risk in T1DM patients. Third, although we have addressed the pathophysiology of DR-related stroke, it should be noted that the analysis conducted here was based on only one study, and this is because most stroke remains a clinical diagnosis. Therefore, data obtained in longitudinal studies that subdivide ischemic stroke into lacunar and cortical will be important to clarify this issue.
Conclusion
The presence of DR is associated with an increased risk of stroke in diabetic patients. The evidence of this association was robust in T2DM patients but uncertain in T1DM patients. Furthermore, with the increasing severity of DR lesions, a significant increasing trend in the risk of stroke is present. Our findings indicate that DR is an important biomarker for the prediction of stroke in clinical practice. To further validate the role of DR in stroke-risk stratification, additional research is required to explore the detailed association between DR stage and stroke risk, and more studies including T1DM patients are necessary.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
BM, KH, and CM conceived and designed the research. WZ, QZ, and KH designed the data collection. MJ, QZ, KH, AW, and GT performed the data collection. QZ, KH, and QG performed the statistical analysis. KH, FM, LZ, FC, WZ, and BM interpreted the data. KH wrote the first draft of the manuscript. All authors revised the manuscript for important intellectual content and read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of China (Number: 81873184). The funders had no role in the study design, interpretation of the data, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the two peer reviewers for their extensive and thoughtful feedback. We thank all members of our study team for their whole-hearted cooperation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.626996/full#supplementary-material
References
1. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. (2010) 376:124–36. doi: 10.1016/S0140-6736(09)62124-3
2. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. (2007) 298:902–16. doi: 10.1001/jama.298.8.902
3. Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. (2008) 27:161–76. doi: 10.1016/j.preteyeres.2007.12.001
4. Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. (2020) 8:337–47. doi: 10.1016/S2213-8587(19)30411-5
5. Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care. (2011) 34:1238–44. doi: 10.2337/dc11-0079
6. Xie J, Ikram MK, Cotch MF, Klein B, Varma R, Shaw JE, et al. Association of diabetic macular edema and proliferative diabetic retinopathy with cardiovascular disease: a systematic review and meta-analysis. JAMA Ophthalmol. (2017) 135:586–93. doi: 10.1001/jamaophthalmol.2017.0988
7. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer C. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. (2020) 8:325–36. doi: 10.1016/S2213-8587(19)30405-X
8. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. (2016) 15:913–24. doi: 10.1016/S1474-4422(16)30073-4
9. Pandian JD, Gall SL, Kate MP, Silva GS, Akinyemi RO, Ovbiagele BI, et al. Prevention of stroke: a global perspective. Lancet. (2018) 392:1269–78. doi: 10.1016/S0140-6736(18)31269-8
10. Zerna C, Thomalla G, Campbell B, Rha JH, Hill MD. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet. (2018) 392:1247–56. doi: 10.1016/S0140-6736(18)31874-9
11. Cheung CY, Ikram MK, Chen C, & Wong TY. Imaging retina to study dementia and stroke. Prog Retinal Eye Res. (2017) 57:89–107. doi: 10.1016/j.preteyeres.2017.01.001
12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
13. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic colour fundus photographs – an extension of the Modified Airlie House Classification. ETDRS Report Number 10. Ophthalmology. (1991) 98:786–806. doi: 10.1016/S0161-6420(13)38012-9
14. Wilkinson CP, Ferris FL III, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. (2003) 110:1677–82. doi: 10.1016/S0161-6420(03)00475-5
15. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Non-randomized Studies in Meta-analyses. (2012). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
16. Cummings P. The relative merits of risk ratios and odds ratios. Arch Pediatrics Adolesc Med. (2009) 163:438–45. doi: 10.1001/archpediatrics.2009.31
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
19. Niedhammer I, Milner A, Witt K, Klingelschmidt J, Khireddine-Medouni I, Alexopoulos EC, et al. Response to letter to the editor from Dr Rahman Shiri: The challenging topic of suicide across occupational groups. Scand J Work Environ Health. (2018) 44:108–10. doi: 10.5271/sjweh.3698
20. Kim YH, Hong MK, Song JM, Han KH, Kang DH, Song JK, et al. Diabetic retinopathy as a predictor of late clinical events following percutaneous coronary intervention. J Invasive Cardiol. (2002) 14:599–602.
21. Lövestam-Adrian M, Hansson-Lundblad C, Torffvit O. Sight-threatening retinopathy is associated with lower mortality in type 2 diabetic subjects: a 10-year observation study. Diabetes Res Clin Practice. (2007) 77:141–7. doi: 10.1016/j.diabres.2006.10.026
22. Davis TM, Millns H, Stratton IM, Holman RR, Turner RC. Risk factors for stroke in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS) 29. Arch Internal Med. (1999) 159:1097–103. doi: 10.1001/archinte.159.10.1097
23. Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke?. Stroke. (2007) 38:398–401. doi: 10.1161/01.STR.0000254547.91276.50
24. Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO mutinational study of vascular disease in diabetes. Diabetologia. (2001) 44(Suppl. 2):S54–S64. doi: 10.1007/PL00002940
25. Landers J, Liu E, Estevez J, Henderson T, Craig JE. Presence of diabetic retinopathy is associated with worse 10-year mortality among Indigenous Australians in Central Australia: The Central Australian ocular health study. Clin Exp Ophthalmol. (2019) 47:226–32. doi: 10.1111/ceo.13375
26. Drinkwater JJ, Davis T, Hellbusch V, Turner AW, Bruce DG, Davis WA. Retinopathy predicts stroke but not myocardial infarction in type 2 diabetes: the Fremantle Diabetes Study Phase II. Cardiovasc Diabetol. (2020) 19:43. doi: 10.1186/s12933-020-01018-3
27. Kawasaki R, Tanaka S, Tanaka S, Abe S, Sone H, Yokote K, et al. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology. (2013) 120:574–82. doi: 10.1016/j.ophtha.2012.08.029
28. Klein BE, Klein R, McBride PE, Cruickshanks KJ, Palta M, Knudtson MD, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Internal Med. (2004) 164:1917–24. doi: 10.1001/archinte.164.17.1917
29. Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. (1999) 117:1487–95. doi: 10.1001/archopht.117.11.1487
30. Chou AY, Liu CJ, Chao TF, Wang KL, Tuan TC, Chen TJ, et al. Presence of diabetic microvascular complications does not incrementally increase risk of ischemic stroke in diabetic patients with atrial fibrillation: a nationwide cohort study. Medicine. (2016) 95:e3992. doi: 10.1097/MD.0000000000003992
31. Petitti DB, Bhatt H. Retinopathy as a risk factor for nonembolic stroke in diabetic subjects. Stroke. (1995) 26:593–6. doi: 10.1161/01.STR.26.4.593
32. Seferovic JP, Bentley-Lewis R, Claggett B, Diaz R, Gerstein HC, Køber LV, et al. Retinopathy, neuropathy, and subsequent cardiovascular events in patients with Type 2 diabetes and acute coronary syndrome in the ELIXA: the importance of disease duration. J Diabetes Res. (2018) 2018:1631263. doi: 10.1155/2018/1631263
33. Gerstein HC, Ambrosius WT, Danis R, Ismail-Beigi F, Cushman W, Calles J, et al. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care. (2013) 36:1266–71. doi: 10.2337/dc12-1311
34. Hitman GA, Colhoun H, Newman C, Szarek M, Betteridge DJ, Durrington PN, et al. Stroke prediction and stroke prevention with atorvastatin in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetic Med. (2007) 24:1313–21. doi: 10.1111/j.1464-5491.2007.02268.x
35. Hankey GJ, Anderson NE, Ting RD, Veillard AS, Romo M, Wosik M, et al. Rates and predictors of risk of stroke and its subtypes in diabetes: a prospective observational study. J Neurol Neurosurg Psychiatry. (2013) 84:281–7. doi: 10.1136/jnnp-2012-303365
36. Hjelmgren O, Strömberg U, Gellerman K, Thurin A, Zetterberg M, Bergström G. Does retinopathy predict stroke recurrence in type 2 diabetes patients: a retrospective study?. PLoS ONE. (2019) 14:e0210832. doi: 10.1371/journal.pone.0210832
37. Su CW, Chang YC, Lin CL, Chen HY. Association of neovascular glaucoma with risk of stroke: a population-based cohort study. J Ophthalmol. (2017) 2017:1851568. doi: 10.1155/2017/1851568
38. Protopsaltis I, Korantzopoulos P, Milionis HJ, Koutsovasilis A, Nikolopoulos GK, Dimou E, et al. Metabolic syndrome and its components as predictors of ischemic stroke in type 2 diabetic patients. Stroke. (2008) 39:1036–8. doi: 10.1161/STROKEAHA.107.498311
39. Bello NA, Pfeffer MA, Skali H, McGill JB, Rossert J, Olson KA, et al. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ. (2014) 2:e000011. doi: 10.1136/bmjdrc-2013-000011
40. Cohen JA, Estacio RO, Lundgren RA, Esler AL, Schrier RW. Diabetic autonomic neuropathy is associated with an increased incidence of strokes. Autonomic Neurosci. (2003) 108:73–8. doi: 10.1016/j.autneu.2003.07.001
41. Ono T, Kobayashi J, Sasako Y, Bando Ko, Tagusari O, Niwaya K, et al. The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol. (2002) 40:428–36. doi: 10.1016/S0735-1097(02)01983-6
42. Bergman M, Jagannathan R, Buysschaert M, Pareek M, Olsen MH, Nilsson PM, et al. Lessons learned from the 1-hour post-load glucose level during OGTT: Current screening recommendations for dysglycaemia should be revised. Diabetes Metab Res Rev. (2018) 34:e2992. doi: 10.1002/dmrr.2992
43. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. (2000) 321:405–412. doi: 10.1136/bmj.321.7258.405
44. Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. (2012) 11:261–71. doi: 10.1016/S1474-4422(12)70005-4
45. Sharma S, Brown GC. Retinal artery obstruction. In: Ryan SJ, editor. Retina, 4th ed. Vol. 2. St. Louis, MO: Mosby (2006). p. 1323–38. doi: 10.1016/B978-0-323-02598-0.50075-6
46. Piriyawat P, Smajsová M, Smith MA, Pallegar S, Al-Wabil A, Garcia NM, et al. Comparison of active and passive surveillance for cerebrovascular disease: the Brain Attack Surveillance in Corpus Christi (BASIC) project. Am J Epidemiol. (2002) 156:1062–9. doi: 10.1093/aje/kwf152
47. Leppälä JM, Virtamo J, Heinonen OP. Validation of stroke diagnosis in the National Hospital Discharge Register and the Register of Causes of Death in Finland. Europ J Epidemiol. (1999) 15:155–60. doi: 10.1023/A:1007504310431
48. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial–revisited. Diabetes. (2008) 57:995–1001. doi: 10.2337/db07-1618
Keywords: diabetic retinopathy, stroke, systematic review, meta-analysis, epidemiology
Citation: Hu K, Jiang M, Zhou Q, Zeng W, Lan X, Gao Q, Mei F, Zhao L, Chen F, Wu A, Tao G, Mou C and Ma B (2021) Association of Diabetic Retinopathy With Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 12:626996. doi: 10.3389/fneur.2021.626996
Received: 07 November 2020; Accepted: 08 February 2021;
Published: 16 March 2021.
Edited by:
Maurizio Acampa, Siena University Hospital, ItalyReviewed by:
Hoang T. Phan, University of Tasmania, AustraliaWeiping Jia, Shanghai Sixth People's Hospital, China
Copyright © 2021 Hu, Jiang, Zhou, Zeng, Lan, Gao, Mei, Zhao, Chen, Wu, Tao, Mou and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenghua Mou, Mjg2MzQxNzE2MiYjeDAwMDQwO3FxLmNvbQ==; Bin Ma, a2l0dHlfbWFiJiN4MDAwNDA7MTYzLmNvbQ==
†These authors share first authorship
 Kaiyan Hu1,2†
Kaiyan Hu1,2† Anhu Wu
Anhu Wu Bin Ma
Bin Ma