- Department of Neurology, Johns Hopkins Comprehensive Epilepsy Center, Johns Hopkins University, Baltimore, MD, United States
Introduction: Acute Encephalitis is associated with a high risk of acute symptomatic seizures, status epilepticus, and remote symptomatic epilepsy. Ketogenic diet therapies (KDT) have been established as a feasible and safe adjunctive management of refractory- and super-refractory status epilepticus. However, the role of KDT in the chronic management of Post-encephalitic epilepsy (PE) and autoimmune-associated epilepsy (AE) is unknown. This study aims to investigate the use of KDT in patients with PE and AE.
Methods: A retrospective single-center case series examining adult patients with PE and AE treated with the modified Atkins diet (MAD), a KDT commonly used by adults with drug-resistant epilepsy.
Results: Ten patients with PE and AE who were treated with adjunctive MAD were included. Four patients had either confirmed or presumed viral encephalitis, five patients had seronegative AE, and one patient had GAD65 AE. The median latency between starting MAD and onset of encephalitis was 6 years (IQR: 1–10). The median duration of MAD was 10 months (IQR: 3.75–36). Three patients (30%) became seizure-free, one patient (10%) achieved 90% seizure freedom, and three patients (30%) achieved a 50–75% reduction in their baseline seizure frequency, while three patients (30%) had no significant benefit. Overall, seven patients (70%) achieved ≥50% seizure reduction.
Conclusion: In addition to its established role in the treatment of RSE, KDT may be a safe and feasible option for the treatment of chronic PE and AE, particularly in those with prior history of SE. Prospective studies are warranted to explore the efficacy of KDT in management of patients with PE and AE.
Introduction
Acute encephalitis is associated with significant morbidity and mortality, with an estimated annual global incidence of 0.07–12.6 cases per 100,000 (1). It is associated with a high risk of acute symptomatic seizures, status epilepticus, and remote symptomatic epilepsy (2). The risk of post-encephalitic epilepsy (PE) ranges from 10 to 40% across several studies (2–5). Over the past two decades, numerous neural auto-antibodies associated with encephalitis, seizures, and epilepsy have been discovered (6). This has led to a significant increase in the incidence of identified patients with autoimmune encephalitis and epilepsy (7). Autoimmune-associated epilepsy (AE) or autoimmune epilepsy is now recognized as an important etiology in a subset of patients with both acute symptomatic seizures and chronic epilepsy (6, 8–10). In a 2018 study, the incidence and prevalence of autoimmune encephalitis were comparable to those of infectious encephalitis (11). Treatment of patients with AE is comprised of multiple lines of immunotherapy (particularly in the acute phase) and anti-seizure drugs when indicated (6).
Ketogenic diets therapies (KDT) are characterized by a reduced carbohydrate intake along with a relative increase in the proportions of fat and protein to promote fat metabolism (12). Since the early 2000s, KDT variants have been investigated as a potential therapeutic option for adult patients with intractable epilepsy and status epilepticus (12, 13).
There are five types of KDT used in adults: the classic ketogenic diet (CKD), and less restrictive diets including the modified ketogenic diet (MKD), medium chain triglyceride (MCT) oil ketogenic diet, low glycemic index treatment (LGIT), and the modified Atkins diet (MAD) (14). In adult patients, MAD is typically prescribed as a net 20 g/day carbohydrate limit, which is equivalent to a ratio of 1–2:1 of fat to protein and carbohydrates combined (15).
Several reports have demonstrated the efficacy of the classic ketogenic diet in patients with super-refractory status epilepticus (SRSE) of multiple etiologies (16, 17). CKD appears to be particularly beneficial in patients with new-onset refractory status epilepticus (NORSE) and specifically Febrile Infection-Related Epilepsy Syndrome (FIRES) (18–21). While the etiology of NORSE in most patients remains unknown, the most common identifiable etiology is either autoimmune or viral encephalitis (19, 22, 23).
Although several reports have studied the use of KDT in the acute settings of encephalitis and SRSE, no prior studies have evaluated its use in patients with chronic post-encephalitic (PE) and autoimmune-associated (AE) epilepsies. This investigation aims to evaluate the use of MAD in patients with post-infectious and autoimmune-associated epilepsies. We present a case series treated at the Johns Hopkins Adult Epilepsy Diet Center along with a brief review of the literature for comparison.
Materials and Methods
Patient Inclusion
This was a retrospective case series performed at the Johns Hopkins Adult Epilepsy Diet Center (AEDC). A prospectively assembled clinical database of all patients evaluated for KDT at the Johns Hopkins Adult Epilepsy Diet Center (AEDC) was approved by the Johns Hopkins Institutional Review Board and all participants or their legally authorized representative provided written consent to be included. Out of this database, we identified adult patients with post-encephalitic (PE) and autoimmune-associated (AE) epilepsies who were evaluated at AEDC from January 2014 to 2020. Patients with childhood (<18 years old) onset of their encephalitis or epilepsy were excluded, those without quantifiable seizures prior to starting MAD, as well as patients who declined participation in the database. PE was defined as persistent seizures after an episode of encephalitis (24), while AE is defined as per the consensus criteria (25). A subset of the patients who met the inclusion and exclusion criteria were previously described in studies evaluating the use of ketogenic diet in super-refractory status epilepticus and new-onset refractory status epilepticus during their acute course while this study focuses on long-term outcomes (16, 17, 19).
Data Collection and Analysis
The electronic medical records of participants were reviewed to extract demographic information, clinical, radiological, and laboratory data. Response to MAD was assessed based on the reported seizure frequency prior to MAD compared to seizure frequency at the time that the records were reviewed.
Statistical Analysis
Descriptive analyses of the categorical and continuous data were performed using proportions, frequencies, medians, and ranges. The statistical analyses were performed using Stata16.0 (College Station, TX).
Results
Fifteen patients with adult-onset PE or AE were evaluated to initiate MAD at the AEDC. Five patients were excluded from analysis for the following reasons: one patient received education on MAD initiation and never started, one patient was lost to follow up, two patients were non-adherent with the diet and stopped it early (within 2–4 weeks), and one patient who was seizure-free on ASDs and was interested in using MAD as adjunctive therapy to potentially lower ASD burden.
Ten patients with PE and AE met the inclusion criteria. Four patients had viral or presumed viral encephalitis (three unknown pathogens and one with California encephalitis), five patients had seronegative AE, and one patient had GAD65 antibody-associated epilepsy. Demographic and clinical characteristics are outlined in Tables 1, 2. The majority of patients presented with an episode of refractory status epilepticus (RSE) (seven patients, 70%). In only three patients (30%), the KDT was started during the acute settings to treat RSE (16, 17, 19).
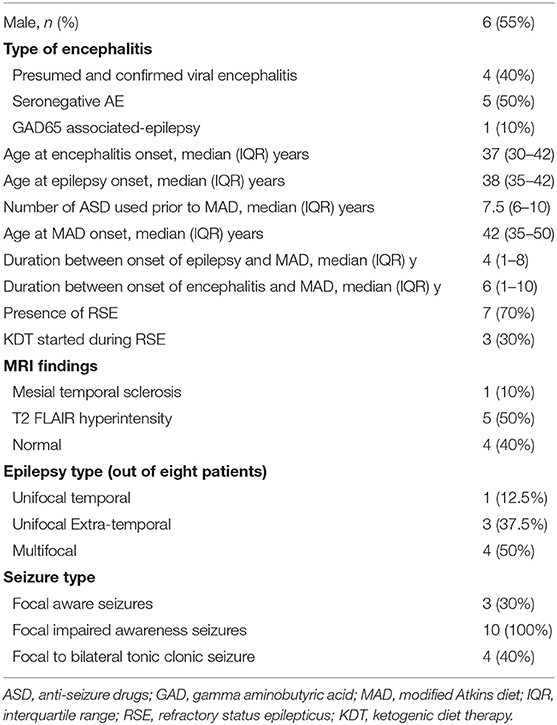
Table 1. Baseline characteristics of patients with post-encephalitic and auto-immune associated epilepsy treated with the modified Atkins diet.
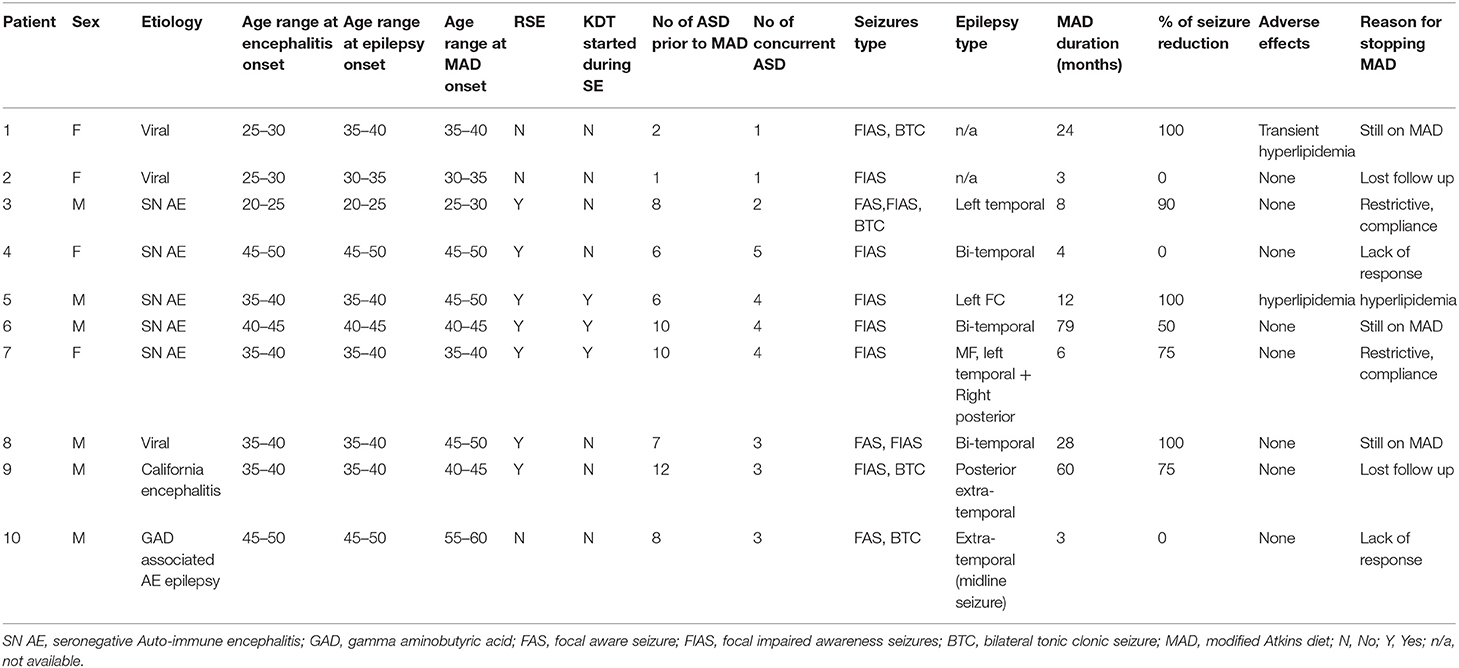
Table 2. Full details of all 10 patients with post-encephalitic and auto-immune associated epilepsy treated with the modified Atkins diet.
The median duration of MAD was 10 months (IQR: 3.75–36). The median age at onset of encephalitis was 37 years (IQR: 30–42). MAD was started at a median of 6 years (IQR: 1–10) after the onset of encephalitis. All 10 patients had focal seizures with impaired awareness, while four patients had additional bilateral tonic-clonic seizures, and three patients had additional focal aware seizures. Eight out of 10 patients had seizures that were localized on EEG. Half of them (four patients) had multifocal epilepsy, three unifocal extra-temporal, and one patient with left temporal localization.
Seizure Outcomes
Out of the 10 patients, three patients (30%) became seizure-free, and one additional patient (10%) achieved 90% seizure freedom with only rare focal seizures with impaired awareness. Four patients (30%) achieved a 50–75% reduction in their baseline seizure frequency on MAD, while two patients (30%) achieved no benefit. None of the patients had an increased frequency of seizures. Overall, seven patients (70%) achieved ≥50% seizure reduction. Additional benefits of MAD included a decrease in seizure severity (in one patient) and improved concentration and attention (in one patient). In reviewing reported adverse effects, the MAD was generally well-tolerated. One patient had transient hyperlipidemia, while another patient had significant LDL elevation prompting discontinuation of the modified Atkins diet. Three patients remain on MAD at last follow up, while two patients were lost to follow up. Reasons of discontinuation included compliance (two patients) and lack of efficacy (two patients).
Discussion
Post-encephalitic and autoimmune-associated epilepsies are increasingly recognized etiologies of drug-resistant focal epilepsy (2–5, 8, 10). In this small case series, the modified Atkins diet was safely used by patients with PE and AE and epilepsy with a 70% responder rate (≥50% seizure reduction).
One third of patients in this study achieved seizure freedom, with another 40% achieving 50–90% seizure reduction. MAD was well-tolerated with only one patient having a persistent and treatment-limiting hyperlipidemia. The median on-diet duration was 10 months (IQR: 4–28), suggesting that MAD was feasible to maintain long-term in this patient population. As previously shown in other studies (26), patients who experience improvement in their seizures had longer on-diet duration. Data extrapolated from children suggests that most responders experience a reduction in seizures on KDT within 14 days from initiation of the diet (27). A consensus recommendation from the International Ketogenic Diet Study Group is to continue the KDT for at least a mean of 3.2 months (±1.3 months SD) before assessing its efficacy (14).
The majority of patients in this study (70%) presented with an episode of refractory status epilepticus as an initial presenting symptom. A classic ketogenic diet was first introduced in 30% of patients studied in the setting of super-refractory status epilepticus. Refractory status epilepticus (RSE) occurs when status epilepticus (SE) persists despite the administration of at least 2 parenteral anti-seizure drugs (including an appropriately-dosed benzodiazepine). About 15–22.6% of cases of SE progress to RSE (28, 29). Of those, another 22% continue to progress to SRSE (30) which is defined as SE persisting or recurred after appropriate anesthetic treatment (31, 32). Both RSE and SRSE carry high rates of morbidity and mortality (33). The prevalence of SE in patients with acute encephalitis ranges between 4 and 20% (34–36), while the prevalence of RSE ranges between 24 and 57% (34, 36) and the prevalence of SRSE has not been well-established.
Several prospective and retrospective studies evaluated the use of KDT in the treatment of SRSE of various etiologies (16, 17, 37). In several case series, a classic ketogenic diet (CKD) was effective in abolishing SE in 73–90% of patients (16, 17, 37). A summary of all adult patients with SRSE due to presumed encephalitic and immune-mediated etiologies treated with adjunctive CKD is presented in Table 3. Thirty-four adult patients received CKD, the median age of patients at the onset of SRSE and CKD initiation was 28.5 (21–40). The majority of the patients responded with cessation of SE, suggesting possible benefit, although there were no control groups used for comparison.
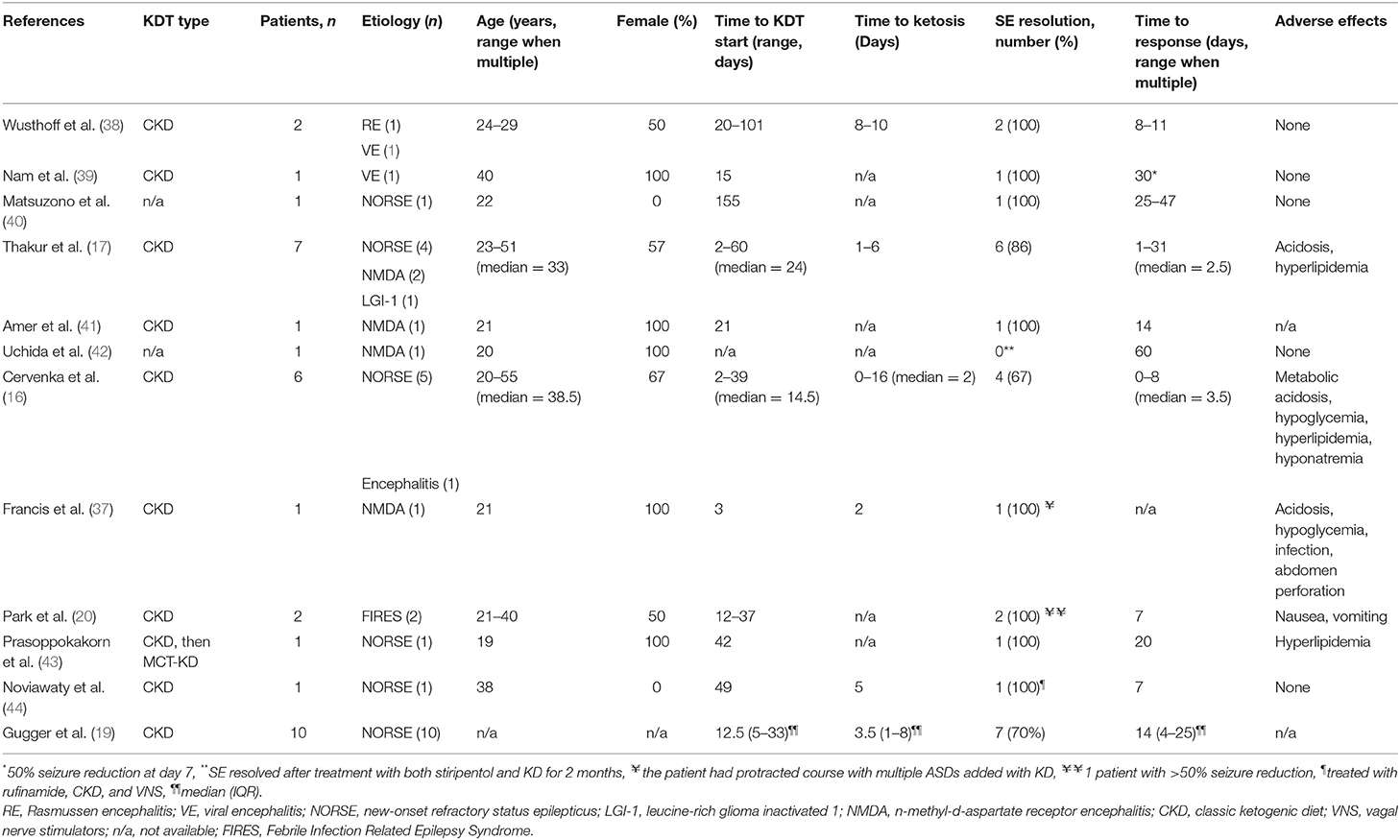
Table 3. Summary of published adult patients with RSE due to presumed infectious and immune-mediated etiologies treated with adjunctive KDT.
The management of chronic PE and AE remains challenging with a significant percentage of patients who develop drug-resistant epilepsy and status epilepticus (3, 45). Patients remain refractory despite treatment with immunotherapy and anti-seizure drugs. A retrospective study showed higher seizure freedom rates with sodium channel agents (carbamazepine, phenytoin, oxcarbazepine, and lacosamide) in patients with AE (46), while there was no difference in the seizure relapse rates between valproate and levetiracetam in another retrospective study (3). Patients with PE and AE are more likely to have multi-focal epilepsy and are therefore not ideal epilepsy surgery candidates, and have been shown to have worse epilepsy surgery outcomes when compared to other etiologies of drug-resistant epilepsy (47, 48). More recently, neuromodulation therapy was reported in patients with AE. Feyissa et al. (49) reported four patients with GAD-65 associated epilepsy treated with responsive neurostimulation (RNS) system. Three patients achieved ≥50% seizure reduction and one patient became seizure-free after RNS data-guided temporal lobectomy (49). In addition to immunotherapy, anti-seizure drugs, and neuromodulation, our case series adds ketogenic diet therapy to the list of potential treatments for epilepsy in patients with PE and AE.
Mechanisms by which KDT may be effective management of seizures in AE and PE are under investigation. The exact pathophysiology of chronic AE and PE which leads to seizures has not been fully elucidated (45). It is hypothesized that varying degrees of persistent inflammation, post-encephalitic structural processes (for example mesial temporal sclerosis), or both processes may contribute (45). There is a growing body of evidence supporting the anti-inflammatory properties of KDT (50). Beta-hydroxybutyrate inhibits NLRP3 inflammasome assembly which in turn decreases caspase-1 activation and the release of pro-inflammatory cytokines (51). KDT increases the levels of polyunsaturated fatty acids that can bind and activate the peroxisome proliferator-activated receptors (PPARs) (50). Additionally, the KDT increases adenosine levels in the brain, which has anti-inflammatory effects (52). Moreover, KDT have antioxidant effects (53) and may modulate mitochondrial function by reducing the production of reactive oxygen species (ROS) (54). Therefore, KDT may be effective for the management of seizures in AE and PE not only because of anti-seizure properties but also by anti-inflammatory mechanisms of action, which could explain the high response rate seen in the patients reported here.
Our study have several limitations aside from its retrospective nature. As a quaternary referral center, patients were often treated following their initial presentation and therefore the exact details surrounding the acute encephalitis episode in some patients were not available. Also, two patients were not monitored on EEG to confirm the diagnosis and localization of focal epilepsy which was determined based on clinical presentation. Moreover, the small sample size and the lack of control group might limit the generalizability of our findings. Finally, patients were on anti-seizure drugs, immunotherapy, and other treatments that were not rigorously documented at the time of treatment and may have impacted seizure control independent of KDT.
Conclusions
In addition to its potential role in the treatment of acute refractory status epilepticus in patients with PE and AE, ketogenic diet therapies may be feasible and safe in the management of chronic post-encephalitic and autoimmune-associated epilepsy. Patients with prior history of SE might respond better to KDT. Further studies are needed to explore the efficacy of KDT in managing seizures in patients with PE and AE. Moreover, whether the early use of KDT can alter the pathophysiology, prognosis, and outcome of patients with encephalitis warrants further exploration.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Johns Hopkins Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KH and MC: study design and critical revisions of the manuscript. KH: data collection, analysis, and drafting of the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
MC receives grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory. Honoraria from American Epilepsy Society, The Neurology Center, Epigenix, LivaNova, and Nutricia. Royalties from Demos/Springer Publishing Company. Consulting for Nutricia, Sage Therapeutics, Glut1 Deficiency Foundation.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Granerod J, Tam CC, Crowcroft NS, Davies NW, Borchert M, Thomas SL. Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology. (2010) 75:924–32. doi: 10.1212/WNL.0b013e3181f11d65
2. Misra UK, Kalita J. Seizures in encephalitis: predictors and outcome. Seizure. (2009) 18:583–7. doi: 10.1016/j.seizure.2009.06.003
3. Peng A, Lai W, Li W, Qiu X, Zhang L, He S, et al. Antiepileptic drugs for acute encephalitic patients presented with seizure. Epilepsy Res. (2020) 164:106347. doi: 10.1016/j.eplepsyres.2020.106347
4. Singh TD, Fugate JE, Hocker SE, Rabinstein AA. Postencephalitic epilepsy: clinical characteristics and predictors. Epilepsia. (2015) 56:133–8. doi: 10.1111/epi.12879
5. Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. (2008) 49(Suppl. 6):13–8. doi: 10.1111/j.1528-1167.2008.01751.x
6. Husari KS, Dubey D. Autoimmune epilepsy. Neurotherapeutics. (2019) 16:685–702. doi: 10.1007/s13311-019-00750-3
7. Dubey D, Toledano M, McKeon A. Clinical presentation of autoimmune and viral encephalitides. Curr Opin Crit Care. (2018) 24:80–90. doi: 10.1097/MCC.0000000000000483
8. Brenner T, Sills GJ, Hart Y, Howell S, Waters P, Brodie MJ, et al. Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia. (2013) 54:1028–35. doi: 10.1111/epi.12127
9. Bien CG, Scheffer IE. Autoantibodies and epilepsy. Epilepsia. (2011) 52(Suppl. 3):18–22. doi: 10.1111/j.1528-1167.2011.03031.x
10. Errichiello L, Perruolo G, Pascarella A, Formisano P, Minetti C, Striano S, et al. Autoantibodies to glutamic acid decarboxylase (GAD) in focal and generalized epilepsy: a study on 233 patients. J Neuroimmunol. (2009) 211:120–3. doi: 10.1016/j.jneuroim.2009.04.010
11. Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. (2018) 83:166–77. doi: 10.1002/ana.25131
12. Husari KS, Cervenka MC. The ketogenic diet all grown up-Ketogenic diet therapies for adults. Epilepsy Res. (2020) 162:106319. doi: 10.1016/j.eplepsyres.2020.106319
13. Felton EA, Cervenka MC. Dietary therapy is the best option for refractory nonsurgical epilepsy. Epilepsia. (2015) 56:1325–9. doi: 10.1111/epi.13075
14. Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. (2018) 3:175–92. doi: 10.1002/epi4.12225
15. Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. (2003) 61:1789–91. doi: 10.1212/01.WNL.0000098889.35155.72
16. Cervenka MC, Hocker S, Koenig M, Bar B, Henry-Barron B, Kossoff EH, et al. Phase I/II multicenter ketogenic diet study for adult superrefractory status epilepticus. Neurology. (2017) 88:938–43. doi: 10.1212/WNL.0000000000003690
17. Thakur KT, Probasco JC, Hocker SE, Roehl K, Henry B, Kossoff EH, et al. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. (2014) 82:665–70. doi: 10.1212/WNL.0000000000000151
18. Gaspard N, Hirsch LJ, Sculier C, Loddenkemper T, van Baalen A, Lancrenon J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. (2018) 59:745–52. doi: 10.1111/epi.14022
19. Gugger JJ, Husari K, Probasco JC, Cervenka MC. New-onset refractory status epilepticus: a retrospective cohort study. Seizure. (2019) 74:41–8. doi: 10.1016/j.seizure.2019.12.002
20. Park EG, Lee J, Lee J. The ketogenic diet for super-refractory status epilepticus patients in intensive care units. Brain Dev. (2019) 41:420–7. doi: 10.1016/j.braindev.2018.12.007
21. Kramer U, Chi CS, Lin KL, Specchio N, Sahin M, Olson H, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. (2011) 52:1956–65. doi: 10.1111/j.1528-1167.2011.03250.x
22. Husari KS, Labiner K, Huang R, Said RR. New-onset refractory status epilepticus in children: etiologies, treatments, and outcomes. Pediatr Crit Care Med. (2020) 21:59–66. doi: 10.1097/PCC.0000000000002108
23. Gaspard N, Foreman BP, Alvarez V, Cabrera Kang C, Probasco JC, Jongeling AC, et al. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. (2015) 85:1604–13. doi: 10.1212/WNL.0000000000001940
24. Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. (2013) 57:1114–28. doi: 10.1093/cid/cit458
25. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
26. Green SF, Nguyen P, Kaalund-Hansen K, Rajakulendran S, Murphy E. Effectiveness, retention, and safety of modified ketogenic diet in adults with epilepsy at a tertiary-care centre in the UK. J Neurol. (2020) 267:1171–8. doi: 10.1007/s00415-019-09658-6
27. Kossoff EH, Laux LC, Blackford R, Morrison PF, Pyzik PL, Hamdy RM, et al. When do seizures usually improve with the ketogenic diet? Epilepsia. (2008) 49:329–33. doi: 10.1111/j.1528-1167.2007.01417.x
28. Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. (2010) 51:251–6. doi: 10.1111/j.1528-1167.2009.02323.x
29. Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. (2019) 381:2103–13. doi: 10.1056/NEJMoa1905795
30. Kantanen AM, Reinikainen M, Parviainen I, Ruokonen E, Ala-Peijari M, Backlund T, et al. Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. (2015) 49:131–4. doi: 10.1016/j.yebeh.2015.04.065
31. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. (2018) 59:739–44. doi: 10.1111/epi.14016
32. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
33. Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. (2012) 135(Pt 8):2314–28. doi: 10.1093/brain/aws091
34. Sonneville R, Mariotte E, Neuville M, Minaud S, Magalhaes E, Ruckly S, et al. Early-onset status epilepticus in patients with acute encephalitis. Medicine (Baltimore). (2016) 95:e4092. doi: 10.1097/MD.0000000000004092
35. Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. (2015) 84:359–66. doi: 10.1212/WNL.0000000000001190
36. Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, et al. Predictors of outcome in acute encephalitis. Neurology. (2013) 81:793–800. doi: 10.1212/WNL.0b013e3182a2cc6d
37. Francis BA, Fillenworth J, Gorelick P, Karanec K, Tanner A. The feasibility, safety and effectiveness of a ketogenic diet for refractory status epilepticus in adults in the intensive care unit. Neurocrit Care. (2019) 30:652–7. doi: 10.1007/s12028-018-0653-2
38. Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. (2010) 51:1083–5. doi: 10.1111/j.1528-1167.2009.02388.x
39. Nam SH, Lee BL, Lee CG, Yu HJ, Joo EY, Lee J, et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. (2011) 52:e181–4. doi: 10.1111/j.1528-1167.2011.03289.x
40. Matsuzono K, Kurata T, Deguchi S, Yamashita T, Deguchi K, Abe K. Ketogenic diet therapy is effective in encephalitis with refractory seizures. Neurol Res. (2014) 36:906–10. doi: 10.1179/1743132814Y.0000000371
41. Amer S, Shah P, Kommineni V. Refractory status epilepticus from NMDA receptor encephalitis successfully treated with an adjunctive ketogenic diet. Ann Indian Acad Neurol. (2015) 18:256–7. doi: 10.4103/0972-2327.150620
42. Uchida Y, Kato D, Toyoda T, Oomura M, Ueki Y, Ohkita K, et al. Combination of ketogenic diet and stiripentol for super-refractory status epilepticus: a case report. J Neurol Sci. (2017) 373:35–7. doi: 10.1016/j.jns.2016.12.020
43. Prasoppokakorn T, Jirasakuldej S, Lakananurak N. Medium-chain triglyceride ketogenic diet is effective for treatment of an adult with super-refractory status epilepticus: a case report and literature review. Eur J Clin Nutr. (2019) 73:1594–7. doi: 10.1038/s41430-019-0471-4
44. Noviawaty I, Olaru E, Rondello C, Fitzsimmons B, Raghavan M. Clinical Reasoning: Ketogenic diet in adult super-refractory status epilepticus. Neurology. (2020) 94:541–6. doi: 10.1212/WNL.0000000000009137
45. Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia. (2020) 61:1341–51. doi: 10.1111/epi.16571
46. Feyissa AM, Lopez Chiriboga AS, Britton JW. Antiepileptic drug therapy in patients with autoimmune epilepsy. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e353. doi: 10.1212/NXI.0000000000000353
47. Carreno M, Bien CG, Asadi-Pooya AA, Sperling M, Marusic P, Elisak M, et al. Epilepsy surgery in drug resistant temporal lobe epilepsy associated with neuronal antibodies. Epilepsy Res. (2017) 129:101–5. doi: 10.1016/j.eplepsyres.2016.12.010
48. Malter MP, Frisch C, Zeitler H, Surges R, Urbach H, Helmstaedter C, et al. Treatment of immune-mediated temporal lobe epilepsy with GAD antibodies. Seizure. (2015) 30:57–63. doi: 10.1016/j.seizure.2015.05.017
49. Feyissa AM, Mirro EA, Wabulya A, Tatum WO, Wilmer-Fierro KE, Won Shin H. Brain-responsive neurostimulation treatment in patients with GAD65 antibody-associated autoimmune mesial temporal lobe epilepsy. Epilepsia Open. (2020) 5:307–13. doi: 10.1002/epi4.12395
50. Koh S, Dupuis N, Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Res. (2020) 167:106454. doi: 10.1016/j.eplepsyres.2020.106454
51. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. (2015) 21:263–9. doi: 10.1038/nm.3804
52. Lusardi TA, Akula KK, Coffman SQ, Ruskin DN, Masino SA, Boison D. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology. (2015) 99:500–9. doi: 10.1016/j.neuropharm.2015.08.007
53. Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. (2009) 59:293–315. doi: 10.1016/j.brainresrev.2008.09.002
Keywords: modified Atkins diet, encephalitis, autoimmune epilepsy, drug-resistant, status epilepticus, SE
Citation: Husari KS and Cervenka MC (2021) Ketogenic Diet Therapy for the Treatment of Post-encephalitic and Autoimmune-Associated Epilepsies. Front. Neurol. 12:624202. doi: 10.3389/fneur.2021.624202
Received: 30 October 2020; Accepted: 19 May 2021;
Published: 16 June 2021.
Edited by:
Kenneth L. Tyler, University of Colorado, United StatesReviewed by:
Jiawei Wang, Capital Medical University, ChinaMaría Magdalena Vaccarezza, MD Hospital Italiano de Buenos Aires, Argentina
Copyright © 2021 Husari and Cervenka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khalil S. Husari, S2h1c2FyaTFAamhtaS5lZHU=
 Khalil S. Husari
Khalil S. Husari Mackenzie C. Cervenka
Mackenzie C. Cervenka