
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 April 2021
Sec. Movement Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.623913
 Liyuan Fan1,2,3†
Liyuan Fan1,2,3† Changhe Shi1,3,4†
Changhe Shi1,3,4† Xinchao Hu1,2,3†
Xinchao Hu1,2,3† Zhongxian Zhang2,5
Zhongxian Zhang2,5 Huimin Zheng1,2,3
Huimin Zheng1,2,3 Haiyang Luo1,2,3
Haiyang Luo1,2,3 Yu Fan1,2,3
Yu Fan1,2,3 Shuo Zhang1,2,3
Shuo Zhang1,2,3 Zhengwei Hu1,2
Zhengwei Hu1,2 Jing Yang1,3
Jing Yang1,3 Chengyuan Mao1,2,3,5*
Chengyuan Mao1,2,3,5* Yuming Xu1,3,4*
Yuming Xu1,3,4*A recent large-scale European-originated genome-wide association study identified 38 novel independent risk signals in 37 loci for Parkinson's disease (PD). However, whether these new loci are associated with PD in Asian populations remains elusive. The present study aimed to explore the relationship between the 12 most relevant loci with larger absolute values for these new risk loci and PD in the Chinese Han population. We performed a case-control study including 527 PD patients and 435 healthy controls. In the allele model, it was found that rs10748818/GBF1 was associated with PD in the Chinese Han population [p = 0.035, odds ratio (OR) 1.221, 95% confidence interval (CI) 1.014–1.472
Parkinson's disease (PD) is the second most common neurodegenerative disorder next to Alzheimer's disease, with a prevalence of 1.7% in the Chinese Han population aged ≥ 65 years (1). The majority of PD cases are sporadic with elusive etiology. Varying factors contribute to the development of PD, including environmental and genetic factors. Mounting evidence has revealed that the latter may provide significant clues to causes of PD (2).
Genome-wide association study (GWAS)-related loci, such as single nucleotide polymorphisms (SNPs) in SNCA, GBA, and LRRK2, are reported to be associated with PD (3). Pooling 17 datasets from PD GWAS available from European ancestry samples, a recent large-scale meta-analysis identified 38 novel independent risk signals in 37 loci for PD (4). However, whether these new loci are associated with PD in Asian populations remains elusive. Referring to the regression coefficient in the results of this meta-analysis, we selected the 12 most relevant loci with larger absolute values for exploration (The detailed information of 38 SNPs was shown in Supplementary Table 2). Consequently, a study including 527 PD patients and 435 healthy controls was performed to investigate the association between the 12 new loci and PD in the Chinese Han population.
A total of 962 subjects of Han Chinese ethnicity were enrolled in the study, including 527 sporadic PD patients and 435 controls. The mean age and sex ratio (male/female) of the two groups were (PD patients: 62.34 ± 9.150 years, 300/227) and (healthy controls: 47.75 ± 10.856 years, 238/197), respectively. To minimize the effect of the familial PD, all patients recruited are sporadic cases. And the young-onset patients were excluded. The cases were defined using the United Kingdom Parkinson's Disease Society Brain Bank criteria. All subjects participating in the study signed written informed consent. This study was approved by the Ethics Committee of First Affiliated Hospital of Zhengzhou University.
Genomic DNA was extracted from peripheral blood collected from the patients and controls using the Blood Genome Extraction Kit (BioTeke Co, Beijing, China). SNPs were genotyped using improved multiple ligase detection reaction (iMLDR) technology (Geneskybiotech, Shanghai, China). All relevant specific polymerase chain reaction (PCR) primers and ligation primers were listed in Supplementary Table 1.
Statistical analysis was performed using IBM SPSS Statistics 26.0. The age difference was assessed using the t test. The Hardy-Weinberg equilibrium in genotype-frequency of controls was assessed using the χ2 test. Logistic regression analysis was used to calculate the risk analysis of each SNP in dominant, recessive models after adjusting for age and gender. Chi-squared tests were adopted to compare differences of sex ratio, genotype frequency, and allele frequency after age and gender-stratified analysis. Multiple tests were performed using the Bonferroni correction method. A 2-tailed p < 0.05 was considered statistically significant.
Frequencies of all 12 variants in the cases and controls met with Hardy-Weinberg equilibrium (p > 0.05, Table 1). In the allele model, the result showed that the rs10748818/GBF1 variant exhibited significant difference between PD patients and the controls [p = 0.035, odds ratio (OR) 1.221, 95% confidence interval (CI) 1.014–1.472, Table 1]. A higher level of G allele was observed in the patients compared with the controls. In dominant and recessive models, rs10748818/GBF1 was not associated with PD after sex and age adjustment via logistic regression [p = 0.275, (OR) 0.834, (CI) 0.601–1.155, Table 1]. In addition, age-stratified analysis showed rs11950533/C5orf24 (genotype model: p =0.034, Table 2) and rs76949143/GS1-124K5·11 (genotype model: p = 0.042, Table 2) were associated with early-onset PD (age < 50 years) and late-onset PD (age ≥ 50 years), respectively.
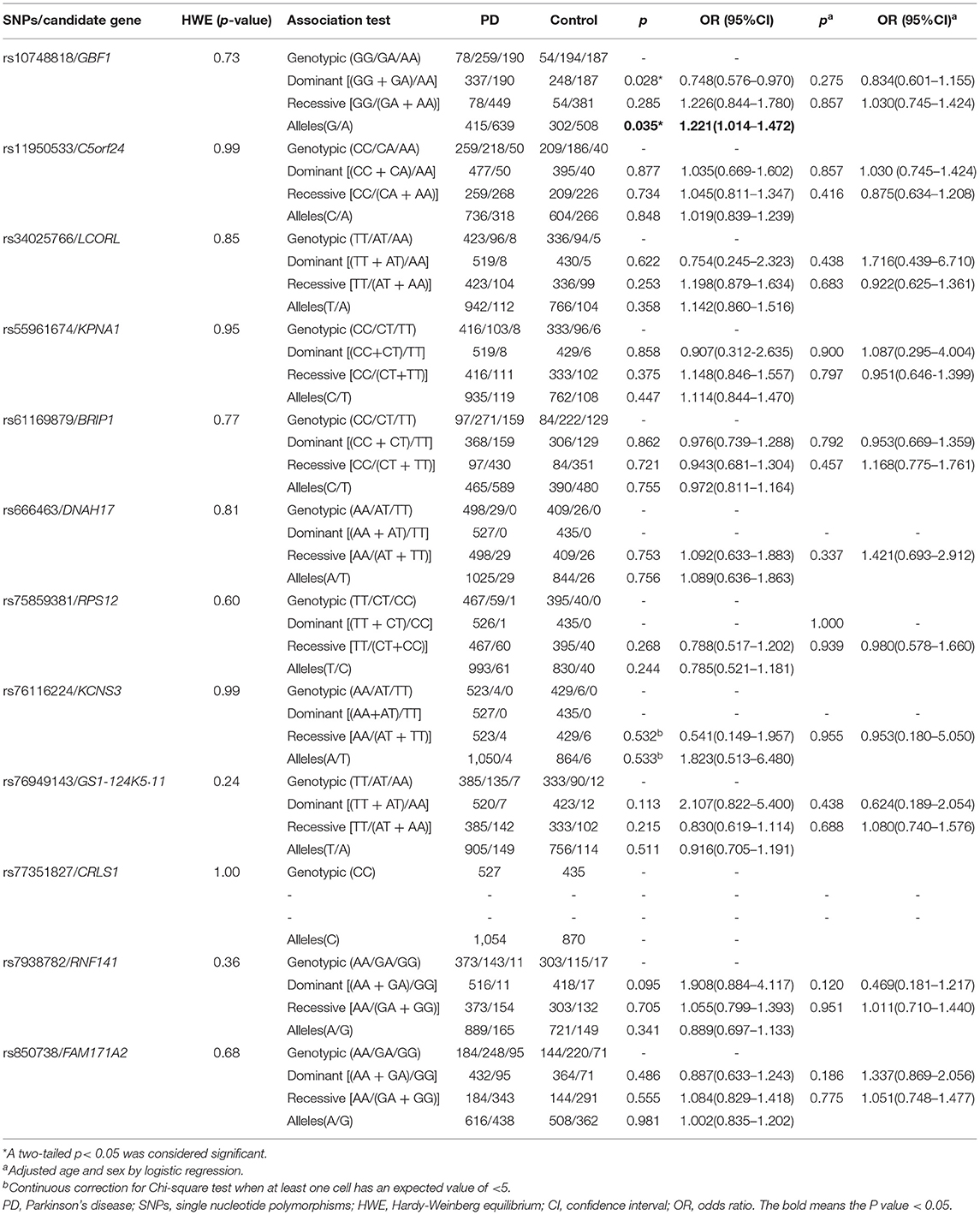
Table 1. Assessment of the relationship level of 12 novel loci with PD in the Chinese Han population.
In contrast, no statistical difference in genotype or allele frequency was detected between PD patients and the controls in the remaining nine loci (rs34025766/LCORL, rs55961674/KPNA1, rs61169879/BRIP1, rs666463/DNAH17, rs75859381/RPS12, rs76116224/KCNS3, rs77351827/CRLS1, rs7938782/RNF141, and rs850738/FAM171A2, Tables 1, 2), neither between groups of the same sex or the same age. All detailed information on the relationship level of 12 loci with PD is shown in Tables 1, 2.
Given the effects of ethnic heterogeneity, our present study investigated the 12 new identified PD-associated variants in a Han Chinese population. We demonstrated that rs10748818/GBF1 exhibited a difference between PD patients and the controls in the allele mode. After age-stratified analysis, rs11950533/C5orf24 and rs76949143/GS1-124K5·11 were associated with early-onset PD and late-onset PD, respectively. To the best of our knowledge, our study is the first to show the association of SNPs in GBF1, C5orf24, and GS1-124K5·11 genes. No statistical difference in genotype or allele frequency was detected between PD patients and the controls in the remaining nine loci. Our study, however, failed to replicate the association of the reported SNPs with PD by Nalls et al. in the European population, which may partially be due to the genetic heterogeneity caused by ethnic and geographical differences (detailed 38 loci information of the GWAS results by Nalls et al. are shown in Supplementary Table 2). Additionally, the interaction between environmental and genetic factors may influence gene expression.
The first Han Chinese GWAS by Foo JN analyzed a total of 22,729 subjects (5,125 PD cases and 17,604 controls) from Singapore, Hong Kong, Malaysia, Korea, mainland China, and Taiwan and replicated associations at SNCA, LRRK2, MCCC1, and 14 other European PD loci but did not identify Asian-specific loci with large effects on PD risk (5). A two-stage meta-analysis of GWAS identified 17 new loci, which were associated with the risk of PD in the European population (26,000 PD patients and 403,000 healthy controls). However, the following study did not find any association between the five most commonly identified candidate variants in the European population with PD in the Chinese population (506 PD patients, 496 MSA patients, and 894 age- and sex-matched healthy controls) (6). Recently, we reported that rs34043159 of IL1R2 and rs4073221 of SATB1 were associated with PD in Chinese Han people (492 PD patients and 524 healthy controls). Further subgroup analysis showed that both rs34043159 of IL1R2 and rs4073221 of SATB1 were associated with late-onset PD. rs34043159 of IL1R2 was associated with PD in female patients, while rs4073221 of SATB1 was associated with PD in both male and female patients (7). The two loci were suggested to be involved in the pathogenesis of PD. But there are still more genetic factors to be identified. Here, we identified another three loci, which were associated with the increased risk of PD.
GBF1, also named ARF1GEF, encodes a member of the Sec7 domain family, which is a guanine nucleotide exchange factor and activates small GTPases of the Arf family. It is involved in regulating the recruitment of proteins to membranes and has been reported to play an essential role in the regulation of the spatial organization and function of mitochondria in a microtubule-dependent manner (8). Numerous studies have implicated that mitochondrial and apoptosis dysfunction are both strongly linked with PD pathogenesis (9). GBF1 localizes at the early Golgi (10) and also links to lipid droplet metabolism (11), plasma membrane signaling, and organelle transport along microtubules with its substrate Arf1. Furthermore, it is involved in the regulation of Golgi fragmentation and is essential for Golgi disassembly and subsequent mitosis entry (12). The fragmentation of the Golgi apparatus is an essential process in the development of apoptosis, which may be related to PD susceptibility. These studies indicated the association of GBF1 with PD.
The C5orf24 is chromosome 5 open reading frame 24, and it has been shown that its DNA methylation level is related to negative affect scores in drug addicts (13). A study identified C5orf24 was upregulated in patients with posttraumatic stress disorder (PTSD) and high intrusion symptoms at baseline and downregulated in participants following treatment (14). However, further investigations are needed to explore the roles of C5orf24 genes played in pathophysiologic pathways of PD.
GS1-124K5·11 is the RAB guanine nucleotide exchange factor 1 pseudogene. The related functional gene of GS1-124K5·11 is RAB guanine nucleotide exchange factor 1 (RABGEF1), which is the upstream factor of the endosomal Rab GTPase cascade. Mutations in Parkin are the second-most-common known cause of PD, and Parkin plays a critical role in mitophagy through ubiquitination of mitochondria. RABGEF1 is recruited to damaged mitochondria via ubiquitin binding downstream of Parkin in mammalian cultured cells and promotes autophagy of damaged mitochondria (15). Overexpression of A53T-Alpha-Synuclein upregulated the expression of RABGEF1 in the mouse midbrain/brainstem (16). However, the role of GS1-124K5·11 in the pathogenesis of PD needs to be further explored.
There are several limitations in the current study, such as the relatively small sample size. Noteworthy, the molecular mechanisms between rs10748818/GBF1, rs11950533/C5orf24, rs76949143/GS1-124K5·11, and PD are still unclear, so more functional experiments should be designed to explore the pathogenesis.
In conclusion, our study demonstrated that the variants of GBF1, C5orf24, and GS1-124K5·11 are associated with PD in the Han Chinese population. It remains to be determined whether geographic or environmental factors are involved in the genetic consequences of these loci. Further genetic analysis and function studies are needed to understand the role of these variants in the pathogenesis of PD.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
LF: data curation, formal analysis, and writing-original draft. CS: resources and funding acquisition. XH and HZ: formal analysis. CM: conceptualization and funding acquisition. YX: funding acquisition and supervision. ZZ: methodology. YF: data curation. HL, SZ, and ZH: writing—review & editing. JY: supervision. All authors: contributed to the study's conception and design.
This work was supported by the National Natural Science Foundation of China (Grants U1904207, 91849115, and 81530037 to YX; Grants 81771290 and 81974211 to CS; and Grant 81901300 to CM), National Key R&D Program of China (Grant 2017YFA0105003 to YX), and the Scientific and Technological Project of Henan Province (Grant SBGJ202003020 to CM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Our work was performed at the Academy of Medical Sciences of Zhengzhou University Translational Medicine Platform.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.623913/full#supplementary-material
PD, parkinson's disease; OR, odds ratio; CI, 95% confidence interval; GWAS, genome-wide association study; SNPs, single nucleotide polymorphisms; PTSD, post-traumatic stress disorder; HWE, Hardy-Weinberg equilibrium; RABGEF1, RAB guanine nucleotide exchange factor 1.
1. Zhang ZX, Roman GC, Hong Z, Wu CB, Qu QM, Huang JB, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. (2005) 365:595–7. doi: 10.1016/S0140-6736(05)17909-4
2. Rocha EM, De Miranda B, Sanders LH. (2018). Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiol Dis. 109(Pt B):249–57. doi: 10.1016/j.nbd.2017.04.004
3. Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet. (2014) 46:989–93. doi: 10.1038/ng.3043
4. Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet Neurol. (2019) 18:1091–102. doi: 10.1016/S1474-4422(19)30320-5
5. Jia Nee F, Tan Louis C, Irwan Ishak D, Wing-Lok A, Low Hui Qi, Prakash Kumar M, et al. (2017). Genome-wide association study of Parkinson's disease in East Asians. Hum Mol Genet. 26:226–32. doi: 10.1093/hmg/ddw379
6. Chen Y, Cao B, Gu X, Ou R, Wei Q, Liu H, et al. No association between 5 new GWAS-linked loci in Parkinson's disease and multiple system atrophy in a Chinese population. Neurobiol Aging. (2018) 67:202.e7–202.e8. doi: 10.1016/j.neurobiolaging.2018.03.027
7. Xinchao H, Chengyuan M, Zhengwei H, Zhongxian Z, Shuo Z, Zhihua Y, et al. Association analysis of 15 GWAS-linked loci with Parkinson's disease in Chinese Han population. Neurosci Lett. (2020) 725:134867. doi: 10.1016/j.neulet.2020.134867
8. Walch L, Pellier E, Leng W, Lakisic G, Gautreau A, Contremoulins V, et al. GBF1 and Arf1 interact with Miro and regulate mitochondrial positioning within cells. Sci Rep. (2018) 8:17121. doi: 10.1038/s41598-018-35190-0
9. Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in parkinson's disease. Prog Neurobiol. (2013) 106-107:17–32. doi: 10.1016/j.pneurobio.2013.04.004
10. Kaczmarek B, Verbavatz JM, Jackson CL. GBF1 and Arf1 function in vesicular trafficking, lipid homoeostasis and organelle dynamics. Biol Cell. (2017) 109:391–9. doi: 10.1111/boc.201700042
11. Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. (2009) 122(Pt 11):1834–41. doi: 10.1242/jcs.045849
12. Mao L, Li N, Guo Y, Xu X, Gao L, Xu Y, et al. AMPK phosphorylates GBF1 for mitotic golgi disassembly. J Cell Sci. (2013) 126(Pt 6):1498–505. doi: 10.1242/jcs.121954
13. Lax E, Warhaftig G, Ohana D, Maayan R, Delayahu Y, Roska P, et al. A DNA methylation signature of addiction in T Cells and its reversal with DHEA intervention. Front Mol Neurosci. (2018) 11:322. doi: 10.3389/fnmol.2018.00322
14. Rusch HL, Robinson J, Yun S, Osier ND, Martin C, Brewin CR, et al. Gene expression differences in PTSD are uniquely related to the intrusion symptom cluster: a transcriptome-wide analysis in military service members. Brain Behav Immun. (2019) 80:904–8. doi: 10.1016/j.bbi.2019.04.039
15. Yamano K, Wang C, Sarraf SA, Munch C, Kikuchi R, Noda NN. Endosomal Rab cycles regulate parkin-mediated mitophagy. Elife. (2018) 7:e31326. doi: 10.7554/eLife.31326
Keywords: Parkinson's disease, single nucleotide polymorphisms, Chinese population, GBF1, C5orf24, GS1-124K5·11
Citation: Fan L, Shi C, Hu X, Zhang Z, Zheng H, Luo H, Fan Y, Zhang S, Hu Z, Yang J, Mao C and Xu Y (2021) Analysis of 12 GWAS-Linked Loci With Parkinson’s Disease in the Chinese Han Population. Front. Neurol. 12:623913. doi: 10.3389/fneur.2021.623913
Received: 30 October 2020; Accepted: 04 March 2021;
Published: 07 April 2021.
Edited by:
Ruey-Meei Wu, National Taiwan University, TaiwanReviewed by:
Chin-Hsien Lin, National Taiwan University Hospital, TaiwanCopyright © 2021 Fan, Shi, Hu, Zhang, Zheng, Luo, Fan, Zhang, Hu, Yang, Mao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuming Xu, eHV5dW1pbmdAenp1LmVkdS5jbg==; Chengyuan Mao, bWFvY2hlbmd5dWFuMjAxNUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.