
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 14 May 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.620096
This article is part of the Research Topic Therapeutic Agents and Targets to Minimize Ischemic Brain Injury View all 18 articles
 Sepide Kashefiolasl1*
Sepide Kashefiolasl1* Marlies Wagner2
Marlies Wagner2 Nina Brawanski1
Nina Brawanski1 Volker Seifert1
Volker Seifert1 Stefan Wanderer3,4
Stefan Wanderer3,4 Lukas Andereggen3,4
Lukas Andereggen3,4 Juergen Konczalla1
Juergen Konczalla1The efficacy of statin-treatment in aneurysmal subarachnoid hemorrhage (SAH) remains controversial. We aimed to investigate the effects of statin-treatment in non-aneurysmal (na)SAH in accordance with animal research data illustrating the pathophysiology of naSAH. We systematically searched PubMed using PRISMA-guidelines and selected experimental studies assessing the statin-effect on SAH. Detecting the accordance of the applied experimental models with the pathophysiology of naSAH, we analyzed our institutional database of naSAH patients between 1999 and 2018, regarding the effect of statin treatment in these patients and creating a translational concept. Patient characteristics such as statin-treatment (simvastatin 40 mg/d), the occurrence of cerebral vasospasm (CVS), delayed infarction (DI), delayed cerebral ischemia (DCI), and clinical outcome were recorded. In our systematic review of experimental studies, we found 13 studies among 18 titles using blood-injection-animal-models to assess the statin-effect in accordance with the pathophysiology of naSAH. All selected studies differ on study-setting concerning drug-administration, evaluation methods, and neurological tests. Patients from the Back to Bedside project, including 293 naSAH-patients and 51 patients with simvastatin-treatment, were recruited for this analysis. Patients under treatment were affected by a significantly lower risk of CVS (p < 0.01; OR 3.7), DI (p < 0.05; OR 2.6), and DCI (p < 0.05; OR 3). Furthermore, there was a significant association between simvastatin-treatment and favorable-outcome (p < 0.05; OR 3). However, dividing patients with statin-treatment in pre-SAH (n = 31) and post-SAH (n = 20) treatment groups, we only detected a tenuously significant higher chance for a favorable outcome (p < 0.05; OR 0.05) in the small group of 20 patients with statin post-SAH treatment. Using a multivariate-analysis, we detected female gender (55%; p < 0.001; OR 4.9), Hunt&Hess ≤III at admission (p < 0.002; OR 4), no anticoagulant-therapy (p < 0.0001; OR 0.16), and statin-treatment (p < 0.0001; OR 24.2) as the main factors improving the clinical outcome. In conclusion, we detected a significantly lower risk for CVS, DCI, and DI in naSAH patients under statin treatment. Additionally, a significant association between statin treatment and favorable outcome 6 months after naSAH onset could be confirmed. Nevertheless, unified animal experiments should be considered to create the basis for developing new therapeutic schemes.
Early brain injury (EBI) followed by subarachnoid hemorrhage (SAH) is caused by transient cerebral ischemia during bleeding. In up to 30% of SAH patients, delayed cerebral ischemia (DCI) is associated with poor clinical outcome or death. DCI is the result of SAH caused secondary effects such as increased intracranial pressure and destruction of brain tissue by intracerebral hemorrhage. A combination of post-SAH complications including cerebral vasospasm, arteriolar constriction and thrombosis, cortical spreading ischemia, and processes triggered by EBI lead to DCI (1–3). Therefore, numerous pharmacological treatments have concentrated on the prevention of EBI and cerebral vasospasm, with disappointing results as of yet (4).
Statins have been identified as a potential treatment option for early brain injury and the associated vasospasm. Based on experimental studies using animal models proving vascular regenerative effects of statins, statin-treatment might reduce EBI and vasospasm in patients after aneurysmal SAH (5, 6).
However, the results of clinical trials have been controversial. A decreased incidence of vasospasm was found in a few Phase II trials, while further clinical studies have failed to confirm these findings (7–9). Additionally, Meta-analyses have also produced inconsistent results. In contrast to clinical studies demonstrating a beneficial effect of statin treatment in only one aspect (10–12), there are additional studies disproving all reported benefits (13–15). Nevertheless, two large-scale multicenter Phase III trials- Simvastatin in Aneurysmal Subarachnoid Hemorrhage (STASH) (16) and High-Dose Simvastatin for Aneurysmal Subarachnoid Hemorrhage (HDS-SAH) (17)—could not detect any beneficial effect of simvastatin treatment independent of statin dose, which has been described as effective in higher drug doses, previously. Therefore, the effect of statins on vasospasm remains unclear.
Considering this discrepancy between experimental and clinical data, we aimed to investigate the effects of statin treatment in SAH in accordance with animal research data. Firstly, a systematic review was conducted to explore the research background of the role of statins in the case of SAH. Detecting the accordance of the applied experimental models with the pathophysiology of naSAH, we analyzed our patient database regarding the effect of statin treatment in non-aneurysmal SAH patients.
Peer-reviewed articles reporting the clinical evidence of efficacy of statins for SAH in experimental animal models were identified. Papers were selected in MEDLINE/Pubmed according to the PRISM statement. The search terms “statin AND SAH” with the filter option for “species” were used to search Pubmed systematically. English language articles up to December 2018 were considered. The literature review wasperformed by two independent reviewers.
All studies working on an experimental SAH model in vivo were included after first review of titles and abstracts. Differentiating between a blood injection and endovascular perforation SAH model (referring to the study design single or double hemorrhage model in case of both methods), animal studies with injection models were included. Blood injection models with the presence of subarachnoid blood in the cisterns around the midbrain mimicking naSAH with the center of the bleeding immediately anterior to the midbrain, with or without extension to the ambient and chiasmatic cisterns, horizontal part of the sylvian fissure and posterior horns of the lateral ventricles, resembling non-aneurysmal SAH in humans, were added to our database.
All included papers were reviewed in full text and availability of coordinates. Reference lists of suitable studies were scrutinized for additional articles.
All eligible studies were analyzed describing the following parameters: animal species, study design containing the exact description of SAH model, size of animal groups, dose of drug, route and time of drugs' administration, employed tests, time of examination, and results.
Regarding the highest number of studies using the SAH blood injection model, in accordance with the pathophysiology of naSAH, we retrospectively analyzed our institutional database of consecutive patients suffering naSAH between 1999 and 2018. Non-aneurysmal Subarachnoid hemorrhage was defined as a spontaneous non-traumatic hemorrhage into the subarachnoid space without any evidence of an intracranial vascular pathology.
The retrospective clinical study was approved by the local ethics committee of the Goethe-University and was performed in accordance with the relevant guidelines and regulations of the regional ethics committee in Frankfurt am Main, Germany. Because of the retrospective design, informed consent was waived by the ethics committee.
All patients with SAH, diagnosed by SAH pattern on CT-scan, or confirmed by lumbar puncture, underwent cerebral digital subtraction angiography (DSA) to rule out intracranial vascular bleeding sources. Patients in whom the bleeding source was detected to be an aneurysm or vascular malformation were excluded from the study and treated by surgical or endovascular aneurysm occlusion based on an interdisciplinary consensus. If absence of intracranial vascular pathology was confirmed by a neuroradiologist, patients received a 2nd angiography regularly ~14–48 days after the ictus. All admitted SAH patients without intracranial vascular pathology were included in this study as non-aneurysmal SAH patients.
According to bleeding patterns, naSAH patients were divided into perimesencephalic- (PM-) and non-perimesencephalic (NPM-) naSAH. A PM-SAH was defined as the presence of subarachnoid blood in the cisterns around the midbrain with the center of the bleeding immediately anterior to the midbrain, with or without extension to the ambient and chiasmatic cisterns, the horizontal part of the sylvian fissure, and posterior horns of the lateral ventricles. Extensive intraventricular hemorrhage and intraparenchymal hemorrhage were exclusive of PM-SAH. Patients with an aneurysmal SAH pattern, described as blood located in the interpeduncular cistern, as well as in the Sylvian cistern, interhemispheric cistern and convexity, or a negative computer tomography with a positive lumbar puncture, and negative digital subtraction angiography (DSA), were classified as NPM-SAH.
Patients not receiving treatment at SAH onset because of advanced brain injury or without clinical follow-up 6 months post-SAH were excluded.
All parameters relevant to this analysis, including statin-pretreatment (medication intake independent of SAH onset), statin-post-treatment after SAH onset (drug: simvastatin 40 mg a day), the occurrence of CVS, DI (CVS-related vs. non-CVS-related), DCI and finally clinical outcome (modified Rankin Scale: mRS after 6 months; favorable outcome mRS 0–2 vs. unfavorable outcome mRS 3–6) in patients with non-aneurysmal SAH, were recorded.
The degree of CVS in patients was uniformly defined by CT-A as a radiological imaging on the basis of arterial narrowing (e.g., >66% for severe CVS). Because of the small number of patients with high or low dose statin treatment [statin dose: 80 mg (n = 2) or 20 mg (n = 3)], these patients were excluded.
Data analyses were performed using the computer software package (IBM SPSS, version 22, IBM SPSS Inc.; Armonk, NY). Multivariable logistic regression analysis with forward stepwise modeling was carried out to identify factors for outcome improvement among above mentioned aspects. Categorical variables were analyzed using the Fisher's exact test and GraphPad Prism (6.0, GraphPad Software Inc., USA). Normal distributed variables were expressed as mean values with standard deviations (SD) and analyzed using a two-tailed t-test. A p < 0.05 was considered statistically significant.
Of the total of 18 English titles with experimental data, 13 studies with the presence of subarachnoid blood in the cisterns around the midbrain in accordance with the pathophysiology of naSAH used SAH blood injection model to assess the statin effect. Only seven studies applied simvastatin as the same drug, but in different doses and application routes. All of these selected studies differ in time of drug administration, employed tests, and time of examination. Evaluation of basilar artery diameter as a unified parameter to detect CVS after SAH was done in seven studies, using cerebral angiography (2/7) vs. measurement of cross-sectional areas (5/7) (Figure 1, PRISMA flow diagram).
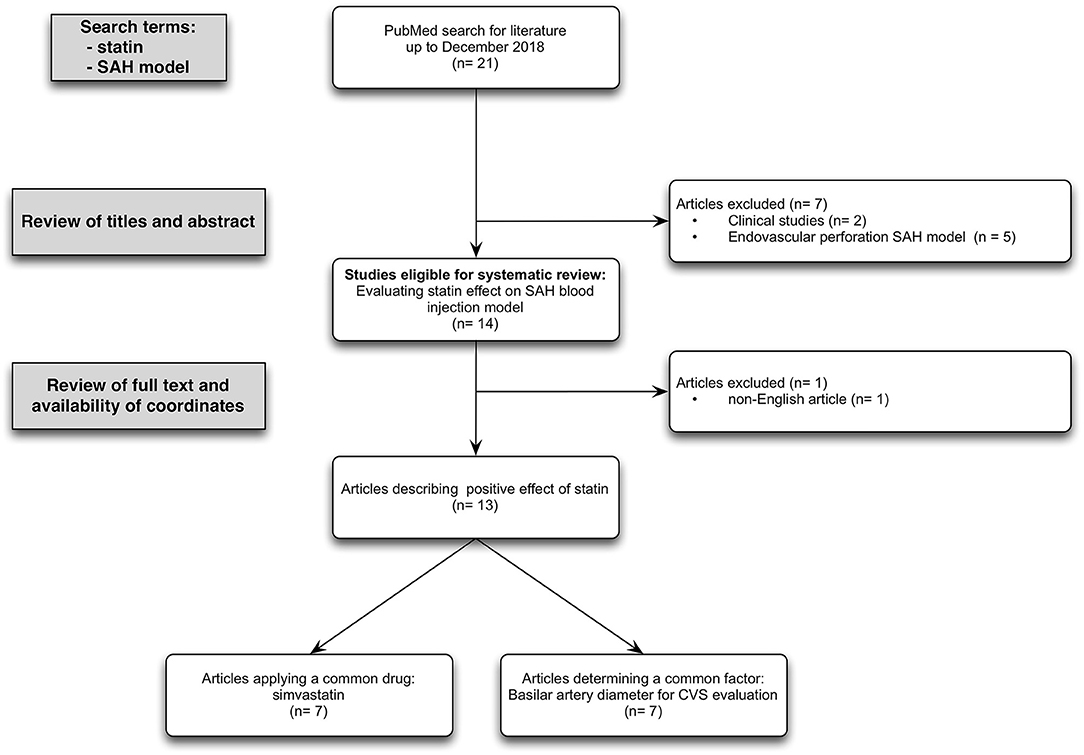
Figure 1. PRISMA flow diagram: Flow chart summarizing the study selection process. Inclusion criteria required animal-based experimental studies focusing on statin effect in case of SAH induced by subarachnoidal blood injection model. Exclusion criteria required studies reporting on the application of an endovascular perforation SAH model and language other than English. Overall, 13 studies met the inclusion and exclusion criterias.
Detailed study data summarized in Table 1 (18–29, 31) demonstrate the disunity of study setting of all included experimental studies determining the statin effect on SAH using blood injection model. There are not only discrepancies in choosing animal species, SAH model (single vs. double blood injection model) and size of animal groups, but also in study setup including choice and dose of drug, route and time of drug administration, employed tests, time of examination, and evaluated results.
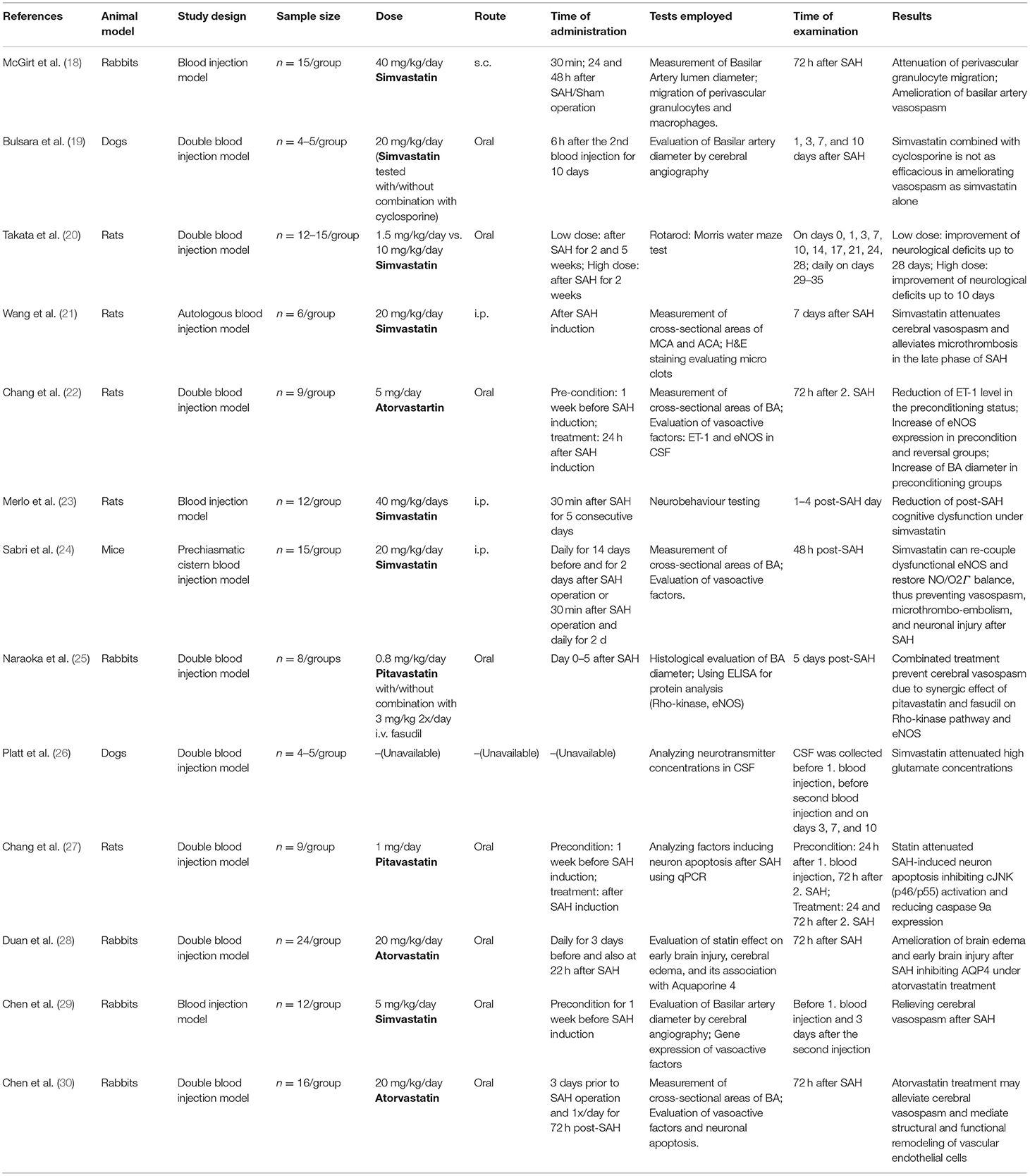
Table 1. Experimental studies assessing neuroprotective effects of Statin on SAH using blood injection model.
A total of 293 patients with naSAH were included. 51 (17%) naSAH patients with simvastatin treatment (40 mg/d) were recruited for analysis, including 31 (61%) patients with pretreatment and 20 (39%) patients with post-treatment after SAH onset. In total 54 patients (18%) developed early hydrocephalus, 62 patients (21%) cerebral vasospasm, 53 patients (18%) had delayed cerebral ischemia (DCI) and 68 patients (23%) suffered from delayed infarction at discharge. Concerning Fisher bleeding pattern on CT-scan at admission, a total of 86 (29%) patients with a Fisher 3 bleeding pattern were included.
Patients under simvastatin treatment were affected by a significantly lower risk of Fisher 3 bleeding pattern on initial CT-scan (P < 0.05; OR 13.5), early hydrocephalus (P < 0.05; OR 2.5); CVS (p < 0.01; OR 3.7), DI (p < 0.05; OR 2.6), and DCI (p < 0.05; OR 3.0). Additionally, we differentiate between CVS-related (22/68, 32%) and non-CVS-related (46/68, 68%) DI describing a trend toward lower risk of CVS-related DI (1/22, 5%) in patients with simvastatin treatment (Table 2A).
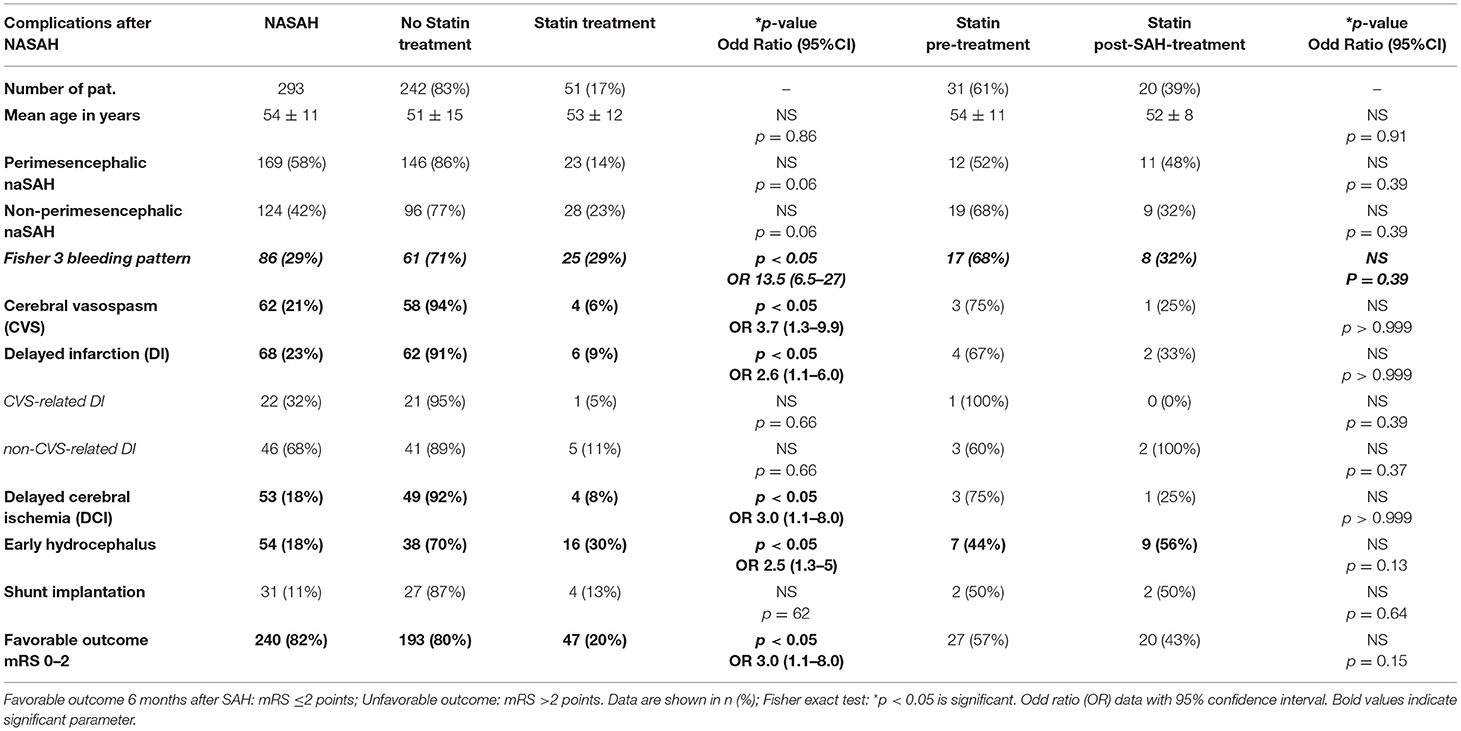
Table 2A. Clinical complications and outcome in NASAH patients in dependence on statin treatment between 1999 and 2018.
Furthermore, our results indicate a significant association between simvastatin treatment and favorable neurological outcome (p < 0.05; OR 3.0). A relevant statistical difference between simvastatin-pretreatment and –post-SAH-treatment was not detected in this small cohort (Table 2A).
Due to the pathomechanism of statin effect as a treatment option after naSAH based on recent clinical studies, we performed Table 2B analyzing SAH followed complications and outcome in case of patients with statin post-SAH treatment compared to untreated group. Even in this small patient group with statin post-SAH treatment (n = 20), we detected a significant higher chance for favorable outcome (p < 0.05; OR 0.05) in these patients and a trend toward lower risk for development of cerebral vasospasm (p = 0.05) (Table 2B). Nevertheless, because of the small number of patients (n = 20) these statistical results should be treated with caution.
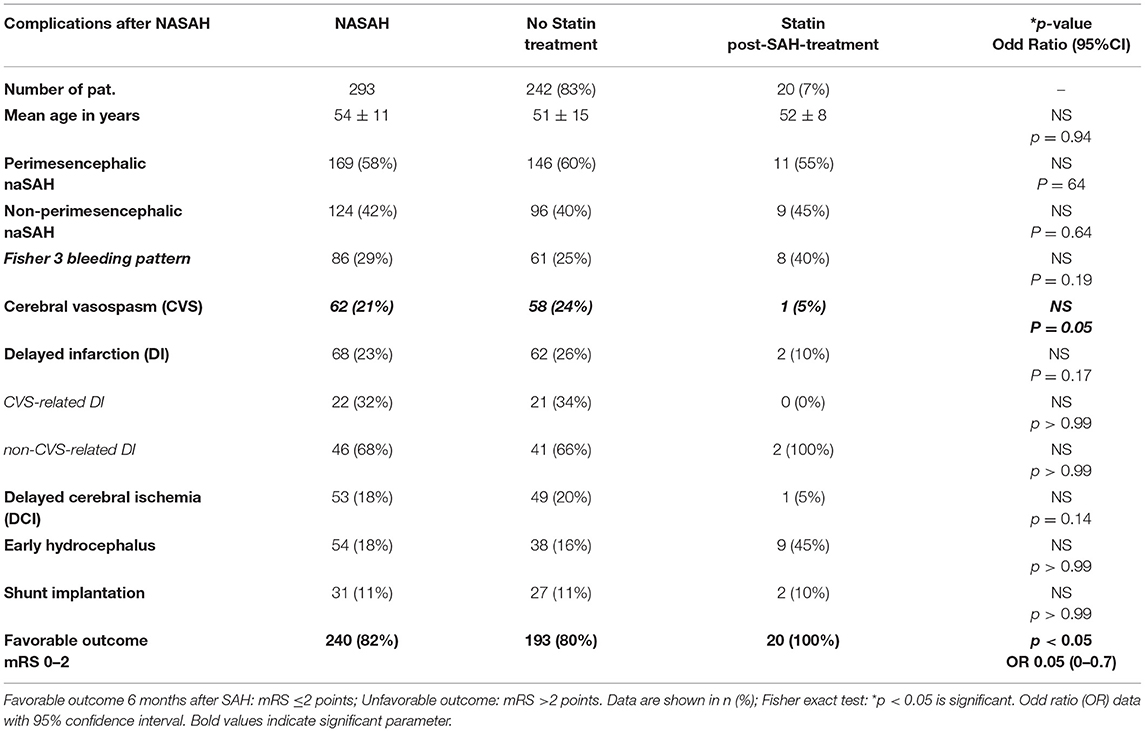
Table 2B. Clinical complications and outcome in NASAH patients in dependence on statin post-SAH-treatment between 1999 and 2018.
Using a multivariate analysis to detect significant patient characteristics associated with favorable outcome, we recognized female gender (55%; p < 0.001; 4.9), Hunt & Hess ≤ III at admission (p < 0.002; 4), no anticoagulation and antiplatelet drug treatment (p < 0.0001; 0.16), and statin treatment (p < 0.0001; 24.2) as main factors improving the clinical outcome of naSAH patients (Table 3).
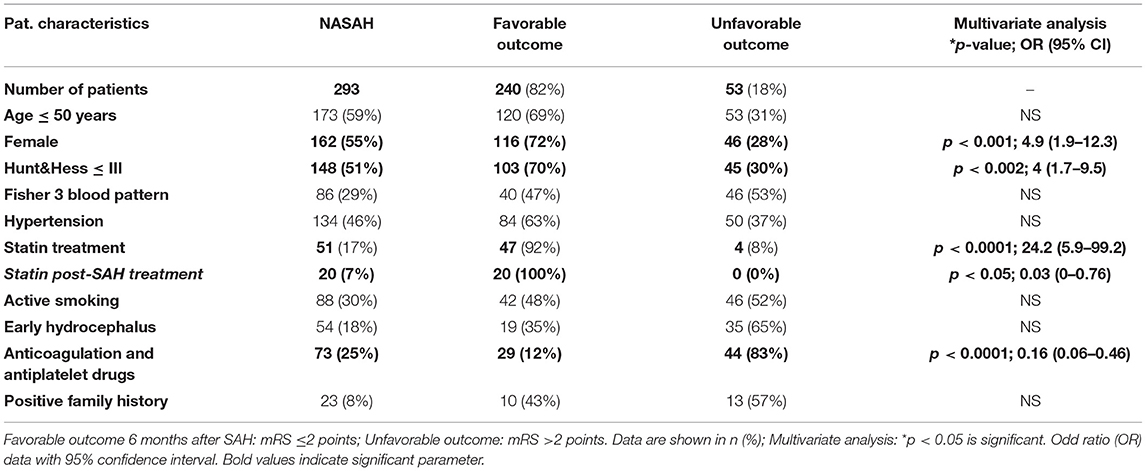
Table 3. Clinical outcome in association with patient characteristics in NASAH patients admitted to the University Hospital (City) between 1999 and 2018.
Creating a scientific basis as a background for the evaluation of data of included naSAH patients, we firstly attempted to collect all experimental studies focused on statin effect in animals using the blood injection model, according to the pathophysiology of naSAH.
We detected 13 experimental studies differing on study design factors including animal species, drug dose and application route, time of drug administration, employed tests, and time of examination. Concerning different conclusions, six studies described an attenuation of cerebral vasospasm after SAH onset, one study could prove ameliorated neurological deficit after SAH, another study dealt with brain edema after SAH, being attenuated under statin treatment and a single publication determined a decreased rate of cognitive dysfunction. Two further included studies focused on the mechanism of statin effect. Taking all studies paying attention to the statin effect on cerebral vasospasm under consideration, we could not unify the results to be analyzed as a metanalysis, not only because of the different study setup, but also because of the different evaluations of severity of vasospasm. Except for two included experimental research studies, performing cerebral angiography in experimental animals after SAH induction as the most precise established method to detect vasospasm, other studies choose to measure cross-sectional areas of vessels histologically, which does not allow an accurate assessment of vessel diameters.
Considering all mentioned factors, independent of all scientific research work invested in these publications, there is no possibility to unify the resulting statements to enable a statistically crucial analysis as a background for further clinical studies.
However, despite all of these discrepancies, most studies concentrating on statin effect following complications after SAH used blood injection models to induce the bleeding, which does mimic non-aneurysmal SAH rather than aneurysmal SAH. All of these experimental studies underlined the positive effect of statin treatment after SAH induction from different points of view. Investigating numerous clinical studies on this aspect in aneurysmal SAH patients and failing to confirm experimental findings, a retrospective analysis to determine the possible reasons of these discrepancies was not in the focus of interest. Even multiple published meta-analyses produced inconsistent results (8–15).
However, two further large-scale multicenter Phase III trials, STASH (16) and HDS-SAH (17), resulted in disappointing findings. The STASH study could not confirm an improved outcome in aneurysmal SAH patients. In addition, authors of the HDS-SAH study denied any benefits of treatment with high doses of simvastatin (80 mg) vs. treatment with low doses (40 mg), as described previously. Nevertheless, both studies had limitations. In the STASH study the evaluation of vasospasm was not described in detail, and in the HDS-SAH study no control group was used. These limiting factors make it difficult to draw evidence-based conclusions (29).
Therefore, the effect of statins on vasospasm after SAH remained unclear; positive effects may have been neutralized through an adverse pathway. However, recognizing this fact and regarding the pathophysiology of the experimental SAH model being used in most of the studies done in this field, we created a translational insight detecting naSAH patients to assess the statin effect.
Previous studies observed a significant increase in the number of patients with NASAH. Non-aneurysmal SAH patients have significantly less DCI and a higher chance for excellent outcomes compared to aneurysmal SAH, but patients with PM and NPM-SAH also developed DCI. Especially patients with an NPM-SAH have, according to the bleeding pattern, an increased risk for DCI and for a worse neurological outcome (32). Therefore, patients with a NASAH should be monitored for new therapeutic options concerning SAH followed complications to increase the chance for a favorable outcome.
This is the first study presenting the statin effect in patients with naSAH in accordance to the pathophysiological background of statin treatment attenuating SAH following complications in animals. We could detect a significantly lower risk for CVS, DCI, and DI in naSAH patients under statin treatment. Additionally, a significant association between statin treatment and favorable outcome 6 months after naSAH onset could be confirmed. According to previous studies, patients with NASAH and a Fisher Grade 3 bleeding pattern had a significantly higher risk for an unfavorable outcome and death (32). However, a multivariate analysis of recent data described the bleeding pattern as a not-significant risk factor for unfavorable outcome.
Although there is a red thread through the data, from bench to bedside, underlining the effect of statin treatment in naSAH patients, there are some limitations in this study, which have to be under consideration. At first, the small number of detected patients did not allow differentiations to be made between patients under different drugs and doses of statin. In addition, an analysis of statin effect as a pre-treatment vs. post-SAH-treatment could not be done. This aspect should be considered as a limiting factor, because detecting 20 patients suffering from naSAH with statin post-treatment could not be sufficiently powered by statistical analysis to prove the therapeutic effect of statins in the case of the clinical course of naSAH patients. Nevertheless, a separated analysis of the small patient group with statin treatment after SAH detected a tenuous significantly higher chance for favorable outcome and a trend toward lower risk for cerebral vasospasm after non-aneurysmal SAH (Table 2B).
However, recent data demonstrate a positive trend in case of statin-effect in naSAH patients according to the known important role of statins in vasculogenesis and vascular regeneration (31).
Furthermore, dividing non-aneurysmal SAH into perimesencepahlic (PM-) SAH, where the blood is strictly around the midbrain or brainstem, and non-perimesencephalic (NPM-) SAH, where the blood extends into the adjacent cistern, it is well-known that NPM-SAH have a different illness course with higher risk of CVS, DCI and poor outcome compared to PM-SAH (32). Concerning this aspect, because of the small number of included patients we could not differ between PM-SAH and NPM-SAH in our multivariate analysis. All these aspects minimize the impact of these results.
Therefore, a combination of pre-/post-treatment with statins may play a role, but a causal relationship cannot, thus far, be proven. Data from other centers are necessary to confirm our findings; however, for this rare condition, the cohort may be considered large.
However, contrary to the most studies in aneurysmal SAH patients, there is a significant association between statin treatment and the occurrence of cerebral vasospasm, delayed infarction, and delayed cerebral ischemia in the case of non-aneurysmal SAH. To analyze propriety and possible application of our results in daily clinical management of naSAH patients, further prospective clinical trials are needed.
Furthermore, by failure of multiple big size clinical studies despite successful experimental data, a translational flashback could ease the way to recognize possible influencing factors. Therefore, unified animal experiments and translational clinical trials should be considered to create the basis for developing new therapeutic schemes, successfully.
Based on this translational study, the injection models mimic non-aneurysmal SAH rather than aneurysmal SAH, and the model might not be suitable to study aneurysmal SAH.
Collecting all experimental studies focused on statin effect in animals using the blood injection model, we firstly detected the accordance of this SAH model with the pathophysiology of naSAH.
Then, we presented the statin effect in patients with naSAH in accordance with the pathophysiological background of statin treatment attenuating SAH following complications in animals. We could detect a significant lower risk for CVS, DCI, and DI in naSAH patients being under statin treatment. Additionally, a significant association between statin treatment and favorable outcome 6 months after naSAH onset could be confirmed. Nevertheless, unified animal experiments should be considered to create the basis for developing new therapeutic schemes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
SK and JK wrote the main manuscript text and SK prepared Figure 1 and Tables 1–3. All authors reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Marina Heibel and Anne Sicking (University Hospital Frankfurt, Department of Neurosurgery) edited the manuscript for non-intellectual content.
1. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. (2014) 10:44–58. doi: 10.1038/nrneurol.2013.246
2. Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology. (1986) 36:329–33. doi: 10.1212/WNL.36.3.329
3. Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. (2005) 36:2024–6. doi: 10.1161/01.STR.0000177879.11607.10
4. Van Gijn J, Rinkel GJE. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. (2001) 124(Pt 2):249–78. doi: 10.1093/brain/124.2.249
5. McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. (2002) 33:29506. doi: 10.1161/01.STR.0000038986.68044.39
6. Sugawara T, Ayer R, Jadhav V, Chen W, Tsubokawa T, Zhang JH. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. (2008) 86:3635–43. doi: 10.1002/jnr.21807
7. Chou SH, Smith EE, Badjatia N, Nogueira RG, Sims JR II, Ogilvy CS, et al. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. (2008) 39:2891–3. doi: 10.1161/STROKEAHA.107.505875
8. Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. (2005) 36:1627–32. doi: 10.1161/01.STR.0000176743.67564.5d
9. Vergouwen MD, Meijers JCM, Geskus RB, Coert BA, Horn J, Stroes ESG, et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab. (2009) 29:1444–53. doi: 10.1038/jcbfm.2009.59
10. Sillberg VAH, Wells GA, Perry JJ. Do statins improve outcomes and reduce the incidence of vasospasm after aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke. (2008) 39:2622–6. doi: 10.1161/STROKEAHA.107.508341
11. Su SH, Xu W, Hai J, Wu Y, Yu F. Effects of statins-use for patients with aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. Sci Rep. (2014) 4:4573. doi: 10.1038/srep04573
12. Tseng MY. Summary of evidence on immediate statins therapy following aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2011) 15:298–301. doi: 10.1007/s12028-011-9596-6
13. Kramer AH, Fletcher JJ. Statins in the management of patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. (2010) 12:285–96. doi: 10.1007/s12028-009-9306-9
14. Liu J, Chen Q. Effect of statins treatment for patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis of observational studies and randomized controlled trials. Int J Clin Exp Med. (2015) 8:7198–208.
15. Vergouwen MD, de Haan RJ, Vermeulen M, Roos YBWEM. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. (2010) 41:e4752. doi: 10.1161/STROKEAHA.109.556332
16. Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD, STASH Collaborators. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. (2014) 13:666–75. doi: 10.1016/S1474-4422(14)70084-5
17. Wong GK, Chan DYC, Siu DYC, Zee BCY, Poon WS, Chan MTV, et al. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke. (2015) 46:382–8. doi: 10.1161/STROKEAHA.114.007006
18. McGirt MJ, Pradilla G, Legnani FG, Thai QA, Recinos PF, Tamargoet RJ, et al. Systemic administration of simvastatin after the onset of experimental subarachnoid hemorrhage attenuates cerebral vasospasm. Neurosurgery. (2006) 58:945–51. doi: 10.1227/01.NEU.0000210262.67628.7E
19. Bulsara KR, Coates JR, Agrawal VK, Eifler DM, Wagner-Mann CC, Durham HE, et al. Effect of combined simvastatin and cyclosporin compared with simvastatin alone on cerebral vasospasm after subarachnoid hemorrhage in a canine model. Neurosurg Focus. (2006) 21:E11. doi: 10.3171/foc.2006.21.3.11
20. Takata K, Sheng H, Borel CO, Laskowitz DT, Warner DS, Lombardet FW. Simvastatin treatment duration and cognitive preservation in experimental subarachnoid hemorrhage. J Neurosurg Anesthesiol. (2009) 21:326–33. doi: 10.1097/ANA.0b013e3181acfde7
21. Wang Z, Chen G, Zhu WW, Bian JY, Shen XO, Zhouet D, et al. Influence of simvastatin on microthrombosis in the brain after subarachnoid hemorrhage in rats: a preliminary study. Ann Clin Lab Sci. (2010) 40:32–42.
22. Chang CZ, Wu SC, Lin CL, Hwang SL, Howng SL, Kwanet al. Atorvastatin preconditioning attenuates the production of endothelin-1 and prevents experimental vasospasm in rats. Acta Neurochir. (2010) 152:1399–406. doi: 10.1007/s00701-010-0652-3
23. Merlo L, Cimino F, Scibilia A, Ricciardi E, Chirafisi J, Speciale A, et al. Simvastatin administration ameliorates neurobehavioral consequences of subarachnoid hemorrhage in the rat. J Neurotrauma. (2011) 28:2493–501. doi: 10.1089/neu.2010.1624
24. Sabri M, Ai J, Marsden PA, Macdonald LR. Simvastatin re-couples dysfunctional endothelial nitric oxide synthase in experimental subarachnoid hemorrhage. PLoS ONE. (2011) 6:e17062. doi: 10.1371/journal.pone.0017062
25. Naraoka M, Munakata A, Matsuda N, Shimamura N, Ohkuma H. Suppression of the Rho/Rho kinase pathway and prevention of cerebral vasospasm by combination treatment with statin and fasudil after subarachnoid hemorrhage in rabbit. Transl Stroke Res. (2013) 4:368–74. doi: 10.1007/s12975-012-0247-9
26. Platt SR, Coates JR, Eifler DM, Edwards GL, Kent M, Bulsaraet KR. Effect of treatment with simvastatin and cyclosporine on neurotransmitter concentrations in cerebrospinal fluid after subarachnoid hemorrhage in dogs. Am J Vet Res. (2013) 74:1111–7. doi: 10.2460/ajvr.74.8.1111
27. Chang CZ, Wu SC, Kwan AL, Lin CL. Preconditioning with pitavastatin, an HMG-CoA reductase inhibitor, attenuates C-Jun N-terminal kinase activation in experimental subarachnoid hemorrhage-induced apoptosis. Acta Neurochir. (2015) 157:1031–41. doi: 10.1007/s00701-015-2399-3
28. Duan H, Zhang J, Li L, Bao S. Effect of simvastatin on proliferation of vascular smooth muscle cells during delayed cerebral vasospasm after subarachnoid hemorrhage. Turk Neurosurg. (2016) 26:538–44. doi: 10.5137/1019-5149.JTN.13650-15.1
29. Chen JH, Yang LK, Chen L, Wang YH, Wu Y, Jiang BJ, et al. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of AQP4 expression in rabbits. Int J Mol Med. (2016) 37:1059–66. doi: 10.3892/ijmm.2016.2506
30. Chen JH, Wu T, Yang LK, Chen L, Zhu J, Li PP, et al. Protective effects of atorvastatin on cerebral vessel autoregulation in an experimental rabbit model of subarachnoid hemorrhage. Mol Med Rep. (2018) 17:1651–9. doi: 10.3892/mmr.2017.8074
31. Wang B, Sun L, Tian Y, Li Z, Wei H, Wang D, et al. Effects of atorvastatin in the regulation of circulating EPCs and angiogenesis in traumatic brain injury in rats. J Neurol Sci. (2012) 319:117–23. doi: 10.1016/j.jns.2012.04.015
32. Konczalla J, Kashefiolasl S, Brawanski N, Lescher S, Senft C, Platz J, et al. Cerebral vasospasm and delayed cerebral infarctions in 225 patients with non-aneurysmal subarachnoid hemorrhage: the underestimated risk of Fisher 3 blood distribution. J Neurointerv Surg. (2016) 8:1247–52. doi: 10.1136/neurintsurg-2015-012153
Keywords: non-aneurysmal SAH, stroke, regenerative medicine, recovery, translational study, statin treatment
Citation: Kashefiolasl S, Wagner M, Brawanski N, Seifert V, Wanderer S, Andereggen L and Konczalla J (2021) Statins Improve Clinical Outcome After Non-aneurysmal Subarachnoid Hemorrhage: A Translational Insight From a Systematic Review of Experimental Studies. Front. Neurol. 12:620096. doi: 10.3389/fneur.2021.620096
Received: 21 October 2020; Accepted: 17 March 2021;
Published: 14 May 2021.
Edited by:
Emmanuel Pinteaux, The University of Manchester, United KingdomReviewed by:
Chia-Yi (Alex) Kuan, Emory University, United StatesCopyright © 2021 Kashefiolasl, Wagner, Brawanski, Seifert, Wanderer, Andereggen and Konczalla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sepide Kashefiolasl, c2VwaWRlLmthc2hlZmlAZ214LmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.