
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 20 May 2021
Sec. Neurocritical and Neurohospitalist Care
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.604686
 Dong-Seok Gwak1†
Dong-Seok Gwak1† Inyoung Chung2†
Inyoung Chung2† Baik-Kyun Kim3
Baik-Kyun Kim3 Sukyoon Lee4
Sukyoon Lee4 Han-Gil Jeong5
Han-Gil Jeong5 Yong Soo Kim6
Yong Soo Kim6 Heeyun Chae6
Heeyun Chae6 Chan-Young Park6
Chan-Young Park6 Moon-Ku Han6,7*
Moon-Ku Han6,7*Background: In general, disease severity has been found to be associated with abnormal chloride levels in critically ill patients, but hyperchloremia is associated with mixed results regarding patient-centered clinical outcomes. We aimed to investigate the impact of maximum serum chloride concentration on the clinical outcomes of critically ill patients with large hemispheric infarction (LHI).
Methods: We conducted a retrospective observational cohort study using prospective institutional neurocritical care registry data from 2013 to 2018. Patients with LHIs involving over two-thirds of middle cerebral artery territory, with or without infarction of other vascular territories, and a baseline National Institutes of Health Stroke Scale score of ≥13 were assessed. Those with a baseline creatinine clearance of <15 mL/min and required neurocritical care for <72 h were excluded. Primary outcome was in-hospital mortality. Secondary outcomes included 3-month mortality and acute kidney injury (AKI) occurrence. Outcomes were compared to different maximum serum chloride levels (5 mmol/L increases) during the entire hospitalization period using multivariable logistic regression analyses.
Results: Of 90 patients, 20 (22.2%) died in-hospital. Patients who died in-hospital had significantly higher maximum serum chloride levels than did those who survived up to hospital discharge (139.7 ± 8.1 vs. 119.1 ± 10.4 mmol/L; p < 0.001). After adjusting for age, sex, and Glasgow coma scale score, each 5-mmol/L increase in maximum serum chloride concentration was independently associated with an increased risk of in-hospital mortality (adjusted odds ratio (aOR), 4.34; 95% confidence interval [CI], 1.98–9.50; p < 0.001). Maximum serum chloride level was also an independent risk factor for 3-month mortality (aOR, 1.99 [per 5 mmol/L increase]; 95% CI, 1.42–2.79; p < 0.001) and AKI occurrence (aOR, 1.57 [per 5 mmol/L increase]; 95% CI, 1.18–2.08; p = 0.002).
Conclusions: High maximum serum chloride concentrations were associated with poor clinical outcomes in critically ill patients with LHI. This study highlights the importance of monitoring serum chloride levels and avoiding hyperchloremia in this patient population.
Large hemispheric infarction (LHI) is a life-threatening condition that affects the entirety or majority of the middle cerebral artery territory, with or without anterior and posterior cerebral artery involvement, and has a mortality rate of 40–80% in untreated LHI patients (1). Cerebral edema occurs in LHI patients, which often leads to increased intracranial pressure, transtentorial herniation, and neurological deterioration (2). Because of the propensity to cause or exacerbate brain edema by hypo-osmolar balanced crystalloids, normal saline (0.9% NaCl solution) is more frequently used and hypertonic saline is commonly used for the management of cerebral edema (3). However, large volume of both normal saline and hypertonic saline may raise the risk of hyperchloremia (4–7).
Hyperchloremia has been reported to be associated with high hospital mortality and poor outcome in general critically ill patients (8–13) and can lead to metabolic acidosis (14), has negative effects on renal blood flow (15), and is associated with acute kidney injury (AKI) development (16–18). Previous studies have shown that hyperchloremia is associated with high rates of in-hospital mortality in patients with subarachnoid hemorrhages (19), intracerebral hemorrhages (20), and traumatic brain injuries (21).
However, whether hyperchloremia may be associated with hospital mortality in patients with LHI remains largely unknown. In this study, we sought to evaluate the impacts of serum chloride level on the clinical outcomes of critically ill patients with LHI.
We retrospectively analyzed patient data from a prospective institutional neurocritical care registry. Among 5,272 consecutive ischemic stroke patients who were admitted to the Seoul National University Bundang Hospital within 7 days after symptom onset between March 2013 and June 2018, we identified patients who fulfilled the following inclusion criteria: (1) admission to a dedicated neurocritical care unit (n = 508), (2) 18 years of age or older (n = 507), (3) National Institutes of Health Stroke Scale (NIHSS) score ≥ 13 at arrival (n = 272), (4) LHI involving over two-thirds of middle cerebral artery territory, with or without infarction of other vascular territories (n = 121), and (5) pre-stroke modified Rankin Scale (mRS) score of 0–1 (n = 96). Patients were excluded from this study if they required neurocritical care for <72 h (n = 3) and had a creatinine clearance (estimated using the Cockcroft and Gault equation) of <15 mL/min or end-stage renal disease requiring dialysis before admission (n = 1). Those with an expected survival duration of <1 year (n = 2) were also excluded (Figure 1). The primary objective of this study was to determine whether the maximum serum chloride level over the entire duration of hospitalization was associated with in-hospital mortality. Our secondary objective was to assess the association between maximum serum chloride levels and 3-month mortality and AKI occurrence. Our study proposal was approved by the institutional review board (IRB approval number: B-1908/556-105). Informed consent was waived because of the retrospective nature of the study, and all study participants were fully de-identified.
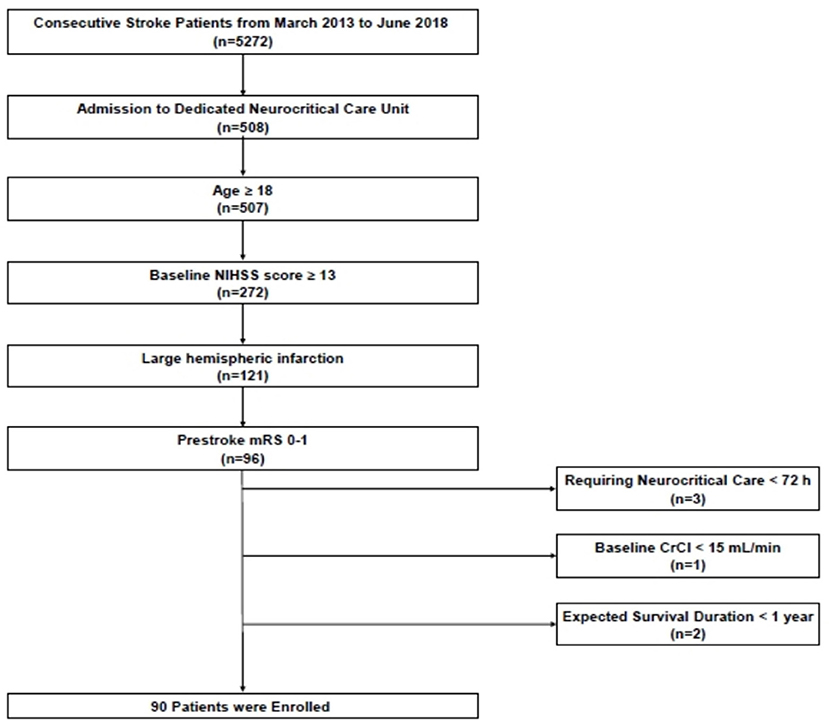
Figure 1. Flowchart of patient screening and enrollment. NIHSS, National Institutes of Health Stroke Scale.
Data on baseline demographic and clinical characteristics that may affect patients' clinical outcomes were obtained from the prospective institutional neurocritical care registry database or through the electronic chart review method. Data on the following variables were collected: age; height; weight; sex; pre-stroke mRS score; onset-to-arrival time; baseline NIHSS score; Glasgow Coma Scale (GCS) score with the worst value at the admission day of neurocritical care unit; systolic and diastolic blood pressure; oxygen requirement (PaO2/FiO2); previous history of stroke, hypertension, diabetes mellitus, hyperlipidemia, smoking, atrial fibrillation, and anemia; treatment variables patients received, including type of recanalization therapy; mannitol, hypertonic saline (11.7% NaCl), and its total dose administrated, fluid balance defined by fluid intake—output, nephrotoxic agents, antibiotics, contrast media; targeted temperature management (TTM), decompressive surgery, renal replacement therapy (RRT), and mechanical ventilation; pneumonia, length of stay in the neurocritical care unit, and duration of hospitalization; imaging variables including initial and maximum infarct volume evaluated by the ABC/2 method (22), and maximum midline shift measured at the level of septum pellucidum (23); and laboratory data, including baseline and maximum serum chloride, sodium, and creatinine levels, baseline and minimum serum bicarbonate levels, maximum base deficit, blood urea nitrogen levels, baseline creatinine clearance (assessed using the Cockcroft and Gault equation), AKI development based on Kidney Disease: Improving Global Outcome (KDIGO) criteria (24), hemoglobin levels, and baseline blood glucose level.
The primary outcome was in-hospital mortality. Secondary outcomes included 3-month mortality and occurrence of any AKI (KDIGO Stage ≥ 1) and moderate to severe AKI (KDIGO stage ≥ 2). AKI stage 1 represents a ≥ 0.3-mg/dl increase in serum creatinine levels over a 48-h period, a 1.5-fold to 2-fold increase from baseline serum creatinine levels, or a urine output of ≤ 0.5 ml/kg/h for more than 6 h. AKI stage 2 was defined as a 2-fold to 3-fold increase from baseline serum creatine levels or a urine output of ≤ 0.5 ml/kg/h for more than 12 h. AKI stage 3 represents a > 3-fold increase in serum creatinine levels from baseline (or a serum creatinine level of ≥ 4.0 mg/dl with an acute increment of at least 0.5 mg/dl), the need for initiating RRT, a urine output of ≤ 0.3 ml/kg/h for 24 h, or a 12-h period of anuria (24).
Bivariate analyses between patients who died in-hospital and those who survived up to hospital discharge were performed using Pearson's chi-square test, Fisher's exact method for categorical variables, Student's t-test, or the Mann–Whitney U-test for continuous variables, as appropriate. Data are expressed as number (%), mean ± standard deviation, or median (interquartile range [IQR]).
Next, we performed univariable logistic regression analyses to assess the association between the maximum serum chloride level during the entire period of hospitalization and in-hospital mortality. Because of the small sample size, we tested several multivariable logistic models with prespecified covariates. Model 1 adjusted for demographics (age and sex) and the well-known predictor of disease severity (GCS score). Model 2 adjusted for imaging variables regarding infarct and edema volumes (initial infarct volume, maximum infarct volume, and midline shift). Model 3 adjusted for well-known risk factors for mortality in critically ill patients (fluid balance and mechanical ventilation). Model 4 adjusted for covariates with a p < 0.1 in the bivariate analysis with the forward stepwise selection method. Model 5 adjusted for hyperchloremia, hypernatremia, age, sex, and GCS score. The cutoff values of hyperchloremia and hypernatremia were determined by the point maximizing Youden's index (sensitivity + specificity −1) in receiver operating characteristic curves (25). The associations of maximum chloride levels with 3-month mortality were tested with the same models. Those who had missing mortality data at 3 months (n = 2) were excluded from the corresponding analysis. Moreover, logistic regression analyses for determining the risk factors for AKI were performed using a forward stepwise selection method among imbalanced variables (p < 0.10) between the groups with and without AKI. A two-sided p < 0.05 was considered statistically significant. All statistical analyses were tested with SPSS version 25.0 for Windows.
A total of 90 LHI patients were enrolled in this study. Of these, 20 (22.2%) patients died in-hospital. Patients' baseline characteristics are shown in Table 1. Those who died during hospitalization had significantly lower GCS scores (3.0 [IQR 3.0–5.0] vs. 6.5 [3.0–9.0]; p = 0.002), higher systolic blood pressure (158.7 vs. 150.2; p = 0.048), and more hyperlipidemia (50.0 vs. 25.7%, p = 0.039) and received treatment via hypertonic saline (95.0 vs. 48.6%; p < 0.001), TTM (75.0 vs. 41.4%; p = 0.008), and mechanical ventilation (90.0 vs. 61.4%; p = 0.016) more frequently than the survivors.
The maximum serum chloride levels (139.7 vs. 119.1 mmol/L; p < 0.001), sodium levels (170.9 vs. 152.7 mmol/L; p < 0.001), and creatinine levels (2.90 vs. 1.20 mmol/L; p < 0.001) were significantly higher, and the minimum value of bicarbonate (15.3 vs. 19.4 mmol/L; p < 0.001) was significantly lower in those who died in-hospital (Table 2). However, baseline laboratory findings including serum chloride, sodium, bicarbonate, creatinine, and creatinine clearance levels were not significantly different between the two groups. AKI occurred significantly more frequently in patients who died in-hospital than in those who survived (90.0 vs. 30.0%; p < 0.001).
Since maximum serum creatinine level and RRT had collinearity with AKI occurrence, they were omitted from the multivariable logistic analysis. Minimum serum bicarbonate level was also excluded to avoid multi-collinearity with maximum base deficit. In the univariable logistic analysis, those who had high maximum serum chloride level were associated with high in-hospital mortality (odds ratio [OR] [per 5 mmol/L], 3.42; 95% confidence interval [CI], 1.87–6.26; p < 0.001; Table 3 and Supplementary Table 1). The association between maximum serum chloride level and in-hospital mortality remained significant throughout the multivariable logistic models 1–4. Additionally, GCS score in models 1 and 4, mechanical ventilation in model 3, and maximum base deficit in model 4 were independently associated with in-hospital mortality. Because of the multicollinearity between serum maximum chloride and sodium level (variance inflation factor of 18.14), these two variables were dichotomized in model 5 to assess the concomitant effect of hyperchloremia (>132.5 mmol/L) with hypernatremia (>162.5 mmol/L) on mortality. Both hyperchloremia and hypernatremia were significantly associated with in-hospital mortality and so was the GCS score.
Maximum serum chloride level was significantly related to 3-month mortality (OR, 2.01 [per 5 mmol/L]; 95% CI, 1.50–2.70; p < 0.001), even after adjusting for confounding variables in models 1–4 (Table 3 and Supplementary Table 2). In model 5, hyperchloremia, not concomitant hypernatremia, was independently associated with 3-month mortality. Further, high maximum serum chloride level was significantly associated with high risk of any AKI development (OR, 1.35 [per 5 mmol/L]; 95% CI, 1.12–1.64; p = 0.002) and remained significant after adjusting for confounders (aOR, 1.57 [per 5 mmol/L]; 95% CI, 1.18–2.08; p = 0.002; Table 4). Moreover, it was independently associated with the development of moderate to severe AKI (aOR, 1.98 [per 5 mmol/L]; 95% CI, 1.40–2.81; p < 0.001). TTM was also independently associated with the development of any AKI and moderate to severe AKI, and the maximum base deficit was significantly related to any AKI development.
In this study, we found that high maximum serum chloride concentration was associated with increased in-hospital mortality in critically ill LHI patients. Every 5-mmol/L increment in maximum chloride level increases the odds of in-hospital mortality risk by 3.42 (1.87–6.26), and the associations remained significant throughout all logistic models. The maximum serum chloride concentration was also significantly associated with 3-month mortality and AKI development.
Previous studies have reported the associations between hyperchloremia and AKI occurrence and mortality in various patient populations (9–13, 16–21). However, the majority of these studies were conducted with general critically ill populations, and their chloride levels were not as high as those in our study. One study including ischemic stroke and intracerebral hemorrhage patients has suggested a possible association between hyperchloremia and increases in serum chloride level within the first 72 h after admission and poor outcomes, but patients treated with hypertonic saline were excluded from the study (26). Moreover, due to the heterogeneity and varying characteristics of the study populations, it was not possible to generalize this study's results for LHI patients. In our study, we observed that high serum chloride levels during the entire hospitalization were associated with an increased risk of poor clinical outcomes in only ischemic stroke patients.
Several explanations regarding potential detrimental effects of hyperchloremia have been proposed. Hyperchloremic metabolic acidosis can depress myocardial function, resulting in reduced cardiac output and organ hypoperfusion (27). This may be a concern for LHI patients who require adequate cerebral perfusion pressure for preventing additional brain ischemia. Another study has shown that hyperchloremic metabolic acidosis during abdominal aortic aneurysm repair has a negative effect on hemostasis (28). Since the chances of hemorrhagic transformation remarkably increases with the large volume of infarctions and edemas (29, 30), caution may be necessary when administering chloride-liberal fluid to LHI patients. Hyperchloremia also causes renal vasoconstriction by inhibiting renin and angiotensin II secretion, leading to a decrease in glomerular filtration rate and AKI development (31).
Patients treated with TTM were also associated with in-hospital mortality and 3-month mortality, although the associations were not statistically significant after adjusting for confounding variables in logistic model 4. Furthermore, TTM was independently associated with AKI development. However, these results should be interpreted with caution. As treatment strategies were determined at the discretion of attending neurologists, patients expected to have larger maximum cerebral edema may have had a higher chance of receiving TTM. Indeed, patients treated with TTM had a lower GCS score, larger maximum infarct volume, larger maximum midline shift, and more received hyperosmolar therapy than did patients who did not receive TTM. Development of pneumonia was not significantly different between the two groups (Supplementary Table 3). Moreover, the hypothermic state may activate potential neuroprotective mechanisms and TTM has been suggested as one of the treatment options for reducing intracranial pressure due to acute ischemic injury (32–34); furthermore, it is not a traditional risk factor for AKI (35).
In our study cohort, the crude maximum sodium level, a well-known predictor of mortality in neurocritically ill patients, was associated with outcomes (36). When evaluating the association of sodium and chloride in combination with in-hospital mortality, only hyperchloremia was an independent risk factor for in-hospital mortality (Model 4). This does not mean that hypernatremia was not associated with outcome but rather that the estimated regression coefficient can be affected due to the study limitation that the maximum chloride and sodium levels were collinear. Indeed, concomitant hypernatremia with hyperchloremia was also related to in-hospital mortality in model 5. Thus, the results could be interpreted as demonstrating that the maximum chloride level was more strongly associated with in-hospital mortality than with the maximum sodium level in our cohort; external validation should be carried out in future studies.
Given the growing evidence of the deleterious effects of hyperchloremia, new strategies for avoiding chloride accumulation might be considered. Recent large randomized controlled trials demonstrated that chloride-restrictive intravenous fluid administration was associated with lower rates of a composite of new RRT, persistent renal dysfunction, and death from any cause than the use of a chloride-liberal strategy for the critically ill (37). However, it is uncertain whether these results apply to neurocritically ill patients, since the proportion of such patients in this trial was small (17.8%). Furthermore, as hypotonic fluids can exacerbate cerebral edemas through high osmotic pressure gradients between the intravascular and extravascular compartment, they are not traditionally recommended for patients with cerebral edema (1, 38, 39). Hyperosmolar therapy with low-chloride hypertonic solutions might represent another option, as 16.4% hypertonic NaCl/Na-Acetate was associated with a lower rate of AKI development than 23.4% NaCl for the management of cerebral edema in patients with subarachnoid hemorrhage (40). Further research is needed to confirm these treatment strategies.
Our study had several limitations. Due to the study's retrospective observational design, we could not assess all potentially relevant variables, prove causal relationships among variables, and exclude selection bias, especially the bias related to the choice of the type of fluid treatment administered. As such, it may be possible for patients with severe cerebral edema to be treated with more hypertonic saline. Furthermore, although all multivariable models showed significant associations between serum maximum chloride level and outcomes, all confounding variables may not be reliably tested due to the small sample size. Because of the multicollinearity of maximum sodium level with chloride level, the effect of concomitant hypernatremia on outcomes could not be fully examined. Moreover, we were also unable to report the exact cumulative amount of chloride burden after patients underwent fluid infusion.
In conclusion, maximum serum chloride concentration may have an impact on the clinical outcomes of critically ill patients with LHI, specifically in-hospital mortality rate, 3-month mortality rate, and AKI occurrence. Our study highlights the importance of monitoring serum chloride concentration and avoiding hyperchloremia in such patients. Further well-designed studies are required to explore the causal relationship between high chloride burden and clinical outcomes and chloride-restrictive strategies for fluid management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Seoul National University Bundang Hospital (IRB approval number: B-1908/556-105). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
D-SG and IC established the study protocol, analyzed and interpreted the data, and wrote the manuscript. B-KK, SYL, H-GJ, YK, HC, and C-YP interpreted the data and revised the manuscript for intellectual content. M-KH established the study idea, interpreted the data, drafted the manuscript, and made critical revisions in the manuscript with intellectual input. All the authors approved the final version of manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Special thanks are given to the physicians and neurocritical care team involved in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.604686/full#supplementary-material
1. Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American stroke association. Stroke. (2014) 45:1222–38. doi: 10.1161/01.str.0000441965.15164.d6
2. Heiss WD. The ischemic penumbra: how does tissue injury evolve? Ann N Y Acad Sci. (2012) 1268:26–34. doi: 10.1111/j.1749-6632.2012.06668.x
3. Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent 'pre-renal' acute kidney injury?. Kidney Int. (2014) 86:1096–105. doi: 10.1038/ki.2014.105
4. Young P, Bailey M, Beasley R, Henderson S, Mackle D, Mcarthur C, et al. Effect of a buffered crystalloid solution vs. saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. (2015) 314:1701–10. doi: 10.1001/jama.2015.12334
5. Kamel H, Navi BB, Nakagawa K, Hemphill JC III, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. (2011) 39:554–9. doi: 10.1097/CCM.0b013e318206b9be
6. Jones GM, Bode L, Riha H, Erdman MJ. Safety of continuous peripheral infusion of 3% sodium chloride solution in neurocritical care patients. Am J Crit Care. (2016) 26:37–42. doi: 10.4037/ajcc2017439
7. Roquilly A, Mahe PJ, Latte DD, Loutrel O, Champin P, Di Falco C, et al. Continuous controlled-infusion of hypertonic saline solution in traumatic brain-injured patients: a 9-year retrospective study. Crit Care. (2011) 15:R260. doi: 10.1186/cc10522
8. Van Regenmortel N, Verbrugghe W, Van Den Wyngaert T, Jorens PG. Impact of chloride and strong ion difference on ICU and hospital mortality in a mixed intensive care population. Ann Intensive Care. (2016) 6:91. doi: 10.1186/s13613-016-0193-x
9. Neyra JA, Canepa-Escaro F, Li X, Manllo J, Adams-Huet B, Yee J, et al. Association of hyperchloremia with hospital mortality in critically Ill septic patients. Crit Care Med. (2015) 43:1938–44. doi: 10.1097/CCM.0000000000001161
10. Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. (2014) 40:1897–905. doi: 10.1007/s00134-014-3505-3
11. Boniatti MM, Cardoso PR, Castilho RK, Vieira SR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. (2011) 26:175–9. doi: 10.1016/j.jcrc.2010.04.013
12. Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med. (2014) 42:1585–91. doi: 10.1097/CCM.0000000000000305
13. Lee JY, Hong TH, Lee KW, Jung MJ, Lee JG, Lee SH. Hyperchloremia is associated with 30-day mortality in major trauma patients: a retrospective observational study. Scand J Trauma Resusc Emerg Med. (2016) 24:117. doi: 10.1186/s13049-016-0311-7
14. Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. (2015) 102:24–36. doi: 10.1002/bjs.9651
15. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. (2012) 256:18–24. doi: 10.1097/SLA.0b013e318256be72
16. Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care. (2016) 20:315. doi: 10.1186/s13054-016-1499-7
17. Marttinen M, Wilkman E, Petaja L, Suojaranta-Ylinen R, Pettila V, Vaara ST. Association of plasma chloride values with acute kidney injury in the critically ill - a prospective observational study. Acta Anaesthesiologica Scandinavica. (2016) 60:790–799. doi: 10.1111/aas.12694
18. Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs. chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. (2012) 308:1566–1572. doi: 10.1001/jama.2012.13356
19. Sadan O, Singbartl K, Kandiah PA, Martin KS, Samuels OB. Hyperchloremia is associated with acute kidney injury in patients with subarachnoid hemorrhage. Crit Care Med. (2017) 45:1382–8. doi: 10.1097/CCM.0000000000002497
20. Riha HM, Erdman MJ, Vandigo JE, Kimmons LA, Goyal N, Davidson KE, et al. Impact of moderate hyperchloremia on clinical outcomes in intracerebral hemorrhage patients treated with continuous infusion hypertonic saline: a pilot study. Crit Care Med. (2017) 45:e947–53. doi: 10.1097/CCM.0000000000002522
21. Ditch KL, Flahive JM, West AM, Osgood ML, Muehlschlegel S. Hyperchloremia, not concomitant hypernatremia, independently predicts early mortality in critically ill moderate-severe traumatic brain injury patients. Neurocrit Care. (2020) 33:533–41. doi: 10.1007/s12028-020-00928-0
22. Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. (2009) 72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329
23. Jeon SB, Kwon SU, Park JC, Lee DH, Yun SC, Kim YJ, et al. Reduction of midline shift following decompressive hemicraniectomy for malignant middle cerebral artery infarction. J Stroke. (2016) 18:328–36. doi: 10.5853/jos.2016.00262
24. Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
25. Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
26. Huang K, Hu Y, Wu Y, Ji Z, Wang S, Lin Z, et al. Hyperchloremia is associated with poorer outcome in critically ill stroke patients. Front Neurol. (2018) 9:485. doi: 10.3389/fneur.2018.00485
27. Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth. (2008) 101:141–50. doi: 10.1093/bja/aen148
28. Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. (2001) 93:817–22. doi: 10.1097/00000539-200110000-00004
29. Wang BG, Yang N, Lin MA, Lu BX. Analysis of risk factors of hemorrhagic transformation after acute ischemic stroke: cerebral microbleeds do not correlate with hemorrhagic transformation. Cell Biochem Biophys. (2014) 70:135–42. doi: 10.1007/s12013-014-9869-8
30. Kerenyi L, Kardos L, Szasz J, Szatmari S, Bereczki D, Hegedus K, et al. Factors influencing hemorrhagic transformation in ischemic stroke: a clinicopathological comparison. Eur J Neurol. (2006) 13:1251–5. doi: 10.1111/j.1468-1331.2006.01489.x
31. Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. (1989) 256:F152–7. doi: 10.1152/ajprenal.1989.256.1.F152
32. Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. (1998) 29:2461–6. doi: 10.1161/01.STR.29.12.2461
33. Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke. (2007) 38:3084–94. doi: 10.1161/STROKEAHA.107.490193
34. Guluma KZ, Oh H, Yu SW, Meyer BC, Rapp K, Lyden PD. Effect of endovascular hypothermia on acute ischemic edema: morphometric analysis of the ICTuS trial. Neurocrit Care. (2008) 8:42–7. doi: 10.1007/s12028-007-9009-z
35. Susantitaphong P, Alfayez M, Cohen-Bucay A, Balk EM, Jaber BL. Therapeutic hypothermia and prevention of acute kidney injury: a meta-analysis of randomized controlled trials. Resuscitation. (2012) 83:159–67. doi: 10.1016/j.resuscitation.2011.09.023
36. Aiyagari V, Deibert E, Diringer MN. Hypernatremia in the neurologic intensive care unit: how high is too high? J Crit Care. (2006) 21:163–72. doi: 10.1016/j.jcrc.2005.10.002
37. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically Ill adults. N Engl J Med. (2018) 378:829–39. doi: 10.1056/NEJMoa1711584
38. Ertmer C, Van Aken H. Fluid therapy in patients with brain injury: what does physiology tell us? Crit Care. (2014) 18:119. doi: 10.1186/cc13764
39. Van Der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. (2016) 20:126. doi: 10.1186/s13054-016-1309-2
Keywords: chloride, critical care, cerebral infarction, mortality, acute kidney injury, brain edema
Citation: Gwak D-S, Chung I, Kim B-K, Lee SY, Jeong H-G, Kim YS, Chae H, Park C-Y and Han M-K (2021) High Chloride Burden and Clinical Outcomes in Critically Ill Patients With Large Hemispheric Infarction. Front. Neurol. 12:604686. doi: 10.3389/fneur.2021.604686
Received: 10 September 2020; Accepted: 14 April 2021;
Published: 20 May 2021.
Edited by:
Barak Bar, University of Wisconsin-Madison, United StatesReviewed by:
Minjee Kim, Northwestern University, United StatesCopyright © 2021 Gwak, Chung, Kim, Lee, Jeong, Kim, Chae, Park and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moon-Ku Han, bWtoYW5Ac251LmFjLmty
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.