- 1Neurology Department, Ibn Sina Hospital, Kuwait, Kuwait
- 2Faculty of Medicine, Kuwait University, Kuwait, Kuwait
- 3Division of Neurology, Department of Medicine, Amiri Hospital, Kuwait, Kuwait
- 4Department of Neurology, Cairo University, Cairo, Egypt
- 5Neuropsychiatry Department, Faculty of Medicine, Minia University, Minia, Egypt
Background: Chronic migraine (CM) affects 5.4% of the Kuwaiti population. It is associated with significant headache-related disability, psychiatric comorbidity and reduced quality of life. The aim of this study is to assess the efficacy of Onabotulinumtoxin A on psychological aspects of chronic migraine patients.
Methods: This prospective study over 36 months included chronic migraine patients in a tertiary headache center. Eligible patients met International Classification of Headache Disorders disorders-third edition, beta version (ICHD-III) revision criteria for chronic migraine. Patients with history of psychiatric or medical problems other than migraine disorders were excluded. Patients who received less than 4 injections cycles of Onabotulinumtoxin A were excluded. Identified patients received 155 units of Onabotulinumtoxin A quarterly according to the Phase III Research Evaluating Migraine Prophylaxis Therapy Trail (PREEMPT) protocol. Quality of life, the seven-item Generalized Anxiety Disorder (GAD-7) scores, the nine-item Patient Health Questionnaire (PHQ9), and the Pittsburgh Sleep Quality Index (PSQI) were collected before injection and at the end of the study. Mean comparison tests were performed using the independent sample t-test to assess the effects of Onabotulinumtoxin A on quality of life and comorbid symptoms of anxiety, depression, and quality of sleep.
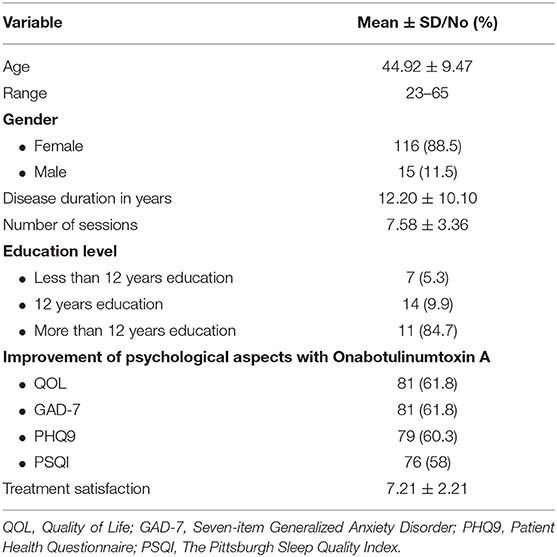
Results: The study identified 131 chronic migraine patients with a mean age of 44.92 years, mean disease duration of 12.20 years and a mean treatment sessions of 7.58. In their last visit, most of our sample showed improvement in quality of life (81%), GAD-7 (81%), PHQ9 (79%), and PSQ1 (76%). The mean score of patient satisfaction was 7.21. Onabotulinumtoxin A treatment for CM improved quality of life significantly (72.92 vs. 103.62; P < 0.0001). It was also associated with significant reduction in anxiety [GAD-7 (12.00 vs. 6.61; P < 0.0001)] and depression [PHQ-9 (17.91 vs. 12.52; P < 0.0001)] scores, as well as reduced difficulty in sleeping [PSQI (12.60 vs. 6.66; P < 0.0001)] at the last visit.
Conclusion: Prophylactic Onabotulinumtoxin A treatment for CM was associated with significant improvement of quality of life, reduction in symptoms of anxiety and depression, as well as improved symptoms of poor sleep.
Background
Migraine, a primary type of headache, is a common disabling disorder that can be divided into episodic and chronic migraine (1). The global prevalence of migraine is 14.4%, for females, and 9.8% for males (2). Global studies show that ~1.4–2.2% of the world's population have been diagnosed with chronic migraine (2). Comparatively, the 1-year prevalence of migraine in Kuwait was 23%, which is significantly higher (3). According to the latest Global Burden of Disease Study, headaches, including migraines, ranked second in the leading causes of disability, showing the large burden of the disorder (4). Migraine majorly affects the everyday lives of its sufferers, negatively affecting productivity, and even schooling (5). This burden is also increased by the comorbid psychiatric conditions that occur in association with it, including depression, anxiety, and sleep disorders. Large scale, population-based studies showed that patients with migraine are 2.2–4.0 times more likely to have depression (6). Moreover, in those who have episodic migraines, depression was associated with an increased risk of developing chronic migraine (7). In addition, the diagnosis of generalized anxiety disorder was significantly more prevalent in migraineurs than in those without migraine (8). Generalized anxiety disorder is often comorbid with major depressive disorder (MDD) and both of them increase the burden of migraine (8). Therefore, patients with migraine and psychiatric comorbidities may benefit from preventive therapy to reduce the attacks.
Sleep has also been demonstrated to have a clear relationship with migraine. Chronic migraine patients (CM) reported shorter nightly sleep periods than those with episodic migraine, and they were more likely to exhibit trouble initiating sleep, staying asleep, and sleep triggering headache. Also, complaints of those insomnia symptoms were at least three-fold greater in those patients than the general population (9). This shows the importance of management in migraine patients in the hope of decreasing this socioeconomic and medical burden. Therefore, patients with migraine and psychiatric comorbidities may benefit from preventive therapy to reduce the attacks.
Onabotulinumtoxin A was approved as a prophylactic therapy in adult patients with chronic migraine by the United State Food and Drug Administration (FDA) in 2010 (10). Onabotulinumtoxin A has been proven to reduce not only headache frequency but also the symptoms of depression, anxiety, as well as poor sleep quality in migraine patients (11).
While Onabotulinumtoxin A is known to cause muscle paralysis, the exact mechanism by which it relieves chronic migraine is not clear as of yet. However, some evidence suggests it does so by reducing local nerve sensitization by local inhibition of neuropeptide release, thus resulting in an indirect reduction of central sensitization (12). The neuropeptide calcitonin gene-related peptide (CGRP), which is known to be a major player in migraine, might be involved in regulating sleep maintenance at night (13, 14). Therefore, Onabotulinumtoxin A treatment may help to improve sleep in migraine patients by repressing CGRP from activated sensory neurons and by directly decreasing the amount of CGRP released from trigeminal neurons (15).
Although the socioeconomic burden of migraine in the Kuwaiti population was previously studied (3), no study to date has assessed the psychological aspects as mood and sleep suffering from migraine in patients in Kuwait. Therefore, this study aims to show the effect of treatment on improvement of mood and sleep in chronic migraine patients who received Onabotulinumtoxin A injections.
Method
This prospective, questionnaire-based study was conducted in a specialized headache clinic in a tertiary hospital in Kuwait. The study started on 1st October 2016 until 30 September 2019. The study population included both male and female patients aged 18–65 years who are diagnosed with CM with or without medication over use headache (MOH). Diagnoses were confirmed by headache specialist according to International Classification of Headache Disorders III (ICHD-III) (1). The study excluded patients with psychiatric disorders or chronic medical problems.
Patients with a diagnosis of another headache disorder, previous use of any Onabotulinumtoxin A for treatment of any headache (at any time) or for any other reason in the last year, pregnant or breast-feeding patients, and patients who did not complete at least three treatment cycles were excluded from the study. In addition, patients who had psychiatric comorbidities, history of previous psychiatric diseases, other migraine comorbidities, such as hypertension, obesity, or smoking, or those taking other medications from Onabotulinumtoxin A for migraine were not eligible for inclusion in the study. Study excluded patients who are on tricyclic antidepressant or selective serotonin reuptake inhibitors as prophylactic treatment for chronic migraine. Also, patients who received <4 injections cycles of Onabotulinumtoxin A were excluded.
Patients were injected intramuscularly with Onabotulinumtoxin A according to the PREEMPT protocol, 155 units divided into 31 injection sites around the head and neck, with sessions occurring in every 3 months (10).
A total of four questionnaires were used to interview patients in order to collect the data for the study. The questionnaires used in the study include The Quality of Life (QOL) (16), Generalized Anxiety Disorder-7 (GAD-7) (17) The Pittsburgh Sleep Quality Index (PSQI) (18) and The Patient Health Questionnaire-9 (PHQ-9) questionnaires (19).
Outcomes measured included change in PHQ-9, GAD-7, PSQI, and QOL from baseline. The patients were asked to answer above mentioned questionnaires twice; initially, prior to their first session in treatment, and the second after at the end of study if they completed at least four cycle of medications. The changes in the answers were compared to determine the effectiveness of the Onabotulinumtoxin A treatment.
Improvement by ≥1 in severity category in the PHQ-9 or GAD-7 score from baseline was considered to be a clinically meaningful improvement (20, 21). On the other hand, a reduction by ≥3-point in the total PSQI score was considered a clinically meaningful improvement (22). Treatment satisfaction was assessed by numeric scale, in which 0 meant no satisfaction at all and 10 means the patient was fully satisfied.
This study was carried out in accordance with the ethical guidelines of Kuwait Ministry of Health. The protocol was approved by the ethical committee of Ibn Sina hospital. All subjects gave a written informed consent in accordance with the Declaration of Helsinki. The study was performed in observation of the latest version of the declaration of Helsinki (23), and all data was anonymous and protected in accordance with the ethical guidelines of the Council for International Organizations of Medical Sciences (24).
Statistical analyses were performed with IBM SPSS Statistics 25.0 software for Mac (SPSS Inc., Chicago, IL, USA). All continuous variables were expressed as means, whereas categorical ones were expressed as proportions and percentages. Paired sample t-test was used to compare between PHQ-9, GAD-7, PSQI, and QOL before and after treatment with Onabotulinumtoxin A. Pearson's correlations were performed for metric and ordinal variables between adverse events and quality of life. A significant difference was set to be at p < 0.05.
Results
This cohort study included 139 CM patients. The eligible patients were131. Table 1 shows demographic and clinical characteristics of our cohort. The participants had mean age of 44.92 years, mean disease duration 12.20 years and a mean number of sessions 8. At the end of the study, most of the patients showed a significant improvement in the quality of life (81%), GAD-7 (81%), PHQ9 (79%), and PSQ1 (76%).
The mean score of patient satisfaction was 7.21. Onabotulinumtoxin A treatment for CM improved the quality of life significantly (72.92 vs. 103.62; P < 0.0001). It was also associated with significant reduction in the generalized anxiety scores (GAD-7) (12.0 vs. 6.6; P < 0.0001); depression scores (PHQ-9) (17.9 vs. 12.52; P < 0.0001) as well as sleep difficulty (PSQI) (12.6 vs. 6.7; P < 0.0001) at last visit (Table 2, Figure 1).
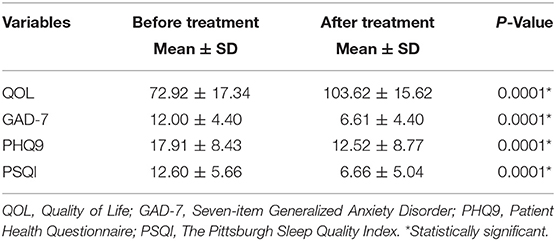
Table 2. Impact of Onabotulinumtoxin A on psychological aspect in chronic migraine patients (N = 113).
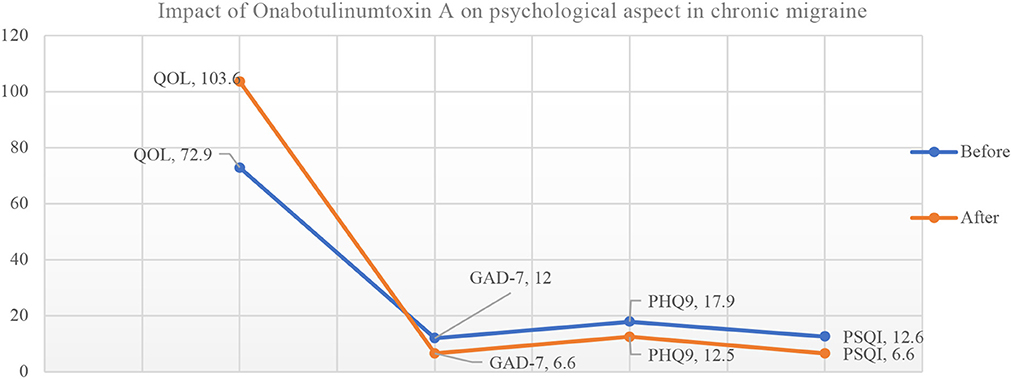
Figure 1. Impact of Onabotulinumtoxin A on psychological aspect in chronic migraine patients (N = 113). QOL, Quality of Life; GAD-7, Seven-item Generalized Anxiety Disorder; PHQ9, Patient Health Questionnaire; PSQI, The Pittsburgh Sleep Quality Index.
Our results did not conclude any significant difference among males and females regarding treatment satisfaction or improvement of psychological aspects (Table 3).
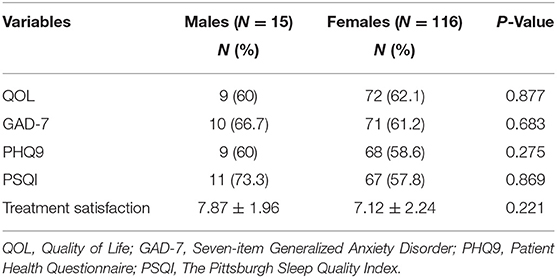
Table 3. Improvement of psychological aspects and treatment satisfaction with Onabotulinumtoxin A in both genders.
We reported significant correlation between satisfaction for treatment and improvement of psychological aspects. However, we did not record any significant correlation between age, gender, education years, disease duration, education, and improvement of psychological aspects (Table 4). This means that improvement of psychological aspects in CM patients lead to treatment satisfaction regardless age, gender, disease duration, or education.
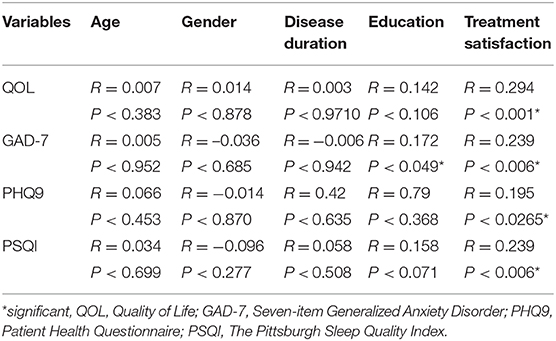
Table 4. Correlation between disease duration and treatment satisfaction or improvement of psychological aspects.
Few patients reported adverse effects related to Onabotulinumtoxin A treatment, which included neck pain and stiffness 3 (2.7%), lateral eyebrow elevation 2 (1.8%), ptosis 5 (4.44%). However, there was no significant correlation with these adverse events and quality of life after treatment (P < 0.62; r = 0.044). They were mild and irreversible.
Discussion
Migraine is a common neurological disease that can be very disabling on the patients and their families. It is associated with reduced quality of life and other psychiatric comorbidities (25). Migraine is primarily managed through three approaches: lifestyle and behavioral modification, acute therapy, and prophylactic therapy to reduce the frequency, severity, and the duration of the attacks, and thus reducing the risk of medication overuse (26). The FDA has approved of Onabotulinumtoxin A for the prophylactic treatment of chronic migraine in 2010 (10). Its efficacy, safety and tolerability, were proven by the largest migraine therapeutic trial PREEMPT (27).
The analyses conducted in this study concluded that the majority of participants with chronic migraine were aged 45 years old on average, which is consistent with previous studies reporting that people in this age group are most likely to be affected by chronic migraine (28, 29). It was also noted that the majority of the participants were satisfied with their treatment. In addition, the study showed a significant improvement in the quality of life of patients with chronic migraine, which can be attributed to the successful migraine relief. This finding is consistent with previously reported studies (27, 30).
The mechanism by which Onabotulinumtoxin A decreases the burden of the disease is not entirely known, but it is theorized that Onabotulinumtoxin A works on the central, and peripheral sensitization and injecting the trigeminally innervated craniofacial cervical region will blocks the peripheral sensitization by inhibiting the release of pain mediating peptides, specifically CGRP (31).
Also, of note, anxiety and mood disorders have been shown to be closely associated with migraine (32, 33). It is documented that generalized anxiety disorder is 2–10 times more likely to affect migraineurs than the general population (32, 34). These psychiatric comorbidities may influence the prognosis of migraine as they have been associated with a poorer quality of life, increased suicide risk, and they are risk factors for progression of migraine from episodic to chronic (19, 32, 34). These results showed that Onabotulinumtoxin A used for the treatment of migraine helped with depression and anxiety in these patients, as there was a significant improvement in the PHQ-9 and GAD-7 questionnaires for depression and anxiety, respectively. This result was consistent with previous published studies (11, 35) that found that Onabotulinumtoxin A is associated with a reduction in the frequency and impact of migraine attacks. The studies also concluded that Onabotulinumtoxin A led to an improvement in the symptoms of depression and anxiety, for which the exact mechanism is not entirely clear yet. However, this may be attributed to either the placebo effect on headache and mood disturbances, or it can be due to the reduction of headaches, with a secondary reduction in depression and anxiety (35). On the other hand, there is some evidence that Onabotulinumtoxin A also can help in depression and anxiety independent of its effect on the improvement of migraine (36, 37). Therefore, using Onabotulinumtoxin A in patients with chronic migraine and other psychiatric comorbidities may be an excellent choice. Studies have also noted the cyclical relationship between migraines, stress, and anxiety, which can mean that the use of Onabotulinumtoxin A can lead to a decrease in migraine episodes both directly and indirectly through its effect of reducing anxiety and depression (38).
Moreover, previous Studies have shown a significant association between migraine and sleep disturbances (39, 40), as sleep disruption is common among migraine patients and can even trigger a migraine attack (41). The results of this study showed a significant reduction in the last visit compared to the first in the PSQI, which was employed to measure the quality and patterns of sleep. This is similar to the finding in a previous cross-sectional case control study that found PSQI scores increased with the increase in the frequency of migraine (41).
Satisfaction for treatment which reflects treatment-related migraine improvement correlated significantly with improvement of the investigated psychological aspects.
Some of the participants reported some of the documented side effects resulting from Onabotulinumtoxin A treatment, which included neck pain and stiffness, lateral eyebrow elevation, ptosis, and these adverse events were mild and reversible. These side effects are well-documented in studies that looked to Onabotulinumtoxin A efficacy (28). However, the number of patients experiencing these side effects was minimal 10(8.8%), and these adverse events was mild and reversible and did not impact quality of life in our cohort.
Conclusion
This cohort study concluded that Onabotulinumtoxin A improves not only migraine symptoms in chronic migraine patients, but it also leads to significant improvements in psychological aspects in chronic migraine patients, leading to improvements in the quality of life, anxiety symptoms, as well as sleep disturbances. We believe that treating migraine patients should be targeting all these aspects since they are definitely synergistic to each other, they are bidirectional and treating one will improve the other and vice versa. Onabotulinumtoxin A may has the ability to do so without the need to use pharmacological agents.
Strengths
This is the first study to be conducted in Kuwait in order to study the association between Onabotulinumtoxin A use for treatment of chronic migraine and psychological aspects and symptoms in chronic migraine. Another strength is the appropriate sample size and prospective study for 3 years. Nevertheless, some limitations were identified.
Limitations
The limitations of the study are as follows: (1) the sample was chosen from the patient list in Ibn-Sina hospital, which may not completely represent all chronic migraine patients in Kuwait; (2) in addition, the study researched symptoms of psychiatric illnesses, whereas future studies can be dedicated to studying the effect of Onabotulinumtoxin A on patients with specific diagnoses concluded by psychiatrists through applying Diagnostic and Statistical Manual of Mental Disorders (DSM-5); (3) the study did not include a placebo group; (4) finally, the study did not report on the use of medication prior to initiation of Onabotulinumtoxin A treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethical committee of Ibn Sina hospital. The participants provided written informed consent to participate in this study.
Author Contributions
JA-H designed the study and reviewed the manuscript. HK, OA, FB, SM, and SA performed data collection and drafted the manuscript. DY and RA reviewed the manuscript, performed data collection and drafted the manuscript. SF performed statistical analysis, drafted, criticized, and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our appreciation to the study participants and to nurse Samia Omar and nurse Enayaat Khairy at headache clinic in Ibn Sina Hospital for actively supporting We would like to thank the study participants and the administrative department in Ibn Sina Hospital for actively participating in the study.
Abbreviations
CGRP, Calcitonin gene-related peptide; CM, Chronic migraine; DSM-5, Diagnostic and Statistical Manual of Mental Disorders; FDA, Food and Drug Administration; ICHD-III, International Classification of Headache Disorders III; MDD, Major depressive disorder; MOH, Medication over use headache; PHQ9, Patient Health Questionnaire; PREEMPT, Phase III Research Evaluating Migraine Prophylaxis Therapy Trail; QOL, Quality of Life; GAD-7, Seven-item Generalized Anxiety Disorder; PSQI, The Pittsburgh Sleep Quality Index.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
2. Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM, et al. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2018) 17:954–76. doi: 10.1016/S1474-4422(18)30322-3
3. Al-Hashel JY, Ahmed SF, Alroughani R. Burden of migraine in a Kuwaiti population: a door-to-door survey. J Headache Pain. (2017) 18:105. doi: 10.1186/s10194-017-0814-2
4. (IHME), I. F. H. M. A. E. Findings from the Global Burden of Disease Study. Seattle, WA: Institute for Health Metrics and Evaluation (2017).
5. Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Clin Proc. (2009) 84:422–35. doi: 10.4065/84.5.422
6. Hamelsk SW LR. (2006). Psychiatric comorbidity of migraine. J Headache Pain. 46:1327–33. doi: 10.1111/j.1526-4610.2006.00576.x
7. Ashina S, Serrano D, Lipton RB, Maizels M, Manack AN, Turkel CC, et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain. (2012) 13:615–24. doi: 10.1007/s10194-012-0479-9
8. Dindo LN, Recober A, Haddad R, Calarge CA. Comorbidity of migraine, major depressive disorder, and generalized anxiety disorder in adolescents and young adults. Int J Behav Med. (2017) 24:528–34. doi: 10.1007/s12529-016-9620-5
9. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache J Head Face Pain. (2005) 45:904–10. doi: 10.1111/j.1526-4610.2005.05159.x
10. Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache J Head Face Pain. (2010) 50:921–36. doi: 10.1111/j.1526-4610.2010.01678.x
11. Blumenfeld AM, Tepper SJ, Robbins LD, Adams AM, Buse DC, Orejudos A, et al. Effects of onabotulinumtoxinA treatment for chronic migraine on common comorbidities including depression and anxiety. J Neurol Neurosurg Psychiatry. (2019) 90:353–60. doi: 10.1136/jnnp-2018-319290
12. Aoki KR, Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat Disord. (2011) 17:S28–33. doi: 10.1016/j.parkreldis.2011.06.013
13. Villalón CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. (2009) 124:309–23. doi: 10.1016/j.pharmthera.2009.09.003
14. Kunst M, Hughes ME, Raccuglia D, Felix M, Li M, Barnett G, et al. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr Biol. (2014) 24:2652–64. doi: 10.1016/j.cub.2014.09.077
15. Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache J Head Face Pain. (2004) 44:35–43. doi: 10.1111/j.1526-4610.2004.04007.x
16. (WHOQOL), T. W. H. O. Q. O. L. A. Development and psychometric properties. Soc Sci Med. (1998) 46:1569–85. doi: 10.1016/S0277-9536(98)00009-4
17. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
18. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
19. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
20. McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord. (2010) 127:122–9. doi: 10.1016/j.jad.2010.04.030
21. Robinson E, Titov N, Andrews G, McIntyre K, Schwencke G, Solley K. Internet treatment for generalized anxiety disorder: a randomized controlled trial comparing clinician vs. technician assistance. PLoS One. (2010) 5:e010942. doi: 10.1371/journal.pone.0010942
22. Hughes CM, McCullough CA, Bradbury I, Boyde C, Hume D, Yuan J, et al. Acupuncture and reflexology for insomnia: a feasibility study. Acupunct Med. (2009) 27:163–8. doi: 10.1136/aim.2009.000760
23. World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. (2008). Available online at: http://www.wma.net/e/policy/b3.htm.
24. Foster CG. International ethical guidelines for biomedical research involving human subjects. J Med Ethics. (1994) 20:123–4. doi: 10.1136/jme.20.2.123
25. Cutrer FM, Bajwa ZH, Sabahat A. Pathophysiology, clinical manifestations, and diagnosis of migraine in adults. Up to Date. (2012).
26. Frampton JE, Silberstein S. OnabotulinumtoxinA: a review in the prevention of chronic migraine. Drugs. (2018) 78:589–600. doi: 10.1007/s40265-018-0894-6
27. Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. (2010) 30:793–803. doi: 10.1177/0333102410364676
28. Russo M, Manzoni GC, Taga A, Genovese A, Veronesi L, Pasquarella C, et al. The use of onabotulinum toxin A (Botox®) in the treatment of chronic migraine at the Parma Headache Centre: a prospective observational study. Neurol Sci. (2016) 37:1127–31. doi: 10.1007/s10072-016-2568-z
29. Buse DC, Manack AN, Fanning KM, Serrano D, Reed ML, Turkel CC, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache J Head Face Pain. (2012) 52:1456–70. doi: 10.1111/j.1526-4610.2012.02223.x
30. Boudreau G, Becker WJ, Graboski C, Ong-Lam M, Finkelstein I, Christie S, et al. Impact of OnabotulinumtoxinA on quality of life, health resource utilization, and work productivity in people with chronic migraine: interim results from a prospective, observational study (PREDICT) (P4.10-023). Neurology. (2019) 92(15 Suppl):4–10.
31. Whitcup SM, Turkel CC, DeGryse RE, Brin MF. Development of onabotulinumtoxinA for chronic migraine. Ann N Y Acad Sci. (2014) 1329:67–80. doi: 10.1111/nyas.12488
32. Peres MF, Mercante JP, Tobo PR, Kamei H, Bigal ME. Anxiety and depression symptoms and migraine: a symptom-based approach research. J Headache Pain. (2017) 18:37. doi: 10.1186/s10194-017-0742-1
33. Vgontzas A, Cui L, Merikangas KR. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache J Head Face Pain. (2008) 48:1451–9. doi: 10.1111/j.1526-4610.2008.01175.x
34. Minen MT, De Dhaem OB, Van Diest AK, Powers S, Schwedt TJ, Lipton R, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. (2016) 87:741–9. doi: 10.1136/jnnp-2015-312233
35. Boudreau GP, Grosberg BM, Mcallister PJ, Lipton RB, Buse DC. Prophylactic onabotulinumtoxinA in patients with chronic migraine and comorbid depression: an open-label, multicenter, pilot study of efficacy, safety and effect on headache-related disability, depression, and anxiety. Int J Gen Med. (2015) 8:79. doi: 10.2147/IJGM.S70456
36. Brin MF, Durgam S, Lum A, James L, Liu J, Thase ME, et al. OnabotulinumtoxinA for the treatment of major depressive disorder: a phase 2 randomized, double-blind, placebo-controlled trial in adult females. Int Clin Psychopharmacol. (2020) 35:19–28. doi: 10.1097/YIC.0000000000000290
37. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. (2018) 20:18r02298. doi: 10.4088/PCC.18r02298
38. Sauro, K. M., and Becker WJ. The stress and migraine interaction. Headache: J Head Face Pain. (2009) 49:1378–86. doi: 10.1111/j.1526-4610.2009.01486.x
39. Ødegård SS, Sand T, Engstrøm M, Stovner LJ, Zwart JA, Hagen K. The long-term effect of insomnia on primary headaches: A prospective population-based cohort study (HUNT-2 and HUNT-3). Headache J Head Face Pain. (2011) 51:570–80. doi: 10.1111/j.1526-4610.2011.01859.x
40. Kim SJ, Han KT, Jang SY, Yoo KB, Kim SJ. The association between migraine and types of sleep disorder. Int J Environ Res Public Health. (2018) 15:2648. doi: 10.3390/ijerph15122648
Keywords: Onabotulinumtoxin A, chronic migraine, depression, anxiety, sleep disturbance
Citation: Al-Hashel JY, Kh Ashkanani H, Almutairi O, Bokubar FA, Mubarak S, Alwazzan S, Alroughani R, Youssry D and Farouk Ahmed S (2021) Onabotulinumtoxin A Improves Psychological Aspects in Chronic Migraine Patients. Front. Neurol. 11:633355. doi: 10.3389/fneur.2020.633355
Received: 25 November 2020; Accepted: 23 December 2020;
Published: 27 January 2021.
Edited by:
Sabina Cevoli, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyReviewed by:
Andrea Negro, Sapienza University of Rome, ItalySimona Guerzoni, University of Modena and Reggio Emilia, Italy
Copyright © 2021 Al-Hashel, Kh Ashkanani, Almutairi, Bokubar, Mubarak, Alwazzan, Alroughani, Youssry and Farouk Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jasem Youssef Al-Hashel, ZHJhbGhhc2hlbCYjeDAwMDQwO2hvdG1haWwuY29t; amFzZW1rdW1zYSYjeDAwMDQwO2hvdG1haWwuY29t; Samar Farouk Ahmed, c2FtZXJlbHNoYXliJiN4MDAwNDA7aG90bWFpbC5jb20=; c2FtYXIuZmFyb3VrJiN4MDAwNDA7bWluaWEuZWR1LmVn
 Jasem Youssef Al-Hashel
Jasem Youssef Al-Hashel Hasan Kh Ashkanani2
Hasan Kh Ashkanani2 Raed Alroughani
Raed Alroughani Doaa Youssry
Doaa Youssry