- 1Scientific Institute for Research, Hospitalization and Healthcare (IRCCS) Mondino Foundation, Pavia, Italy
- 2Faculty of Law, Giustino Fortunato University, Benevento, Italy
- 3Department of Electrical, Computer, and Biomedical Engineering, University of Pavia, Pavia, Italy
- 4Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
The COVID-19 pandemic is a global health problem that is radically transforming public and private healthcare organizations around the world, negatively affecting the rehabilitative treatments of non-COVID pathologies as well. In this situation, it becomes crucial to be able to guarantee the continuity of care also to all those patients with neurodegenerative diseases unable to reach healthcare services. Remote communication technologies are gaining momentum as potentially effective options to support health care interventions—including cognitive rehabilitation—while patients can stay safely at home. In this context, we are implementing HomeCoRe (i.e., Home Cognitive Rehabilitation software) in order to offer an innovative approach and a valid support for home-based cognitive rehabilitation in neurodegenerative diseases, such as mild cognitive impairment and early dementia. HomeCoRe has been developed within a research project between engineers and clinicians in order to obtain a usable and safe cognitive rehabilitation tool. This software has multiple advantages for patients and therapists over traditional approaches, as shown in its use in hospital settings. HomeCoRe could then represent an opportunity for accessing cognitive rehabilitation in all those situations where patients and therapists are not in the same location due to particular restrictions, such as COVID-19 pandemic.
Introduction
With the rise in life expectancy during the last decades, we are witnessing a steady increase in the number of older adults in the total population with a high risk of developing neurodegenerative diseases (1). In particular, among these, dementia represents one of the major health problems in older adults, with progressive deterioration of cognition, daily functioning, and behavior that together lead to disability. This is further exacerbated by the existing link among cognitive decline, hospitalization, and mortality, resulting in a considerable challenge to patients, caregivers, and the health system in term of resources allocation (2). The transitional phase between normal and pathological cognitive aging is a clinical condition called Mild Cognitive Impairment (MCI), which represents a risk factor for the development of dementia (3). Although not all MCI patients progress to dementia, interventions at this pre-dementia stage may be able to reduce/slow down the deterioration along the continuum of MCI and dementia (4).
Because of the limited effectiveness of pharmacological therapies, attempts have been made to provide identification of other factors in patients' care that may delay the onset and slow progression of cognitive decline in MCI. In particular, non-pharmacological interventions have received increasing attention in recent years (5). Particularly, there is evidence that cognitive training is an effective intervention strategy in improving or at least maintaining cognitive level in MCI patients, thus slowing the progression to dementia (6, 7). Cognitive training and enhancement activities can indeed activate brain compensation mechanisms to tackle the physiological and pathological neuro-degeneration processes (8). Traditional cognitive training includes paper-and-pencil exercises usually administered in hospital settings and, less frequently, at patients' homes (9). Since this kind of intervention has some limitations—e.g., time, costs, and patients' accessibility, to name a few—their provision outside the clinical setting is often reduced (10, 11). In recent years, the development of Information and Communication Technologies (ICT) has kindled interest in alternative rehabilitative approaches. In particular, computer-based cognitive training allows one to overcome the limits of traditional paper-and-pencil techniques providing patient-tailored interventions that can be easily delivered not only in-person but also remotely at patients' homes (12). It means that they could simplify the therapist's work in terms of the planning, design, and management of the cognitive intervention also outside from the clinical setting.
To date, unprecedented new challenges to patients' care have been determined by the COVID-19 pandemic, including difficulties accessing routine treatments, such as cognitive rehabilitation, for individuals with neurodegenerative diseases. Hence, in parallel to the increase in the number of studies that claim for ICTs implementation in patient management, their effective integration in the routine clinical practice is still limited (13). The aim of this perspective article is to explore current evidence-based recommendations on the efficacy of ICT-based cognitive rehabilitation to achieve/continue adequate cognitive stimulation in the current pandemic. In this context, it is also offered a perspective about an innovative approach and a valid support for home-based cognitive rehabilitation in neurodegenerative diseases, which is HomeCoRe (i.e., Home Cognitive Rehabilitation software).
Telemedicine and Telerehabilitation
Telemedicine is defined as an interface in a virtual patient-clinician relationship to provide primary and secondary care by ICT (14). It is not intended to replace the healthcare model based on face-to-face interaction, but rather it is its declination varying according to patients' needs and characteristics (15). Telemedicine could be useful in the management of chronic diseases having high social impact and issues related to continuous long-term care, including diseases related to aging, such as dementia and other neurodegenerative disorders (14).
In particular, telerehabilitation (TR) is a young telemedicine subfield that could be defined as the set of instruments and protocols aimed at providing rehabilitation at a distance (16). Allowing remote delivery of different rehabilitation services in different medical conditions, TR provides benefits for the healthcare system and patients in terms of cost-effectiveness and feasibility for large-scale implementations. TR can use different types of technologies, such as sensor-based technology, tele/video-conference, specific ad hoc development software, or virtual reality (17).
Narrowing down the field to neurology, the main pathology treated by TR is stroke followed by traumatic brain injury, multiple sclerosis, and Parkinson's disease (18). For instance, some evidence suggests that physical and speech therapy delivered by TR to post-stroke patients is no worse than conventional in-person interventions in terms of reliability and effectiveness (19, 20). Even if motor and speech/voice impairments have been the main targets of TR (18), the interest for the treatment of other disabilities, such as the cognitive deficit following acquired neurological or neurodegenerative diseases, is growing steadily (21). In this field, the cognitive TR literature is more recent and mostly focuses on treating cognitive impairment in patients with stroke (22), multiple sclerosis (23), and brain tumors (24, 25).
Cognitive Telerehabilitation in Neurodegenerative Diseases
So far, few studies have been conducted to assess feasibility and efficacy of cognitive TR in older people with neurodegenerative diseases, such as MCI, Alzheimer's disease, and frontotemporal dementia. With the growing interest in this field, many study protocols have recently proliferated in the literature [e.g., (26)]. Only two systematic reviews (27, 28) are available on this topic. Cognitive TR has comparable effects in terms of efficacy, validity, and reliability to conventional in-person rehabilitation. However, as reported by Maresca and colleagues (28), most studies are characterized by small samples and lack of standardized procedures, aims, and targets. Accordingly, further randomized controlled trials (RCT) are strongly needed to improve our knowledge of how to use home-based cognitive TR effectively to delay the progression of cognitive impairment in people with MCI and dementia.
This necessity is further supported by the fact that some concerns have slowed the integration of cognitive TR into clinical practice (29, 30), but the existing literature gives some recommendations to overcome them.
First, the loss of human contact with the clinician and the limited flexibility in the adoption of devices most appropriate for patients' differing needs could hinder adherence to TR (17). Similarly, people with advanced age or cognitive deficit might have poor computer skills and difficulties managing technological devices on their own (31). Furthermore, patients' characteristics, such as hearing and vision impairments and the level and type of cognitive impairment may influence the number of post-rehabilitation benefits. All these factors could in fact be an important cause of distraction, especially for older people who may have little or no experience or confidence using technology (32). Hence, platforms should be developed in order to be accessible and user-friendly; duration and frequency of rehabilitation activities should be tailored according to patients' characteristics (33); therapists should monitor adherence and performance of each session remotely during the whole period of treatment (34). In any case, there is evidence that cognitive TR is a valuable and well-accepted methodology, and comparable effects have been found between TR and in-hospital treatment in terms of global cognitive performance in patients with early phases of cognitive deterioration (35).
Second, even if caregivers are supportive and facilitate adherence to TR in daily routines (36), it is important to avoid their excessive involvement to limit the burden of the approach and to prevent thwarting the benefits of the treatment itself. Furthermore, patients without a compliant caregiver could be excluded by the use of TR, representing a selection bias for this kind of intervention (37). However, there is evidence also about the possibility of using telemedicine devices in MCI patients living alone. In particular, it seems that in this case patients' compliance depends on the level of monitoring he/she remotely receives (38). In addition, it is important to consider that easy-access TR tools can produce benefits (e.g., autonomy, mood, self-efficacy, quality of life, etc.) in patients, with consequent positive effects also for caregivers (39).
With these considerations in mind, TR constitutes a unique opportunity in the field of cognitive rehabilitation. It indeed represents a replacement for in-person treatment or its continuation, providing equitable access to care for patients with neurodegenerative diseases (40, 41). Such an opportunity could be useful not only for older patients with dementia or physical disabilities, but also for those presenting pre-dementia symptoms while of working age or geographically remote. More generally, TR could have a pivotal role in the clinical practice in all those situations where patients and therapists cannot be in the same location, due to patients' requirements or, as in the case of the COVID-19 pandemic, because of particular emergencies.
Cognitive Telerehabilitation During Covid-19 Pandemic
The COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus, is a global health problem that has radically transformed public and private healthcare organizations around the world (42). This enduring health emergency, and the consequent adaptation of healthcare facilities, negatively influences the rehabilitative treatments of non-COVID pathologies, with an impact on the quality of life of patients (especially those with cognitive symptoms) and their families. In particular, social isolation, a long confinement period, and personal experiences combined with pre-existing diseases may play an important role in exacerbating cognitive decline and dementia (43). As an urgent response to provide continuity of care and social connectedness during the COVID-19 pandemic, new alternative options of cognitive rehabilitation are needed. To this end, remote communication technologies are increasingly considered as potentially effective options to support healthcare interventions, among which is cognitive rehabilitation, directly at the patient's home, reducing risks of possible infections (44–46). Aging per se is, in fact, associated with vulnerabilities of a physical, psychosocial, and environmental nature (47), determining more comorbidities and hospitalizations and, as a consequence, an increasing chance of being infected with COVID-19 (48). Such a susceptibility to morbidity and mortality from COVID-19 becomes more pronounced in those older adults with dementia (49). Hence, rehabilitation via remote communication technologies may represent a viable alternative tool to access care while reducing the risk of COVID-19 infection and avoiding unnecessary travel and discomfort to the patient and other family members (50).
Within this framework, cognitive TR may be viewed as a valid recovery tool (51) deriving from the reshape of cognitive rehabilitation with the use of technologies (52). Hence, based on these promising results and forced by the COVID-19 pandemic contingency, new studies and a larger diffusion of cognitive TR approaches are expected (53–55). To date, most efforts have been devoted to using telemedicine/telerehabilitation to address patients' recovery after COVID-19, which is very important in order to monitor and manage resulting deficits (56–61). For instance, Salawu and colleagues (60) have proposed a multidisciplinary TR program for patients with COVID-19 discharged from hospitals with residual rehabilitation needs. However, telemedicine and telerehabilitation should be implemented also in non-COVID patients in various settings of neurological care (36, 62, 63). From this perspective, Motolese and colleagues (36) explored the feasibility of a smartphone application for monitoring motor and cognitive performance of non-demented Parkinson's disease outpatients during the lockdown. Ramalho and colleagues (64) proposed a protocol of telemental healthcare to be applied to populations with different levels of needs, including older adults in need of constant home-based assistance. Again, in the field of pathological aging, other recommendations pertain to the management of behavioral and psychological symptoms of dementia or long-term care of older adults living in nursing homes via telemedicine (65). To the best of our knowledge, no experience has been published on the use of TR in older adults with cognitive impairment during COVID-19, even if strongly recommended (53–55).
A Perspective for Future Cognitive Rehabilitation: HomeCoRe
During the past years, we have implemented and used a computer-supported cognitive training program (Cognitive Rehabilitation—CoRe—software) for in-person sessions in the hospital setting (66, 67). CoRe has been developed within a research project between engineers and clinicians. We reported that CoRe was safe and effective with respect to cognition in inpatients with Parkinson Disease-Mild Cognitive Impairment (68, 69) and also in older adults with other forms of early cognitive impairment (70). Following these encouraging results observed in the hospital setting, we recently developed a TR version of CoRe that allows the provision of treatment at patients' homes: HomeCoRe (71).
HomeCoRe is a patient-tailored intervention aimed at stimulating several cognitive abilities (e.g., logical-executive functions, attention/processing speed, working memory, and episodic memory) through a series of sessions of 2D exercises planned remotely for multiple advantages for therapists and patients. It is timesaving, ready to use, and able to set exercises for each training session automatically. Exercises take place in an adaptive mode. In particular, during their dynamic generation, the individual patients' performance data (accuracy and number of aids required) are analyzed in order to set the appropriate difficulty level. Furthermore, for each exercise and each level, thresholds are defined so as to allow difficulty levels to be progressively increased. In addition, the system calculates an “overall weighted score (WS),” taking into account the correctness of the answers, the execution time, and the difficulty of the exercises. The WS informs the therapist about each patient's performance in a single value. Hence, WS represents a useful and advantageous index that can be used to assess and monitor both the overall outcome of a training session and the global trend of the rehabilitation (Figure 1).
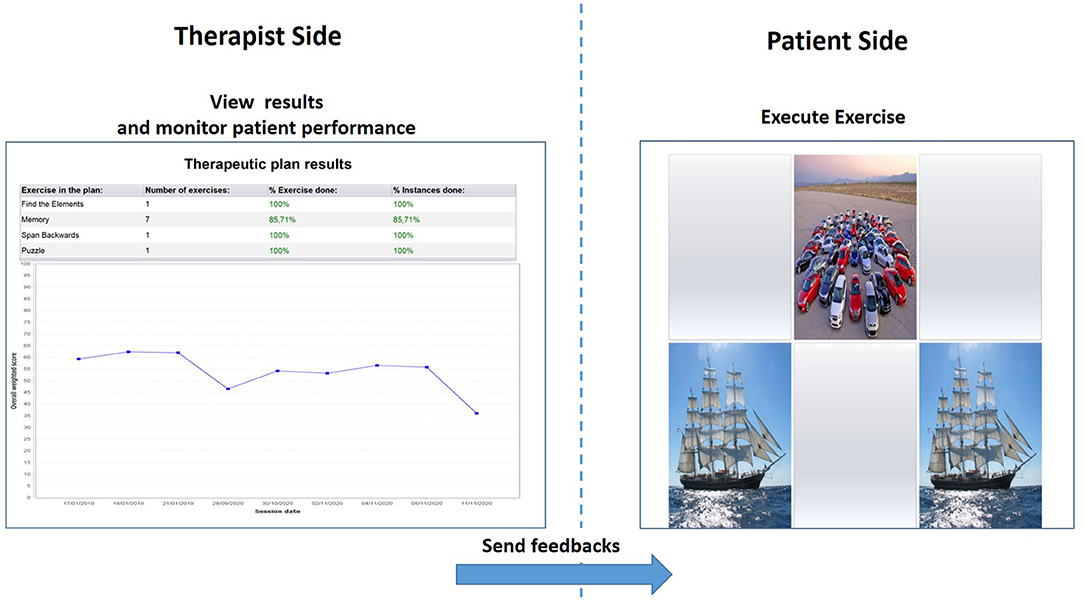
Figure 1. Therapist interface for monitoring results and patient performance in terms of overall Weighted Score (left) and patient interface for the execution of the memory exercise (right).
The HomeCoRe architecture includes two main components, the therapist side and the patient/caregiver side, as well as the communication channels between them. The therapist side of the interface allows the remote setting and monitoring of all requirements of the treatment plan (e.g., frequency and duration of the plan, types of exercise, and difficulty level) (see Figure 2). The patients/caregiver side of the interface is very simple to use, and it allows for viewing and executing the exercises of the day and communicating with the therapist (see Figure 2).
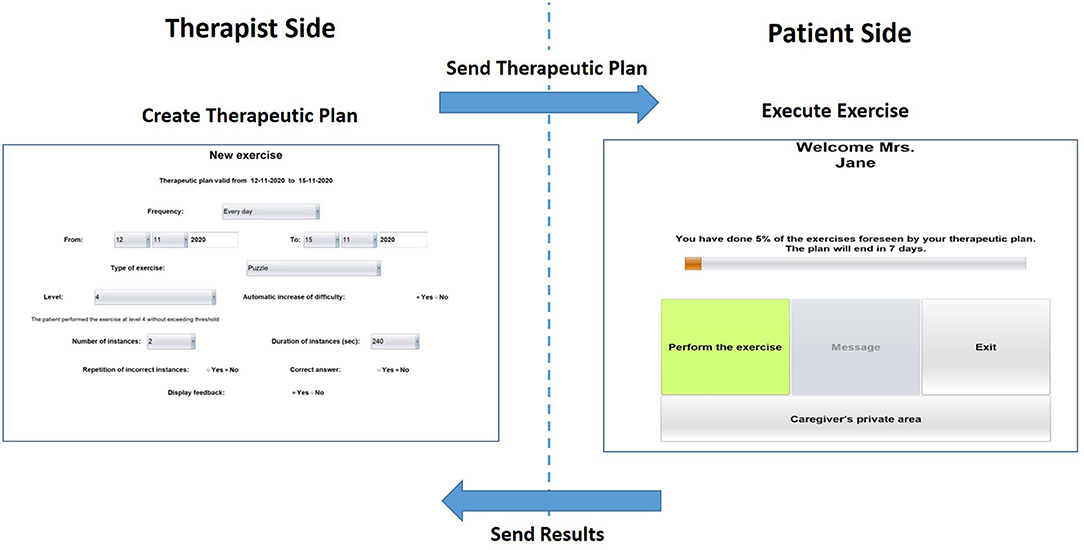
Figure 2. Home page of the therapist-side of the interface for setting the requirements for the exercise plan (left) and home page of the patient/caregiver-side of the interface (right).
In a recent work (71), we interviewed and surveyed inpatients to investigate their willingness to continue rehabilitation at home by using HomeCoRe after discharge. Caregivers were also interviewed, due to their role in both supporting and motivating patients. The survey results showed that most of both patient participants and caregivers appreciated HomeCoRe and intended to have a further home commitment. Subsequently, we tested the functionality and usability of HomeCoRe by using in-hospital workstations that simulated home sessions. Currently, we are carrying out a pilot study on a small sample of patients in the early stage of cognitive deterioration testing HomeCoRe directly at patients' homes. This will allow evaluating both patients' and caregivers' experience (e.g., compliance, benefits) and the cognitive effects of HomeCoRe rehabilitation. The output of this pilot study will inform a randomized clinical trial to explore the cost-effectiveness of cognitive telerehabilitation via HomeCoRe compared with in-person cognitive rehabilitation in patients with neurodegenerative diseases.
HomeCoRe promises to qualify as an innovative approach and a valid support for cognitive rehabilitation in neurodegenerative diseases. In addition, as a TR tool, HomeCoRe will allow extending the duration of the rehabilitation treatment of inpatients beyond the hospital discharge, which often coincides with treatment interruption, due to the scarcity of healthcare personnel for homecare. It also offers a unique opportunity to deliver cognitive rehabilitation to people who live in remote areas or who cannot reach healthcare services due to physical impairments or particular restrictions, such as the COVID-19 pandemic.
Conclusion
Due to the progressive aging of our population, the number of people with MCI or dementia is expected to grow consistently, with a social impact and economic burden on the healthcare system. Therefore, the World Health Organization (WHO) stresses taking global action against cognitive decline and dementia, encouraging governments worldwide to focus on prevention and to improve healthcare services. In addition, in line with the new health and social order that has been determined since the COVID-19 pandemic, it is crucial to offer a cognitive rehabilitation modality that can be used directly at home, in a condition of distance and safety for both family members and the patient her/himself. To this end, remote communication technologies are increasingly regarded as potentially effective options—with the appropriate recommendations (29, 30)—to support cognitive rehabilitation (53–55). In this framework, HomeCoRe is software for cognitive rehabilitation in neurodegenerative diseases that could be incorporated into clinical routine protocols as a complementary non-pharmacological therapy to support the continuum of care from the hospital to the patient's home.
In conclusion, the COVID-19 pandemic has determined new chances to embrace technology allowing people to maintain their connection with the outside world during isolation (72, 73). Such opportunities can also be extended to the delivery of care for neurodegenerative diseases, producing a technological evolution in the healthcare system (74–76) and dementia practice (53–55, 77), in the coming years.
Data Availability Statement
The original contributions generated in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by San Matteo Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SBe and SBo have conceived the work. All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication.
Funding
This project was funded by the Current Research Fund of the Italian Ministry of Health at the National Neurological Institute C. Mondino Foundation (Ricerca Corrente 2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Stenberg K, Hanssen O, Edejer TT-T, Bertram M, Brindley C, Meshreky A, et al. Financing transformative health systems towards achievement of the health sustainable development goals: a model for projected resource needs in 67 low-income and middle-income countries. Lancet Glob Heal. (2017) 5:e875–87. doi: 10.1016/S2214-109X(17)30263-2
2. Olazarán J, Muñiz R, Reisberg B, Peña-Casanova J, del Ser T, Cruz-Jentoft AJ, et al. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology. (2004) 63:2348–53. doi: 10.1212/01.wnl.0000147478.03911.28
3. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. (1999) 56:303–8. doi: 10.1001/archneur.56.3.303
4. Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. (2014) 30:421–42. doi: 10.1016/j.cger.2014.04.001
5. Karakaya T, Fußer F, Schröder J, Pantel J. Pharmacological treatment of mild cognitive impairment as a prodromal syndrome of Alzheimer's disease. Curr Neuropharmacol. (2013) 11:102–8. doi: 10.2174/157015913804999487
6. Faucounau V, Wu YH, Boulay M, De Rotrou J, Rigaud AS. Cognitive intervention programmes on patients affected by mild cognitive impairment: a promising intervention tool for MCI? J Nutr Health Aging. (2010) 14:31–5. doi: 10.1007/s12603-010-0006-0
7. Mowszowski L, Batchelor J, Naismith SL. Early intervention for cognitive decline: can cognitive training be used as a selective prevention technique? Int Psychogeriatrics. (2010) 22:537–48. doi: 10.1017/S1041610209991748
8. Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. (2011) 134:1591–609. doi: 10.1093/brain/awr039
9. Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. (2003) 4:CD003260. doi: 10.1002/14651858.CD003260
10. García-Casal JA, Loizeau A, Csipke E, Franco-Martín M, Perea-Bartolomé MV, Orrell M. Computer-based cognitive interventions for people living with dementia: a systematic literature review and meta-analysis. Aging Ment Health. (2017) 21:454–67. doi: 10.1080/13607863.2015.1132677
11. Zokaei N, MacKellar C, Cepukaityte G, Patai EZ, Nobre AC. Cognitive training in the elderly: bottlenecks and new avenues. J Cogn Neurosci. (2017) 29:1473–82. doi: 10.1162/jocn_a_01080
12. Irazoki E, Contreras-Somoza LM, Toribio-Guzmán JM, Jenaro-Río C, van der Roest H, Franco-Martín MA. Technologies for cognitive training and cognitive rehabilitation for people with mild cognitive impairment and dementia. A systematic review. Front Psychol. (2020) 11:648. doi: 10.3389/fpsyg.2020.00648
14. Chirra M, Marsili L, Wattley L, Sokol LL, Keeling E, Maule S, et al. Telemedicine in neurological disorders: opportunities and challenges. Telemed e-Health. (2019) 25:541–50. doi: 10.1089/tmj.2018.0101
15. Nesbitt TS, Hilty DM, Kuenneth CA, Siefkin A. Development of a telemedicine program: a review of 1,000 videoconferencing consultations. West J Med. (2000) 173:169–74. doi: 10.1136/ewjm.173.3.169-a
16. Zampolini M, Todeschini E, Bernabeu Guitart M, Hermens H, Ilsbroukx S, Macellari V, et al. Tele-rehabilitation: present and future. Ann Ist Super Sanita. (2008) 44:125–34.
17. Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol. (2017) 4:e7. doi: 10.2196/rehab.7511
18. Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: a literature overview of technologies and clinical applications. NeuroRehabilitation. (2010) 27:287–304. doi: 10.3233/NRE-2010-0612
19. Cherney LR, van Vuuren S. Telerehabilitation, virtual therapists, and acquired neurologic speech and language disorders. Semin Speech Lang. (2012) 33:243–57. doi: 10.1055/s-0032-1320044
20. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. (2009) 31:427–47. doi: 10.1080/09638280802062553
21. Caltagirone C, Zannino GD. Telecommunications technology in cognitive rehabilitation. Funct Neurol. (2008) 23:195–9.
22. Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev. (2020) 1:CD010255. doi: 10.1002/14651858.CD010255.pub3
23. Yeroushalmi S, Maloni H, Costello K, Wallin MT. Telemedicine and multiple sclerosis: a comprehensive literature review. J Telemed Telecare. (2020) 26:400–13. doi: 10.1177/1357633X19840097
24. Galiano-Castillo N, Arroyo-Morales M, Lozano-Lozano M, Fernández-Lao C, Martín-Martín L, Del-Moral-Ávila R, et al. Effect of an Internet-based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Support Care Cancer. (2017) 25:3551–9. doi: 10.1007/s00520-017-3782-9
25. van der Linden SD, Sitskoorn MM, Rutten G-JM, Gehring K. Feasibility of the evidence-based cognitive telerehabilitation program Remind for patients with primary brain tumors. J Neurooncol. (2018) 137:523–32. doi: 10.1007/s11060-017-2738-8
26. Fabbri L, Mosca IE, Gerli F, Martini L, Pancani S, Lucidi G, et al. The games for older adults active life (GOAL) project for people with mild cognitive impairment and vascular cognitive impairment: a study protocol for a randomized controlled trial. Front Neurol. (2019) 10:1–9. doi: 10.3389/fneur.2018.01040
27. Cotelli M, Manenti R, Brambilla M, Gobbi E, Ferrari C, Binetti G, et al. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer's disease and frontotemporal dementia: a systematic review. J Telemed Telecare. (2019) 25:67–79. doi: 10.1177/1357633X17740390
28. Maresca G, Maggio MG, De Luca R, Manuli A, Tonin P, Pignolo L, et al. Tele-neuro-rehabilitation in Italy: state of the art and future perspectives. Front Neurol. (2020) 11:563375. doi: 10.3389/fneur.2020.563375
29. Gentry MT, Lapid MI, Rummans TA. Geriatric telepsychiatry: systematic review and policy considerations. Am J Geriatr Psychiatry. (2019) 27:109–27. doi: 10.1016/j.jagp.2018.10.009
30. Standing C, Standing S, McDermott M-L, Gururajan R, Kiani Mavi R. The paradoxes of telehealth: a review of the literature 2000-2015. Syst Res Behav Sci. (2018) 35:90–101. doi: 10.1002/sres.2442
31. Richardson M, Zorn TE, Weaver K. Senior's Perspectives on the Barriers, Benefits and Negatives Consequences of Learning and Using Computers. Hamilton: Department of Management Communication, University of Waikato (2002).
32. Peel NM, Russell TG, Gray LC. Feasibility of using an in-home video conferencing system in geriatric rehabilitation. J Rehabil Med. (2011) 43:364–6. doi: 10.2340/16501977-0675
33. Jeong IC, Liu J, Finkelstein J. Factors affecting adherence with telerehabilitation in patients with multiple sclerosis. Stud Health Technol Inform. (2019) 257:189–93. doi: 10.3233/978-1-61499-951-5-189
34. Gil-Pagés M, Solana J, Sánchez-Carrión R, Tormos JM, Enseñat-Cantallops A, García-Molina A. A customized home-based computerized cognitive rehabilitation platform for patients with chronic-stage stroke: study protocol for a randomized controlled trial. Trials. (2018) 19:191. doi: 10.1186/s13063-018-2577-8
35. Jelcic N, Agostini M, Meneghello F, Bussè C, Parise S, Galano A, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer's disease: a pilot study. Clin Interv Aging. (2014) 9:1605–11. doi: 10.2147/CIA.S68145
36. Motolese F, Magliozzi A, Puttini F, Rossi M, Capone F, Karlinski K, et al. Parkinson's disease remote patient monitoring during the COVID-19 lockdown. Front Neurol. (2020) 11:567413. doi: 10.3389/fneur.2020.567413
37. Moo LR, Gately ME, Jafri Z, Shirk SD. Home-based video telemedicine for dementia management. Clin Gerontol. (2020) 43:193–203. doi: 10.1080/07317115.2019.1655510
38. Smith GE, Lunde AM, Hathaway JC, Vickers KS. Telehealth home monitoring of solitary persons with mild dementia. Am J Alzheimer's Dis Other Dementias®. (2007) 22:20–6. doi: 10.1177/1533317506295888
39. Nijland N, van Gemert-Pijnen J, Boer H, Steehouder MF, Seydel ER. Evaluation of internet-based technology for supporting self-care: problems encountered by patients and caregivers when using self-care applications. J Med Internet Res. (2008) 10:e13. doi: 10.2196/jmir.957
40. Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer's disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychol Rev. (2013) 23:48–62. doi: 10.1007/s11065-013-9227-4
41. Ramos-Ríos R, Mateos R, Lojo D, Conn DK, Patterson T. Telepsychogeriatrics: a new horizon in the care of mental health problems in the elderly. Int Psychogeriatrics. (2012) 24:1708–24. doi: 10.1017/S1041610212000981
42. WHO. (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (accessed November 30, 2020).
43. Korczyn AD. Dementia in the COVID-19 Period. J Alzheimers Dis. (2020) 75:1071–2. doi: 10.3233/JAD-200609
44. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. (2020) 382:1679–81. doi: 10.1056/NEJMp2003539
45. Tozzi AE, Gesualdo F, D'Ambrosio A, Pandolfi E, Agricola E, Lopalco P. Can digital tools be used for improving immunization programs? Front public Heal. (2016) 4:36. doi: 10.3389/fpubh.2016.00036
46. Woodall T, Ramage M, LaBruyere JT, McLean W, Tak CR. Telemedicine services during COVID-19: considerations for medically underserved populations. J Rural Health. (2020) doi: 10.1111/jrh.12466. [Epub ahead of print].
47. Banerjee D. “Age and ageism in COVID-19”: elderly mental health-care vulnerabilities and needs. Asian J Psychiatr. (2020) 51:102154. doi: 10.1016/j.ajp.2020.102154
48. Banerjee D. The impact of Covid-19 pandemic on elderly mental health. Int J Geriatr Psychiatry. (2020) doi: 10.1002/gps.5320. [Epub ahead of print].
49. Bauer K, Schwarzkopf L, Graessel E, Holle R. A claims data-based comparison of comorbidity in individuals with and without dementia. BMC Geriatr. (2014) 14:10. doi: 10.1186/1471-2318-14-10
50. Maggio MG, De Luca R, Manuli A, Calabrò RS. The five ‘W' of cognitive telerehabilitation in the Covid-19 era. Expert Rev Med Devices. (2020) 17:473–5. doi: 10.1080/17434440.2020.1776607
51. Gamito P, Oliveira J, Coelho C, Morais D, Lopes P, Pacheco J, et al. Cognitive training on stroke patients via virtual reality-based serious games. Disabil Rehabil. (2017) 39:385–8. doi: 10.3109/09638288.2014.934925
52. Bartolo M, Intiso D, Lentino C, Sandrini G, Paolucci S, Zampolini M, et al. Urgent measures for the containment of the coronavirus (Covid-19) epidemic in the neurorehabilitation/rehabilitation departments in the phase of maximum expansion of the epidemic. Front Neurol. (2020) 11:423. doi: 10.3389/fneur.2020.00423
53. Bloem BR, Dorsey ER, Okun MS. The coronavirus disease 2019 crisis as catalyst for telemedicine for chronic neurological disorders. JAMA Neurol. (2020) 77:927–8. doi: 10.1001/jamaneurol.2020.1452
54. Mantovani E, Zucchella C, Bottiroli S, Federico A, Giugno R, Sandrini G, et al. Telemedicine and virtual reality for cognitive rehabilitation: a roadmap for the COVID-19 pandemic. Front Neurol. (2020) 11:926. doi: 10.3389/fneur.2020.00926
55. Platz T, Sandrini G. Specialty grand challenge for neurorehabilitation research. Front Neurol. (2020) 11:349. doi: 10.3389/fneur.2020.00349
56. Negrini S, Kiekens C, Bernetti A, Capecci M, Ceravolo MG, Lavezzi S, et al. Telemedicine from research to practice during the pandemic “instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur J Phys Rehabil Med. (2020) 56:327–30. doi: 10.23736/S1973-9087.20.06331-5
57. de Sire A, Andrenelli E, Negrini F, Negrini S, Ceravolo MG. Systematic rapid living review on rehabilitation needs due to COVID-19: update as of April 30th, 2020. Eur J Phys Rehabil Med. (2020) 56:354–60 doi: 10.23736/S1973-9087.20.06378-9
58. Curci C, Pisano F, Bonacci E, Camozzi DM, Ceravolo C, Bergonzi R, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehabil Med. (2020) 56:633–41. doi: 10.23736/S1973-9087.20.06339-X
59. Turolla A, Rossettini G, Viceconti A, Palese A, Geri T. Musculoskeletal physical therapy during the COVID-19 pandemic: is telerehabilitation the answer? Phys Ther. (2020) 100:1260–4. doi: 10.1093/ptj/pzaa093
60. Salawu A, Green A, Crooks MG, Brixey N, Ross DH, Sivan M. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int J Environ Res Public Health. (2020) 17:4890. doi: 10.3390/ijerph17134890
61. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. (2020) 52:jrm00089. doi: 10.2340/16501977-2727
62. Assenza G, Lanzone J, Brigo F, Coppola A, Di Gennaro G, Di Lazzaro V, et al. Epilepsy care in the time of COVID-19 pandemic in Italy: risk factors for seizure worsening. Front Neurol. (2020) 11:737. doi: 10.3389/fneur.2020.00737
63. Stipa G, Gabbrielli F, Rabbito C, Di Lazzaro V, Amantini A, Grippo A, et al. The Italian technical/administrative recommendations for telemedicine in clinical neurophysiology. Neurol Sci. (2020). doi: 10.1007/s10072-020-04732-8. [Epub ahead of print].
64. Ramalho R, Adiukwu F, Gashi Bytyçi D, El Hayek S, Gonzalez-Diaz JM, Larnaout A, et al. Telepsychiatry during the COVID-19 pandemic: development of a protocol for telemental health care. Front Psychiatry. (2020) 11:552450. doi: 10.3389/fpsyt.2020.552450
65. Soares WB, Silvestre IT, Lima AM, de O, de Almondes KM. The Influence of telemedicine care on the management of behavioral and psychological symptoms in dementia (BPSD) risk factors induced or exacerbated during the COVID-19 pandemic. Front psychiatry. (2020) 11:577629. doi: 10.3389/fpsyt.2020.577629
66. Alloni A, Sinforiani E, Zucchella C, Sandrini G, Bernini S, Cattani B, et al. Computer-based cognitive rehabilitation: the CoRe system. Disabil Rehabil. (2017) 39:407–17. doi: 10.3109/09638288.2015.1096969
67. Alloni A, Quaglini S, Panzarasa S, Sinforiani E, Bernini S. Evaluation of an ontology-based system for computerized cognitive rehabilitation. Int J Med Inform. (2018) 115:64–72. doi: 10.1016/j.ijmedinf.2018.04.005
68. Bernini S, Alloni A, Panzarasa S, Picascia M, Quaglini S, Tassorelli C, et al. A computer-based cognitive training in mild cognitive impairment in Parkinson's disease. NeuroRehabilitation. (2019) 44:555–67. doi: 10.3233/NRE-192714
69. Bernini S, Panzarasa S, Barbieri M, Sinforiani E, Quaglini S, Tassorelli C, et at. A double-blind randomized controlled trial of the efficacy of cognitive training delivered using two different methods in mild cognitive impairment in Parkinson's disease: preliminary report of benefits associated with the use of a computerized tool. Aging Clin Exp Res. (2020) doi: 10.1007/s40520-020-01665-2. [Epub ahead of print].
70. Rodella C, Bernini S, Panzarasa S, Sinforiani E, Picascia M, Quaglini S, et al. A Double-Blind Randomized Controlled Trial Combining Cognitive Training (CoRe) and Neurostimulation (tDCS) in the Early Stages of Cognitive Impairment, Unpublished Paper. (2020)
71. Quaglini S, Panzarasa S, Alloni A, Sacchi M, Sinforiani E, Bottiroli S, et al. HomeCore: bringing cognitive rehabilitation at home. Stud Health Technol Inform. (2019) 264:1755–6. doi: 10.3233/SHTI190632
72. Shao YK, Mang J, Li PL, Wang J, Deng T, Xu ZX. Computer-based cognitive programs for improvement of memory, processing speed and executive function during age-related cognitive decline: a meta-analysis. PLoS ONE. (2015) 10:e0130831. doi: 10.1371/journal.pone.0130831
73. Ting DSW, Carin L, Dzau V, Wong TY. Digital technology and COVID-19. Nat Med. (2020) 26:459–61. doi: 10.1038/s41591-020-0824-5
74. Bashshur R, Doarn CR, Frenk JM, Kvedar JC, Woolliscroft JO. Telemedicine and the COVID-19 pandemic, lessons for the future. Telemed J E Health. (2020) 26:571–3. doi: 10.1089/tmj.2020.29040.rb
75. Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Heal Surveill. (2020) 6:e18810. doi: 10.2196/18810
76. Smith AC, Thomas E, Snoswell CL, Haydon H, Mehrotra A, Clemensen J, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. (2020) 26:309–13. doi: 10.1177/1357633X20916567
Keywords: telerehabilitation, telemedicine, information and communication technologies, COVID-19 pandemic, cognitive impairment, cognitive rehabilitation, cognitive training, neurodegenerative diseases
Citation: Bernini S, Stasolla F, Panzarasa S, Quaglini S, Sinforiani E, Sandrini G, Vecchi T, Tassorelli C and Bottiroli S (2021) Cognitive Telerehabilitation for Older Adults With Neurodegenerative Diseases in the COVID-19 Era: A Perspective Study. Front. Neurol. 11:623933. doi: 10.3389/fneur.2020.623933
Received: 30 October 2020; Accepted: 03 December 2020;
Published: 14 January 2021.
Edited by:
Giovanni Assenza, Campus Bio-Medico University, ItalyReviewed by:
Jacopo Lanzone, Policlinico Universitario Campus Bio-Medico, ItalyAlessandro de Sire, University of Eastern Piedmont, Italy
Copyright © 2021 Bernini, Stasolla, Panzarasa, Quaglini, Sinforiani, Sandrini, Vecchi, Tassorelli and Bottiroli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Bernini, c2FyYS5iZXJuaW5pQG1vbmRpbm8uaXQ=
 Sara Bernini
Sara Bernini Fabrizio Stasolla
Fabrizio Stasolla Silvia Panzarasa3
Silvia Panzarasa3 Silvana Quaglini
Silvana Quaglini Elena Sinforiani
Elena Sinforiani Giorgio Sandrini
Giorgio Sandrini Tomaso Vecchi
Tomaso Vecchi Cristina Tassorelli
Cristina Tassorelli Sara Bottiroli
Sara Bottiroli