
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 14 January 2021
Sec. Neurorehabilitation
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.618330
This article is part of the Research Topic Neuropsychological and Cognitive-Behavioral Assessment of Neurodegenerative Disease and Rehabilitation Using New Technologies and Virtual Reality. View all 14 articles
 Sonia Di Tella1
Sonia Di Tella1 Sara Isernia1*
Sara Isernia1* Chiara Pagliari1
Chiara Pagliari1 Johanna Jonsdottir1
Johanna Jonsdottir1 Carlotta Castiglioni2
Carlotta Castiglioni2 Patrizia Gindri2
Patrizia Gindri2 Cristina Gramigna3
Cristina Gramigna3 Samuela Canobbio3
Samuela Canobbio3 Marco Salza2
Marco Salza2 Franco Molteni3and
Franco Molteni3and  Francesca Baglio1 on behalf of HEAD study group
Francesca Baglio1 on behalf of HEAD study groupAims: We aimed to identify the significant predictors of ecological memory amelioration after the Human Empowerment Aging and Disability (HEAD) rehabilitation program, a multidimensional treatment for chronic neurological diseases.
Materials and Methods: Ninety-three patients with Parkinson disease (n = 29), multiple sclerosis (n = 26), and stroke (n = 38) underwent a multidimensional rehabilitation. We focused on changes after treatment on ecological memory (outcome measure) evaluated by Rivermead Behavioral Memory Test, Third Edition (RBMT-3). Minimal clinically important difference (MCID) after treatment were calculated for RBMT-3. The change score on RBMT-3 was categorized in positive effect, stabilization, or no effect of the treatment. Random forest classification identified who significantly benefited from treatment against who did not in terms of ecological memory functioning. Accordingly, logistic regression models were created to identify the best predictors of the treatment effect. A predicted probability value was derived, and the profile of the ideal candidate of HEAD protocol was shown by combining different ranks of significant predictors in a 3 × 3 matrix for each pair of predictors.
Results: A significant number of cases reported positive effect of the treatment on ecological memory, with an amelioration over the MCID or a stabilization. The random forest analysis highlighted a discrete accuracy of prediction (>0.60) for all the variables considered at baseline for identifying participants who significantly benefited and who did not from the treatment. Significant logistic regression model (Wald method) showed a predictive role of Montreal Cognitive Assessment (MoCA; p = 0.007), 2-Minute Walk Test (2MWT; p = 0.038), and RBMT-3 (p < 0.001) at baseline on HEAD treatment effect. Finally, we observed a high probability of success in people with higher residual cognitive functioning (MoCA; odds ratio = 1.306) or functional mobility (2MWT; odds ratio = 1.013).
Discussion: The HEAD program is a rehabilitation with effects on multiple domains, including ecological memory. Residual level of cognitive and/or motor functioning is a significant predictor of the treatment success. These findings confirm the intrinsic relationship subsisting between motor and cognitive functions and suggest the beneficial effects of physical activity on cognitive functions and vice versa.
Recent reports alarmingly pointed out the age-related increment of years of life with diseases (1). Since 1990, mortality rates declined concomitantly with the growth of non-fatal diseases, leading people to cope with chronic conditions and consequently chronic care needs throughout life. Parkinson disease (PD), multiple sclerosis (MS), and post-stroke are the most prevalent chronic neurological conditions (2–4) that weigh heavily on the personal burden and the healthcare costs (5). Especially, Global Burden of Diseases' studies recently reported a global prevalence of more than 6 million PD cases (1), more than two million of MS patients (6), and about 1 million adults living with stroke (2). Although these conditions are characterized by different epidemiology and etiopathology, they are united by a high level of motor and cognitive disability accounting for a consistent loss of quality of life. Regarding the cognitive profile, cognitive deficits are heterogeneous, but memory and executive dysfunctions are frequently reported in all of them (7–9). It is of great importance to cope with the cognitive deficits considering their significant impact on daily living (10). Specifically, everyday memory difficulties are frequent and common in MS, PD, and stroke diseases (11–13). Intact memory skills are required to complete many everyday activities; thus, impairments in memory functioning can have important negative effects on the individual ability to live independently and negative implications for quality of life. Given the chronic course of the disease, people living with these conditions must cope with disability for the remainder of their lives. For this reason, new rehabilitative solutions for such individuals to preserve or improve cognitive status and everyday functioning are crucial; especially, it is important to evaluate their efficacy adopting an ecological assessment. Recent evidence suggests (1) the extensive beneficial effects of multidimensional rather than unidimensional treatment, (2) the positive results from the integration of virtual reality (VR) systems into the conventional rehabilitation in people with chronic neurological diseases, and (3) the importance of characterizing the profile of the ideal candidate for these novel approaches.
First, because of the multidimensional pathology-related difficulties, often impacting motor, cognitive, and behavioral functionality, multidisciplinary models of care are taken in consideration (14–17). Recently proposed integrated treatments involve a multidisciplinary team to offer a personalized systemic care for the disabled person. This holistic approach provides beneficial effects in everyday living, and, for this reason, tools to detect changes in daily functioning after treatments need to be considered. In fact, in the last few years, it has become increasingly clear that standard paper-and-pencil neuropsychological tests are limited in predicting what occurs in patients' everyday life. Only weak associations were reported between results on classical tests and subjects' complaints of everyday problems (18–21). To overcome these difficulties and to better describe how cognitive deficits may affect daily functioning, an innovative approach has been proposed, which entails the administration of more ecological tasks (22, 23).
The second evidence regards the adoption of VR solutions. Rehabilitation with these tools seems to be promising in terms of patient involvement and treatment efficacy (24, 25). The utilization of these VR tools helps facilitate engagement and increase patient satisfaction during the training (26–31), by creating a virtual environment eliciting realistic perceptions and reactions (32). In this framework, the Human Empowerment Aging and Disability (HEAD) protocol is a VR multidimensional rehabilitation intervention for people with chronic neurological conditions conceived for both clinical and home settings (i.e. telerehabilitation). A previous study demonstrated its feasibility (33) and its efficacy in PD populations (24). However, as an integrated treatment proposed for different pathologies and grades of disabilities, a secondary investigation on the predictors of treatment success on everyday functions can provide extensive information on the population target for the HEAD rehabilitation.
The last consideration focused on the lack of clinical consensus regarding the characteristics of the population for targeting these types of VR treatments. It is extremely useful to identify which clinical features are prognostic of treatment success. Along these lines, a new field of investigation aims to individualize significant predictors of treatments (34, 35). This approach establishes the profile of the ideal candidate for a given rehabilitation intervention. This strategy will facilitate the possibility to a priori differentiate between patients who will potentially benefit from the treatment and those who will not. The implications of these studies are large and favor the personalization of intervention targeted for the patient, by ensuring a high probability of treatment success.
The present study aims to characterize the profile of the ideal candidate for the VR-multidimensional treatment who will benefit the most with a high probability on ecological measures. Accordingly, we performed a secondary analysis on a large cohort of patients who completed a VR multidimensional treatment (the HEAD program) by adopting an ecological measure of cognitive functioning, one of the more disabling aspects of the chronic neuropathological conditions.
This study consists of a secondary analysis on data related to a multicenter interventional protocol of integrated rehabilitation for people with chronic neurological diseases whose efficiency and efficacy findings are described elsewhere (24, 33). In this context, we focus on the first part of the study design in which patients underwent a 1-month rehabilitation period in and outpatient setting, consisting in 45-min sessions three times per week, for a total of 12 sessions (ClinicHEAD). The entire dataset from the three recruiting centers of the original study (Valduce Hospital Villa Beretta Rehabilitation Center in Lecco, IRCCS Don Carlo Gnocchi Foundation in Milan and the District Clinic San Camillo in Turin) was utilized for the present work. The study was carried out under the norms of the Declaration of Helsinki; it was approved by the local ethics committees; each participant was adequately informed about the study and offered their collaboration and signed a written informed consent.
The sample of the present study consists of people with chronic neurological conditions meeting the following inclusion criteria: diagnosis of MS with an Expanded Disability Status Scale score ≤ 5.5, or diagnosis of PD with a Hoehn and Yahr score ≤ 2, or diagnosis of chronic stroke at least 6 months after the event; ages between 18 and 80 years; Mini-Mental State Examination score >20; absence of disabling pain; severe deficit of visual acuity or auditory perception or in communication; and absence of severe dysmetria.
Patients were enrolled during their periodical clinical visit by the neurologists, periodically receiving neurological follow-up.
All subjects took part in an experimental clinical trial between 2016 and 2017 consisting of multidimensional rehabilitation with VR activities in the clinic, lasting 1 month, 3 times a week for 12 sessions. The intervention, extensively detailed elsewhere (33), took place in the clinic, with the presence of clinical professionals: the neurologist, the physiotherapist, and the neuropsychologist. Motor and cognitive rehabilitation activities were proposed while interacting with virtual scenarios and watching short video clips. The rehabilitation dimensions targeted by the treatment included balance, endurance, speed and strength of both upper and lower limbs, executive functions, memory, language, and dual-task capabilities.
Cognitive performance outcome was obtained by the Montreal Cognitive Assessment [MoCA; (36)] and the Rivermead Behavioral Memory Test, Third Edition [RBMT-3; (37)]. The MoCA (36), is a sensitive tool for global cognitive level assessment, by screening different domains, such as executive functions, memory, language, visual–spatial abilities, attention, calculation, abstraction, and spatial and temporal orientation (scores range from 0 to 30). Two parallel forms of this instrument (38) were utilized for the assessment at T0 and T1. Following Santangelo et al. (39) scores correction procedure, we obtained a age and education adjusted score of the MoCA subdomains: visuospatial abilities (AVS), executive functions (EF), memory (ME), attention (ATT), language (LANG), and orientation (OR).
RBMT-3 is an ecological battery for the assessment of everyday memory performance with relatively short times of administration, and parallel forms and is applicable to patients with motor deficits (37). The RBMT-3 consists of 14 subtests (scores range from 51 to 147): names (remembering the first and second names of two portrait photos), belongings (remembering to ask for two personal belongings at the end of the evaluation session), appointments (asking two questions when an alarm rings 25 min later), picture recognition (delayed recognition of line drawings against distractors), story (immediate and delayed recall of a short story), faces (delayed recognition of photographs of faces against distractors), route (immediate and delayed recall of a short route in the examination room), message (immediate and delayed remembering to pick up an envelope and book), orientation and date (orientation to person, place and time), and novel task (immediate and delayed recall of puzzle pieces positioned in a specific order within a template). In addition to the scaled scores on the subtests, the Global Memory Index (GMI) was calculated as an overall memory performance measure.
Motor performance outcome was evaluated by the Berg Balance Scale [BBS; (40)], 10-Meter Walk Test [10MWT; (41)], and 2-Minute Walk Test [2MWT; (42)]. The BBS is a measure of static balance and the risk of falling. It consists of a 14-item 4-point scale, with a total score ranging from 0 to 56. 10MWT is a quantitative analysis of the walking speed, measuring the speed in meters per second over 10 m. It is considered an assessment of functional mobility. The 2MWT provides a quantitative analysis of gait speed and endurance. The walking distance walked in 2 min is registered as a functional mobility measure.
Measures of quality of life and affectivity were also considered: the Positive Affect and Negative Affect Schedule [PANAS; (43)]. The PANAS scale consists of 20 items that evaluate two independent dimensions: positive affect and negative affect. The range for each scale (10 items on each) is from 10 to 50.
Statistical analyses on outcome measures were performed with IBM SPSS Statistics software (version 24) and JASP (JASP Team 2020, JASP version 0.11.1).
Means, frequencies, and standard deviations were computed to describe sample characteristics. χ2-test and univariate analysis of variance were used to verify whether the three pathologies included in the sample were balanced for age, education, and sex distribution.
For each outcome measure, changes scores (Δ change) from T1 to T0 were calculated. Minimal Clinical Important Difference (MCID) was derived separately for each pathology computing one-half of the deviation standard, according to Katajapuu et al. (44) and Shikiar et al. (45). After that, each change score was categorized into one of three categories: positive effect of the treatment (Δ change > MCID), stable after treatment (–MCID ≤ Δ change ≤ MCID), and no effect of the treatment (Δ change < MCID). Frequencies and χ2-test were run to show effectiveness results of the treatment on the whole sample and separately for each pathology.
Random forest (RF) classification was applied to the data as an exploratory analysis including all demographic and clinical variables assessed at the baseline, as an overall prediction approach in identifying subjects who significantly benefited from treatment in the RBMT-3 (Δ change > MCID). For this purpose, the RBMT-3 outcome was considered dichotomously (Δ change > MCID vs. Δ change ≤ MCID). We built RFs with the default parameter values in JASP (version 0.11.1), with the exception of the data split for which we partitioned the data set into a training (50%), validation (20%), and test set (30%). In relation to the number of trees, we selected an optimal number of trees [Ntrees (maximum) = 100], optimized with respect to the out-of-bag accuracy. Classification accuracy represents the proportion of the instances that were classified correctly.
Performance of the classification model was also evaluated by carrying out a receiver operating characteristic (ROC) analysis. The area under the ROC curve (AUC) provides a measure of overall prediction accuracy and corresponds to random chance when AUC is equal to 0.5 and represents perfect accuracy when AUC be 1. Precision represents the proportion of true positives among all the instances classified as positive; F1 score indicates the harmonic mean of precision and recall, and recall is the proportion of cases that were classified as positive, among all instances that truly were positives.
To further explore the link between the dichotomic variable of the outcome RBMT-3 and possible predictors at baseline, point-biserial correlation analyses were performed for continuous variables and χ2-test for categorical variables to select variables for insertion in the following regression model. A p < 0.10 was preferred to the conventional threshold p < 0.05 to avoid excluding potential significant predictors.
A logistic regression model was utilized to identify the best predictors of treatment effect. Regardless the statistical significance of association in the previous phase (χ2-test), the pathology (PD, SM, stroke) was cautiously considered in the regression model as a possible predictor. Wald forward option was used as a stepwise selection method. A predicted probability value was derived from the logistic model for each subject. Finally, significant baseline predictors were organized in three-tile ranks, and the mean predicted probability values were shown by combining the different ranks of predictors (in a 3 × 3 matrix for each pair of significant predictors).
Ninety-three of the 112 subjects of the original dataset were considered for the present study as they had no missing data (29 with PD, 26 with MS, and 38 with a stroke in the chronic phase). The three pathologies were balanced in terms of sex distribution and level of education. The age of the MS group significantly differed from PD and stroke (Table 1).
The global cognitive level at the Mini-Mental State Examination was comparable between the three groups. However, when considering the MoCA total score, patients with PD showed higher global cognitive functioning than stroke. Moreover, the memory profile at RBMT-3–GMI was slightly different between MS and the other two conditions (MS < PD/stroke). The specific profile on MoCA and RBMT-3 subscores is detailed in Table 2.
The three groups showed an equal level of affectivity, whereas a major impairment in motor functioning was observed in stroke (Table 1).
Changes between T1 and T0 were classified in one of three categories: patients who significantly benefited from treatment (Δ change > MCID), patients who substantially remained stable after treatment (–MCID ≤ Δ change ≤ MCID), and patients with a significant worsening over time (Δ change < MCID).
Percentages of treatment success for each outcome measure are reported in Table 3. The results showed a significantly higher number of cases with treatment success and who remained stable after treatment vs. patients with a significant worsening over time in all outcomes related to cognitive and motor functioning, and affectivity. Table 4 reports percentages of treatment success separately for each pathology.
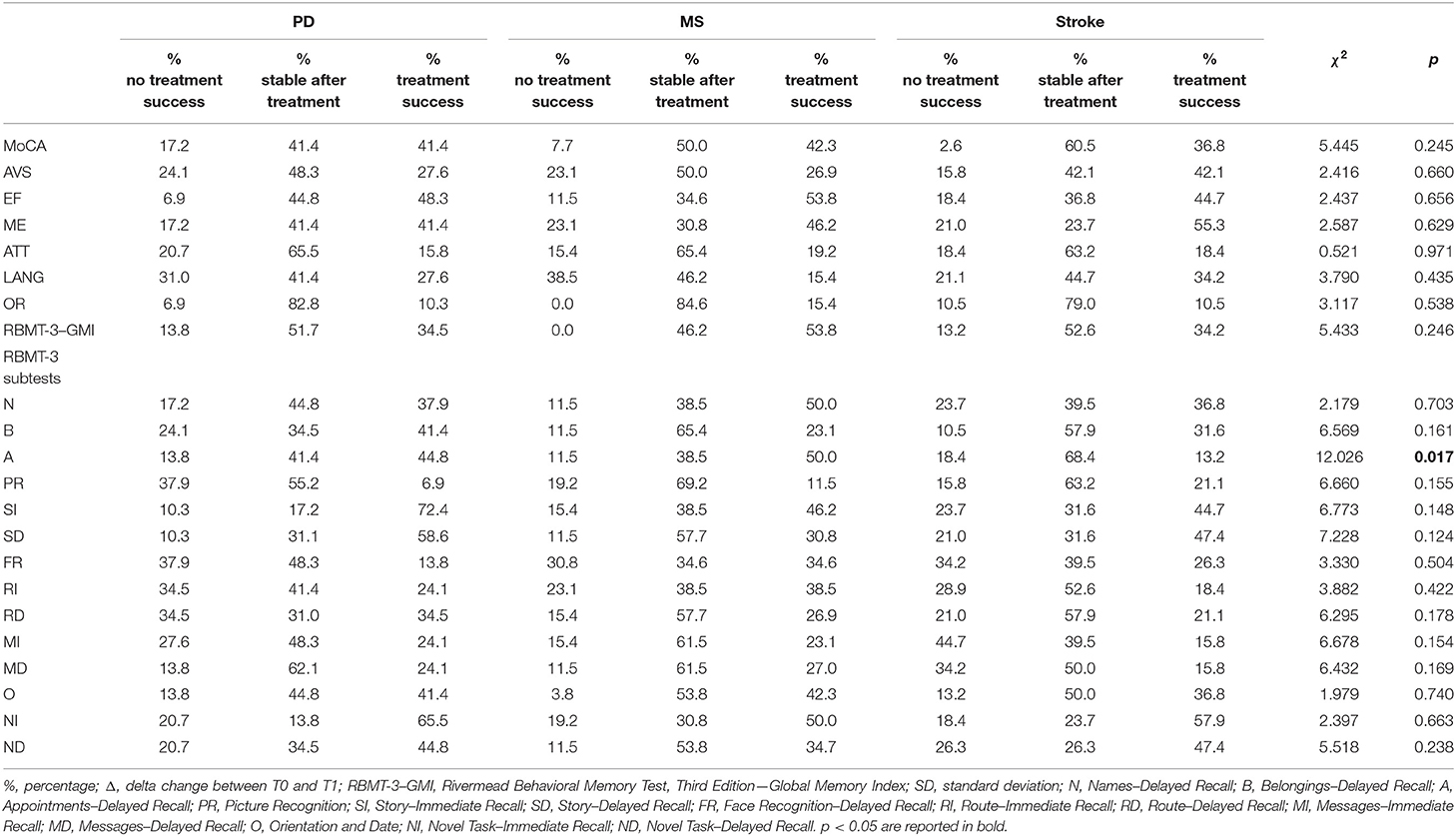
Table 4. Changes within each pathology between T0 and T1 and comparison results of treatment effect vs. no effect cases.
The RF analyses revealed an overall good accuracy (77.8 %) of the classification model built to identify subjects who significantly benefited from treatment in the RBMT-3 (Δ change > MCID vs. Δ change ≤ MCID). Table 5 shows the predictive performances of RF in terms of Precision, Recall, F1 Score and AUC. Precision was above 60% for both classes of patients who benefited and did not benefit from treatment.
By adopting an explorative approach, point-biserial correlations (rpb) and χ2-test, as appropriate, were run between the dichotomic variable of the outcome RBMT-3–GMI (Δ change > MCID vs. Δ change ≤ MCID) and clinical and demographic data in order to detect potential predictors at baseline.
Results highlighted a link between Δ RBMT-3–GMI (Δ change > MCID vs. Δ change ≤ MCID) and MoCA at baseline (rpb = 0.178, p = 0.087), visuospatial subdomain (AVS) of MoCA at baseline (rpb = 0.211, p = 0.042), attention subdomain (ATT) of MoCA at baseline (rpb = 0.210, p = 0.043), RBMT-3–GMI at baseline (rpb = −0.250, p = 0.016) (2MWT (r = 0.274, p = 0.008), BBS at baseline (r = 0.196, p = 0.060), and 10MWT at baseline (r = −0.264, p = 0.011).
Two logistic regression models were computed considering significant results of correlations and the pathology (PD, SM, stroke) as possible predictors of the outcome RBMT-3–GMI (Δ change > MCID vs. Δ change ≤ MCID). In the first model, the following variables were included in the logistic regression: 2MWT, RBMT-3–GMI, MoCA, BBS, and 10MWT. Instead, in the second model, the MoCA was substituted by the subdomains that resulted significantly associated to RBMT-3–GMI Δ change in the preliminary correlation analysis: AVS and ATT. With respect to the first regression, the final third step (Cox and Snell R2 = 0.247, Nagelkerke R2 = 0.334) correctly classified 73.12% of patients. Variables excluded from the third final step were BBS and 10MWT scores at baseline. The binary logistic regression revealed a significant link between RBMT-3–GMI change after rehabilitation and outcome measure at baseline, which was confirmed with a predictive effect for the RBMT-3–GMI, MoCA and 2MWT scores. β-value indicated an inverse relation between the outcome RBMT-3–GMI (Δ change) and the RBMT-3–GMI at baseline, whereas a direct relation was observed between the outcome RBMT-3–GMI (Δ change) and the MoCA and 2MWT scores at baseline the variables (see Table 6 for details).
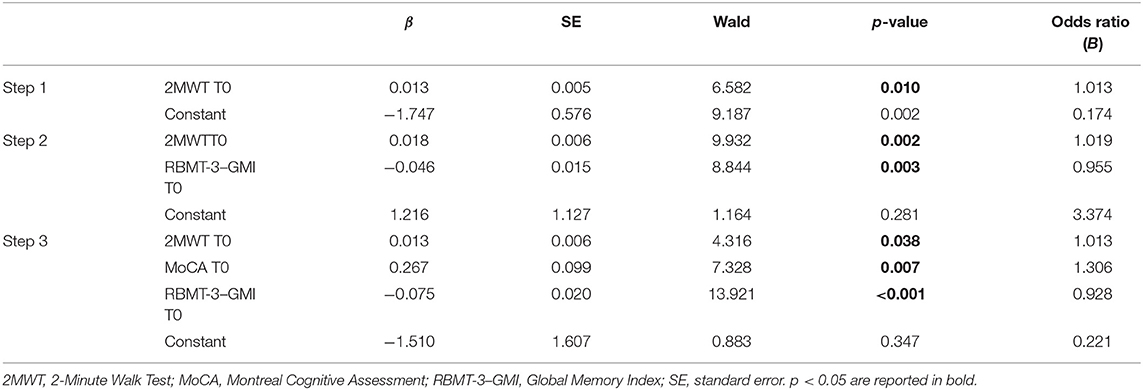
Table 6. Binary logistic regression model to test best predictors of the RBMT-3–GMI change after rehabilitation.
In the second regression, the final third step (Cox and Snell R2 = 0.235, Nagelkerke R2 = 0.318) correctly classified 73.12% of patients. Variables excluded from the third final step were BBS, 10MWT, and ATT scores at baseline (see Table 7 for details).
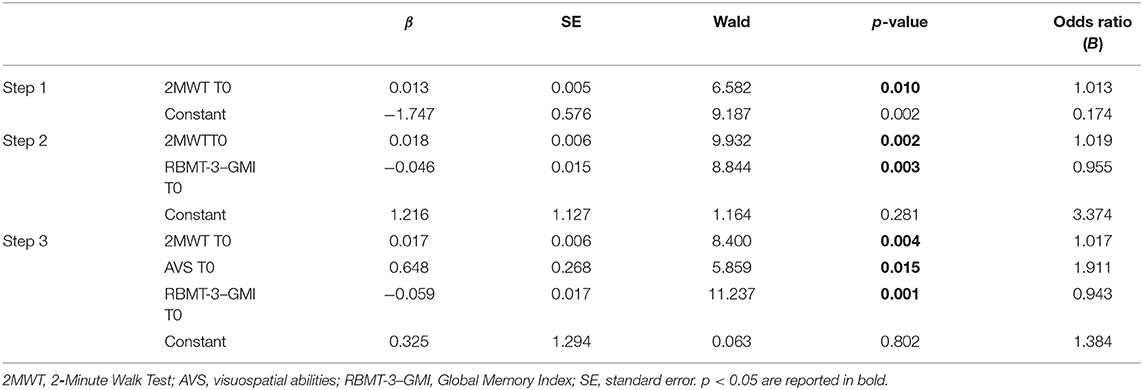
Table 7. Binary logistic regression model to test best predictors of the RBMT-3–GMI change after rehabilitation.
Finally, when considering three-tile ranks of significant baseline predictors (RBMT-3–GMI, MoCA, and 2MWT scores–Figure 1), the ideal candidate for the HEAD treatment in the clinical setting was a person with higher residual cognitive functioning (predicted probability of success: 0.856, Figure 1, panel A) or functional mobility (predicted probability of success: 0.733, Figure 1, panel C). Moreover, an ideal candidate is a person with a higher functional mobility with a moderate level of cognitive decline (predicted probability of success: 0.583, Figure 1, panel B).
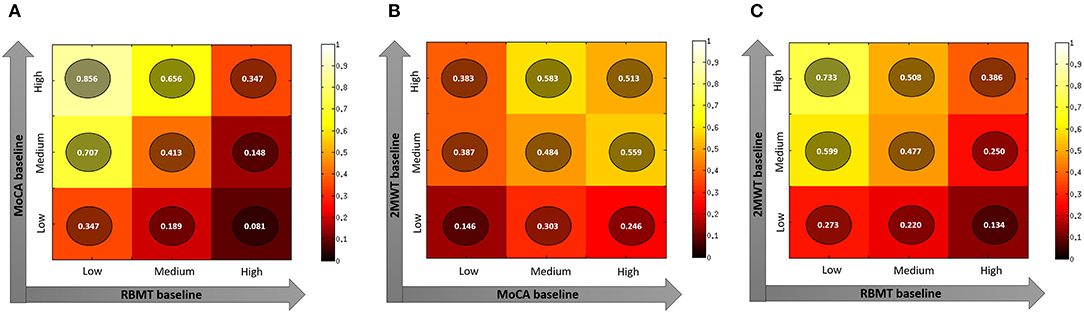
Figure 1. Plots representing predicted probability of different combination of best predictors (Panel A: MoCa and RBMT; Panel B: 2MWT and MoCA; Panel C: 2MWT and RBMT) of the RBMT-3–GMI change after rehabilitation. Panel (A) MoCA value at baseline and RBMT value at baseline; Panel (B) 2MWT value at baseline and MoCA value at baseline; Panel (C) 2MWT value at baseline and RBMT value at baseline. 2MWT, 2-Minute Walk Test; MoCA, Montreal Cognitive Assessment; RBMT-3–GMI, Rivermead Behavioral Memory Test-3–Global Memory Index.
In the present study, we aimed to identify predictors of the HEAD treatment success considering changes in RBMT-3, an ecological measure of functional memory, and to characterize the profile of the ideal candidate for HEAD treatment.
Overall, in line with our previous reports (24), the present work clearly showed that a relatively large number of patients benefited from the HEAD treatment in the clinical setting, with a stable condition or a significant improvement, above the MCID, in all cognitive, motor, and affective domains. It is worth noting that participants were people with chronic diseases who tend to have a stable or worsening disease course over time.
Our findings on predictors of treatment success highlighted the role both of cognitive and motor abilities on the improvement in functional memory. In more detail, when delineating the profile of the ideal candidate for the HEAD treatment in clinic, we found that the prototypical patient who can report beneficial effects with a high probability is a person with more preserved general cognitive functioning and/or higher functional mobility.
Patients with higher MoCA score at baseline are not only patients with more global residual cognitive abilities, but also people with greater cognitive control. In fact, the MoCA test is a screening test highly sensitive to executive functioning, attention and visuospatial abilities (46). Individuals with higher MoCA scores should present with higher capability in representing and maintaining information about goals to be achieved over time, such as rehabilitation goals (47, 48). On the contrary, patients with less cognitive control are likely to encounter difficulties in maintaining representations of task objectives and also in shifting attention between different stimuli in the same task or between different tasks (task-shifting). Therefore, ultimately, they are unfortunately likely to benefit less from a rehabilitative treatment. Similarly, best responders in regaining functional memory after the HEAD treatment are patients with better functional mobility. In fact, persons with higher 2MWT scores at baseline are also persons with higher aerobic capacity and endurance, which represent other relevant prerequisites to perform HEAD activities and thus to achieve rehabilitation goals.
Interestingly, we observed that a high level of residual abilities in one of the two domains (cognitive or motor functioning) was sufficient to compensate the initial decay in the other one. Especially, the best treatment responders were participants with high residual level of motor abilities and a moderate residual level of cognitive functions, especially visuospatial abilities. Vice versa, people with high level of residual cognitive functions but moderate motor abilities benefited from the treatment with a considerable probability of success. This cognitive–motor balance underlines the critical role of the rearrangement mechanisms of the residual resources in the pathological conditions.
Our results also shed light on the intrinsic relationship subsisting between motor and cognitive functions, as well as reported in the literature. In fact, evidence showed the beneficial effects of physical activity on cognitive functions in healthy and pathological conditions (49, 50), indicating also the motor enhancement as a protective factor against cognitive impairment. The underlying biological mechanisms comprise the increment of neurotrophin level (51), the neurogenesis (52), the vascularization and angiogenesis (53), and increased activation in the frontoparietal network and a decreased activation in the default-mode network (54).
Accordingly, high cognitive control and motor abilities allowed performing motor–cognitive dual-task activities included in the HEAD treatment, demanding a discrete level of residual motor and cognitive resources. Although the potential of dual-task training has been demonstrated in different clinical populations (55, 56), potential downsides have been noted in terms of motor and cognitive interference in people with moderate disability, such as increased episodes of falls and sway (57). Moreover, motor–cognitive interference is particularly frequent in some clinical conditions, such as MS (58).
Finally, the VR devices of HEAD rehabilitation required patients to carry out quite sophisticated movements during cognitive activities, as well as visual exploration during motor and cognitive tasks. It is well-known that VR treatments particularly engage visuospatial abilities (59). This aspect could have represented a practical limitation for people with a severe disability. In fact, although there are numerous advantages related to these innovative tools, a recent study indicated also some possible barriers (60), including the need of adaptation of the technological devices to the patient's disability and the patient's additional effort in learning how to interact with the technological system.
The fact that demographic characteristics, such as age and pathology, were not significant predictors was unexpected. The lack of significant impact of age and pathology as predictors could be related to the intrinsic nature of HEAD. This treatment was conceived and developed to ensure a good level of personalization in terms of activities' contents, types, and difficulty level in clinic and at home (i.e. telerehabilitation). This aspect of the treatment allowed adapting the program session-to-session according to the patient's profile and performance. Especially, the personalization of the treatment was designed also on the basis of the pathology, in terms of the activities most effective for the specific clinical conditions (such as “finger-tapping” task for PD patients), and age, in terms of VR contents to be selected for the task (e.g., more or less up-to-date video clips). The selection of the activity's multimedia content could also be tailored to engage the patients by considering motivational aspects. Accordingly, positive outcomes related to VR rehabilitation have been reported, giving the opportunity to set numerous parameters through technological systems (61) in favor of the personalization of rehabilitation.
This work is not without limitations. We considered three clinical populations, and therefore the selection of the outcome measures for this study purposely excluded tests and scales mainly sensible to particular characteristics of a single clinical population (such as Box and Block Test for stroke). Moreover, our results are only related to cognitive outcomes and to the application of the VR in the clinical context. Future studies could adopt this approach and apply it to compare different rehabilitation settings (clinic vs. home), for detecting the impact of VR on different outcomes (i.e., quality of life, gait, affectivity…) and different cognitive domains.
To conclude, our findings will support clinical decision by identifying patients who can be targeted with high probability of VR rehabilitation success on ecological memory functioning. The ideal candidate for HEAD treatment is a person with residual capabilities on motor or cognitive domain, confirming the considerable importance of a prompt multidimensional rehabilitation and the intrinsic relationship subsisting between motor and cognitive functions. Especially, when a domain is impaired, the residual capability allows a compensative mechanism to help facilitate a successful outcome of the rehabilitation process, confirming the beneficial effects of physical activity on cognitive functions and vice versa.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committees of IRCCS Don Gnocchi Foundation, the province of Lecco, Como and Sondrio Ethics Committees, and Città della Salute e della scienza of Turin Ethics Committees. The patients/participants provided their written informed consent to participate in this study.
FB, FM, and MS conceived the study. CG, JJ, and PG carried out the study. CC, CP, and SC collected data. SD, SI, and FB performed statistical analysis and interpreted results. SD, FB, and SI wrote the first draft of manuscript. All authors reviewed and approved the final manuscript.
This study had been funded by the Fondazione Cariplo, Italy, and by Italian Ministry of Health (IRCCS–Ricerca Corrente). This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all patients who participated to the study. We would also thank the HEAD study group to make possible to conduct the present study (alphabetic order): S. Aggujaro, G. Barra, M. Bellomo, R. Bertoni, S. Boccini, M. Bonanima, P. Borgogno, T. Bowman, A. Castagna, M. Covarrubias, A. Del Principe, L. Enei, A. Ferrari, M. Ferrarin, M. Fini, N. Gencarelli, A. Giordano, C. Manfredini, C. Marino, L. Martina, L. Mendozzi, P. Mocarelli, A. Montesano, R. Nemni, G. Palumbo, G. Perini, M. Peverelli, D. Proserpio, L. L. Pugnetti, E. Ripamonti, M. Rossini, G. Ruffin, F. L. Saibene, D. Trombini, A. Zanfini.
1. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1603–58. doi: 10.1016/S0140-6736(16)31460-X
2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the american heart association. Association Circulation. (2018) 137:e67–492. doi: 10.1161/CIR.0000000000000558
3. Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis. (2018) 4:21. doi: 10.1038/s41531-018-0058-0
4. National Multiple Sclerosis Society. MS prevalence. (2017). Available online at: https://www.nationalmssociety.org/About-the-Society/MS-Prevalence
5. Wynford-Thomas R, Robertson NP. The economic burden of chronic neurological disease. J Neurol. (2017) 264:2345–7. doi: 10.1007/s00415-017-8632-7
6. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:269–85. doi: 10.1016/S1474-4422(18)30443-5
7. Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. (2020) 19:860–71. doi: 10.1016/S1474-4422(20)30277-5
8. Leung IH, Walton CC, Hallock H, Lewis SJ, Valenzuela M, Lampit A. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. (2015) 85:1843–51. doi: 10.1212/WNL.0000000000002145
9. Tang EY, Amiesimaka O, Harrison SL, Green E, Price C, Robinson L, et al. Longitudinal effect of stroke on cognition: a systematic review. J Am Heart Assoc. (2018) 7. doi: 10.1161/JAHA.117.006443
10. Mitchell AJKS, Benito-Leon J, Reuber M. The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatrica. (2010) 22:2–13. doi: 10.1111/j.1601-5215.2009.00439.x
11. Higginson CI, Arnett PA, Voss WD. The ecological validity of clinical tests of memory and attention in multiple sclerosis. Arch Clin Neuropsychol. (2000) 15:185–204. doi: 10.1093/arclin/15.3.185
13. Lincoln NB, Kneebone II, Macniven JA, Morris RC. Psychological Management of Stroke. Hoboken, NJ: John Wiley & Sons (2011). doi: 10.1002/9781119961307
14. Di Tella S, Pagliari C, Blasi V, Mendozzi L, Rovaris M, Baglio F. Integrated telerehabilitation approach in multiple sclerosis: a systematic review and meta-analysis. J Telemed Telecare. (2020) 26:385–99. doi: 10.1177/1357633X19850381
15. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. (2013) 12:716–26. doi: 10.1016/S1474-4422(13)70123-6
16. van der Marck MA, Munneke M, Mulleners W, Hoogerwaard EM, Borm GF, Overeem S, et al. Integrated multidisciplinary care in Parkinson's disease: a non-randomised, controlled trial (IMPACT). Lancet Neurol. (2013) 12:947–56. doi: 10.1016/S1474-4422(13)70196-0
17. Vluggen T, van Haastregt JCM, Verbunt JA, van Heugten CM, Schols J. Feasibility of an integrated multidisciplinary geriatric rehabilitation programme for older stroke patients: a process evaluation. BMC Neurol. (2020) 20:219. doi: 10.1186/s12883-020-01791-4
18. Jacoby LL, Jennings JM, Hay LF. Dissociating automatic and consciously controlled processes: implications for diagnosis andrehabilitation of memory deficits. In L. Erlbaum & Associates (Eds.), Basic and Applied Memory Research: Theory in Context. Mahwah, NJ (1996). p. 161–193.
19. Pearman A, Storandt M. Predictors of subjective memory in older adults. J Gerontol B Psychol Sci Soc Sci. (2004) 59:P4–6. doi: 10.1093/geronb/59.1.P4
20. Realdon O, Serino S, Savazzi F, Rossetto F, Cipresso P, Parsons TD, et al. An ecological measure to screen executive functioning in MS: the Picture Interpretation Test (PIT) 360°. Sci Rep. (2019) 9:5690. doi: 10.1038/s41598-019-42201-1
21. Serino S, Baglio F, Rossetto F, Realdon O, Cipresso P, Parsons TD, et al. Picture Interpretation Test (PIT) 360: an innovative measure of executive functions. Sci Rep. (2017) 7:16000. doi: 10.1038/s41598-017-16121-x
22. Burgess PW, Alderman N, Forbes C, Costello A, Coates LM, Dawson DR, et al. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. J Int Neuropsychol Soc. (2006) 12:194–209. doi: 10.1017/S1355617706060310
23. Spooner DM, Pachana NA. Ecological validity in neuropsychological assessment: a case for greater consideration in research with neurologically intact populations. Arch Clin Neuropsychol. (2006) 21:327–37. doi: 10.1016/j.acn.2006.04.004
24. Isernia S, Di Tella S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, et al. Effects of an innovative telerehabilitation intervention for people with Parkinson's disease on quality of life, motor, and non-motor abilities. Front Neurol. (2020) 11:846. doi: 10.3389/fneur.2020.00846
25. Massetti T, da Silva TD, Crocetta TB, Guarnieri R, de Freitas BL, Bianchi Lopes P, et al. The clinical utility of virtual reality in neurorehabilitation: a systematic review. J Cent Nerv Syst Dis. (2018) 10:1179573518813541. doi: 10.1177/1179573518813541
26. Botella C, Riva G, Gaggioli A, Wiederhold BK, Alcaniz M, Baños RM. The present and future of positive technologies. Cyberpsychol Behav Soc Netw. (2012) 15:78–84. doi: 10.1089/cyber.2011.0140
27. Maggio MG, Latella D, Maresca G, Sciarrone F, Manuli A, Naro A, et al. Virtual reality and cognitive rehabilitation in people with stroke: an overview. J Neurosci Nurs. (2019) 51:101–5. doi: 10.1097/JNN.0000000000000423
28. Matamala-Gomez M, Maisto M, Montana JI, Mavrodiev PA, Baglio F, Rossetto F, et al. The role of engagement in teleneurorehabilitation: a systematic review. Front Neurol. (2020) 11:354. doi: 10.3389/fneur.2020.00354
29. O'Neil O, Fernandez MM, Herzog J, Beorchia M, Gower V, Gramatica F, et al. Virtual reality for neurorehabilitation: insights from 3 European clinics. PMR. (2018) 10(9 Suppl. 2), S198–206. doi: 10.1016/j.pmrj.2018.08.375
30. Riva G, Baños RM, Botella C, Wiederhold BK, Gaggioli A. Positive technology: using interactive technologies to promote positive functioning. Cyberpsychol Behav Soc Netw. (2012) 15:69–77. doi: 10.1089/cyber.2011.0139
31. Kefaliakos A, Pliakos I, Kiekkas P, Charalampidou M, Diomidous M. Virtual reality in the rehabilitation of patients with neurological disorders. Stud Health Technol Inform. (2016) 226:45–7. doi: 10.3233/978-1-61499-664-4-45
32. Tieri G, Morone G, Paolucci S, Iosa M. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert Rev Med Devices. (2018) 15:107–17. doi: 10.1080/17434440.2018.1425613
33. Isernia S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, Gramigna C, et al. Efficiency and patient-reported outcome measures from clinic to home: the human empowerment aging and disability program for digital-health rehabilitation. Front Neurol. (2019) 10:1206. doi: 10.3389/fneur.2019.01206
34. Adams EV, Van Puymbroeck M, Walter A, Hawkins BL, Schmid AA, Sharpe JL. Predictors of functional improvements after therapeutic yoga intervention for people with Parkinson's disease. Int J Yoga Therap. (2019) 30:9–18. doi: 10.17761/2020-D-18-00005
35. Li YC, Liao WW, Hsieh YW, Lin KC, Chen CL. Predictors of clinically important changes in actual and perceived functional arm use of the affected upper limb after rehabilitative therapy in chronic stroke. Arch Phys Med Rehabil. (2020) 101:442–9. doi: 10.1016/j.apmr.2019.08.483
36. Conti S, Bonazzi S, Laiacona M, Masina M, Coralli MV. Montreal Cognitive Assessmen (MoCA)-Italian version: regression based norms and equivalent scores. Neurol Sci. (2015) 36:209–14. doi: 10.1007/s10072-014-1921-3
37. Wilson BAGE, Clare L, Baddeley A, Cockburn J, Watson P, et al. (2008). Rivermead Behavioural Memory Test – Third Edition. Manual. Firenze: Giunti O.S. Organizzazioni Speciali (Italian edition in by Beschin, N, Urbano, T and Treccani, B).
38. Siciliano M, Chiorri C, Passaniti C, Sant'Elia V, Trojano L, Santangelo G. Comparison of alternate and original forms of the Montreal Cognitive Assessment (MoCA): an Italian normative study. Neurol Sci. (2019) 40:691–702. doi: 10.1007/s10072-019-3700-7
39. Santangelo G, Siciliano M, Pedone R, Vitale C, Falco F, Bisogno R, et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci. (2015) 36:585–91. doi: 10.1007/s10072-014-1995-y
40. Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada. (1989) 41:304–11. doi: 10.3138/ptc.41.6.304
41. Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. (1997) 26:15–9. doi: 10.1093/ageing/26.1.15
42. Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). (1982) 284:1607–8. doi: 10.1136/bmj.284.6329.1607
43. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. (1988) 54:1063–70. doi: 10.1037/0022-3514.54.6.1063
44. Katajapuu N, Heinonen A, Saltychev M. Minimal clinically important difference and minimal detectable change of the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) amongst patients with chronic musculoskeletal pain. Clin Rehabil. (2020) 34:1506–11. doi: 10.1177/0269215520942573
45. Shikiar R, Harding G, Leahy M, Lennox RD. Minimal important difference (MID) of the Dermatology Life Quality Index (DLQI): results from patients with chronic idiopathic urticaria. Health Qual Life Outcomes. (2005) 3:36. doi: 10.1186/1477-7525-3-36
46. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
47. Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav. (2002) 26:809–17. doi: 10.1016/S0149-7634(02)00067-2
48. Braver TS, West R. Working memory, executive control, and aging. In: Craik FIM, Salthouse TA editors. The handbook of aging and cognition. Psychology Press. (2008). p. 311–372.
49. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. (2012) 78:1323–9. doi: 10.1212/WNL.0b013e3182535d35
50. Groot C, Hooghiemstra AM, Raijmakers PG, van Berckel BN, Scheltens P, Scherder EJ, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. (2016) 25:13–23. doi: 10.1016/j.arr.2015.11.005
51. Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. (2013) 57:47–55. doi: 10.1016/j.nbd.2012.06.011
52. Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. (2014) 9:51–62. doi: 10.2147/CIA.S39506
53. Stevenson ME, Miller CC, Owen HA, Swain RA. Aerobic exercise increases sprouting angiogenesis in the male rat motor cortex. Brain Struct Funct. (2020) 225:2301–14. doi: 10.1007/s00429-020-02100-y
54. Ishihara T, Miyazaki A, Tanaka H, Matsuda T. Identification of the brain networks that contribute to the interaction between physical function and working memory: an fMRI investigation with over 1,000 healthy adults. Neuroimage. (2020) 221:117152. doi: 10.1016/j.neuroimage.2020.117152
55. Fritz NE, Cheek FM, Nichols-Larsen DS. Motor-cognitive dual-task training in persons with neurologic disorders: a systematic review. J Neurol Phys Ther. (2015) 39:142–53. doi: 10.1097/NPT.0000000000000090
56. Jonsdottir J, Gervasoni E, Bowman T, Bertoni R, Tavazzi E, Rovaris M, et al. Intensive multimodal training to improve gait resistance, mobility, balance and cognitive function in persons with multiple sclerosis: a pilot randomized controlled trial. Front Neurol. (2018) 9:800. doi: 10.3389/fneur.2018.00800
57. Etemadi Y. Dual task cost of cognition is related to fall risk in patients with multiple sclerosis: a prospective study. Clin Rehabil. (2017) 31:278–84. doi: 10.1177/0269215516637201
58. Postigo-Alonso B, Galvao-Carmona A, Benítez I, Conde-Gavilán C, Jover A, Molina S, et al. Cognitive-motor interference during gait in patients with Multiple Sclerosis: a mixed methods Systematic Review. Neurosci Biobehav Rev. (2018) 94:126–48. doi: 10.1016/j.neubiorev.2018.08.016
59. Riva G, Mancuso V, Cavedoni S, Stramba-Badiale C. Virtual reality in neurorehabilitation: a review of its effects on multiple cognitive domains. Expert Rev Med Devices. (2020) 6:1–18. doi: 10.1080/17434440.2020.1825939
60. Threapleton K, Drummond A, Standen P. Virtual rehabilitation: what are the practical barriers for home-based research? Digit Health. (2016) 2:2055207616641302. doi: 10.1177/2055207616641302
Keywords: rehabilitation, telerehabilitation, virtual reality, multiple sclerosis, stroke, Parkinson disease, digital health, cognition
Citation: Di Tella S, Isernia S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, Gramigna C, Canobbio S, Salza M, Molteni F and Baglio F (2021) A Multidimensional Virtual Reality Neurorehabilitation Approach to Improve Functional Memory: Who Is the Ideal Candidate? Front. Neurol. 11:618330. doi: 10.3389/fneur.2020.618330
Received: 16 October 2020; Accepted: 07 December 2020;
Published: 14 January 2021.
Edited by:
Sara Bottiroli, Neurological Institute Foundation Casimiro Mondino (IRCCS), ItalyReviewed by:
Anna Giardini, Clinical Scientific Institutes Maugeri (ICS Maugeri), ItalyCopyright © 2021 Di Tella, Isernia, Pagliari, Jonsdottir, Castiglioni, Gindri, Gramigna, Canobbio, Salza, Molteni and Baglio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Isernia, c2lzZXJuaWFAZG9uZ25vY2NoaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.