
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 January 2021
Sec. Neuro-Ophthalmology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.615252
This article is part of the Research Topic Seeing Beyond the Eye: The Brain Connection View all 23 articles
Purpose: This study aimed to assess the macula structure and capillaries in the macula and optic nerve head in recent small subcortical infarct (RSSI) patients.
Methods: This observational cross-sectional study included 40 RSSI patients and 46 healthy controls. Optical coherence tomography angiography was used to image the capillaries in the macula and optic nerve head. An inbuilt algorithm was used to measure the densities in the microvasculature of the macula [superficial retinal capillary plexus (SRCP) and deep retinal capillary plexus (DRCP)] and optic nerve head [radial peripapillary capillary (RPC)] and thickness around the optic nerve head, peripapillary retinal nerve fiber layer (pRNFL).
Results: Densities in RPC (P < 0.001), SRCP (P = 0.001), and DRCP (P = 0.003) were reduced in RSSI patients when compared with healthy controls. The pRNFL thickness was thinner (P < 0.001) in RSSI patients than healthy controls. In the RSSI group, the SRCP density significantly correlated with the DRCP density (rho = 0.381, P = 0.042). The pRNFL thickness displayed a significant relationship with the RPC density (rho = 0.482, P = 0.003) in the RSSI group.
Conclusions: RSSI patients showed interrupted capillary plexuses leading to its significant impairment and neurodegeneration. Our report provides insight into the macula capillary microcirculation changes in RSSI.
Recent small subcortical infarct (RSSI), a common radiological marker of cerebral small vessel disease (SVD), is one of the major cerebrovascular diseases in the aging community currently. RSSI, which affects the perforating arterioles, venules, and capillaries of the brain, causes cerebral vascular impairment (1, 2). With the clinical complications and unstable clinical symptoms associated with RSSI, foremost inhibition has been recommended to have the greatest influence on the general public and healthcare. Till now, there is still lacking reliable and reproducible imaging tools to monitor suppleness of the brain and cognitive function and to monitor asymptomatic participants who have a high probability of having ischemic stroke (3). Impairment of the microvasculature, as seen in lacunar infarction, has been implicated in the pathogenesis of ischemic stroke (4). Despite the improvement in the cerebral imaging modalities, the cerebral microcirculation still remains difficult to directly visualize the cerebral microcirculation, and postmortem findings are usually late-stage changes. Moreover, insufficient treatment options have driven the identification of structural biomarkers to help in the observation and prevention of participants in the very early phase of the disease.
The inner retina microvasculature provides a route to assess the cerebral microvasculature directly and non-invasively in vivo, because reports have shown that the retinal microvasculature shares similar physiological, embryological, and anatomical features with the cerebral microvasculature (5). Patients who have suffered from stroke have been reported to show a higher prevalence of retinal microangiopathic conditions when matched with healthy controls (6); ophthalmological findings such as retinal microaneurysms have been reported to be some of the clinical manifestations associated with ischemic stroke. Reports from the past decade used retinal imaging to study cerebral diseases such as pre-asymptomatic stroke (7–9). These reports showed that the retinal vasculature caliber, tortuosity, and fractal dimension reflect the cerebral vasculature during the disease cascade of ischemic stroke. Previous retinal vascular reports on ischemic stroke used fluorescein angiography (FA) to image and visualize the retinal vasculature. Due to the resolution of FA, imaging is limited to the superficial portion of the macula. Furthermore, it has been reported that retinal vascular changes usually seen on the fundus camera are late indicators of cerebral diseases such as stroke (10, 11).
Optical coherence tomography angiography (OCTA) is an imaging tool that provides an in-depth non-invasive imaging of the retinal microvasculature; it allows a three-dimensional view of the retinal microvasculature in different layers of the retina without dye injection (12); it also provides perfusion of the retinal blood flow. Additionally, the OCTA provides imaging and perfusion of the blood flow in and around the optic nerve head (ONH), which has been reported to serve as additional diagnostic parameters in systemic disorders such as neuromyelitis optica spectrum disorders (13).
Our study utilized the OCTA to measure and summarize the macula microvasculature in patients with RSSI; we also evaluated the macula thickness and microvasculature around the ONH.
This observational cross-sectional study was conducted from November 2019 to June 2020 at the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University in China. The study was permitted by the Ethics Committee of Second Affiliated Hospital and Yuying Children's Hospital and adhered to the Declaration of Helsinki. All participants in our study brought written informed consent before being enrolled in our study.
The participants in this study were recruited from the Yuying Children's Hospital of Wenzhou Medical University from November 2019 to June 2020. RSSI was defined as a classic lacunar syndrome as previously reported (14). Cerebral imaging was also done to confirm the diagnosis and kind of stroke enrolled in our study (15). Where the clinical classification differed from the radiologic classification, cerebral imaging classification was used because the use of only clinical criteria may result in misclassification. Patients included in our study were in the age range of 35 to <80 years. Exclusion criteria also included: (1) patients with contraindications to MRI or unable to cooperate with ophthalmology examination; (2) except for atherosclerosis and small vascular disease, other causes such as cardiogenicity and vasculitis or patients with unknown causes of cerebral infarction; (3) patients with obvious medical diseases such as liver failure, kidney failure, heart failure, serious infections, and malignant diseases; and (4) patients with eye diseases (such as glaucoma, age-related macular degeneration, and diabetic retinopathy) or eye surgery (such as cataract extraction and laser surgery) that affects the retinal microcirculation.
Healthy controls, also finished with brain MRI and with a similar age range from the working staff of both hospitals, were enrolled in our study after consenting. The criteria for inclusion in the control group were as follows: absence of neurological disorders upon evaluation, an Mini-Mental State Examination (MMSE) score of ≥ 26 (cognitively normal), absence of glaucoma or any other eye disease, normal appearance of the ONH and normal thickness of peripapillary retinal nerve fiber layer (pRNFL), intraocular pressure (IOP) below 21.0 mm Hg, and no losses characteristic for glaucoma in visual field testing. Cerebral imaging was also done to rule out any visible cerebral disorders. Other exclusion criteria were as follows: (1) contraindications of MRI examination or inability to cooperate with eye examination; (2) head MRI showed stroke or white matter lesions (Fazekas score ≥ 2); (3) complicated liver failure, kidney failure, heart failure, severe infection, malignant disease, etc.; (4) patients with severe medical diseases; (5) patients with eye diseases (glaucoma, age-related macular degeneration, diabetes with retinopathy, etc.) or patients undergoing eye surgery (such as cataract extraction and laser surgery) that affects the retinal microcirculation.
Participants' basic and clinical information was recorded at admission, such as sex, age, stroke severity [National Institutes of Health Stroke Scale (NIHSS)], and body mass index [calculated as measured weight (kg) divided by the square of measured height (m2)]. Vascular risk factors comprised history of diabetes mellitus, hypertension, transient ischemic attack (TIA), coronary heart disease, cigarette smoking, and alcohol consumption. Ophthalmology-related indicators included IOP, visual acuity (VA), and fundus examination.
Each patient enrolled in our current study underwent assessment of cognitive function using the MMSE by a well-trained neurologist.
The RSSI patients were patients with recent (within 3–4 months) clinical lacunar ischemic stroke. All patients were assessed by a well-trained neurologist (Dr. Han, a stroke physician). Recruitment, testing, and imaging of ischemic patients followed a previous report (14). Assessment of the severity of stroke was under the NIHSS (16); the classification of stroke followed the Oxfordshire Community Stroke Project classification (15).
Measurement of each participant's VA was done using the Snellen chart at a 3.2 m distance. Each eye (both right and left) was measured for its VA.
Fundus camera (CR-DGi; Canon USA Inc., Lakes Success, NY) was used to image the fundus and optic disc of all participants. Patients who presented with the following were excluded: retinal hemorrhages, exudates (soft or hard), macular edema, and swelling of the optic disc.
The imaging of the pRNFL was done with the spectral domain OCT (SD-OCT). OCTA was used to image the capillaries in the macula [superficial retinal capillary plexus (SRCP) and deep retinal capillary plexus (DRCP)] and ONH [radial peripapillary capillaries (RPCs)] The Avanti RTVue-XR tool (Optovue, Fremont, California, USA; software V.2017.100.0.1) was used for imaging. An inbuilt software in the OCT was used to measure the thickness, microvascular density, and area of foveal avascular zone (FAZ). For the inclusion criteria in our present study, high-quality images with signal quality (SQ) ≥ 6 were accepted according to the OSCAR-IB criteria (17).
Both eyes of each patient were included in the data analyses. Among the two groups, generalized estimating equation (GEE) was used to compare the OCT and OCTA parameters while adjusting for risk factors (sex, age, intereye dependencies, hypertension, and diabetes) and SQ. Association between OCT parameters was assessed by Pearson's correlation. P < 0.05 were considered as significant.
Seventy-five eyes from 38 RSSI patients and 92 eyes from 46 healthy controls were included for data analyses. One eye of RSSI patient was not included because it could meet the imaging signal criteria of our study (SQ < 6). A significant difference was seen in the best corrected VA (BCVA) when both groups were compared as seen in Table 1.
Cross-sectional images from the macula OCTA with noticeable vascular flow motions through the angiograms of the macula were not the same among the two groups. RSSI patients showed reduced visible flow signals than did the healthy controls as seen in Figure 1. En-face angiograms between the two groups showed that the macular microvasculature in the RSSI patients was more interrupted in the SRCP than that in the healthy controls.
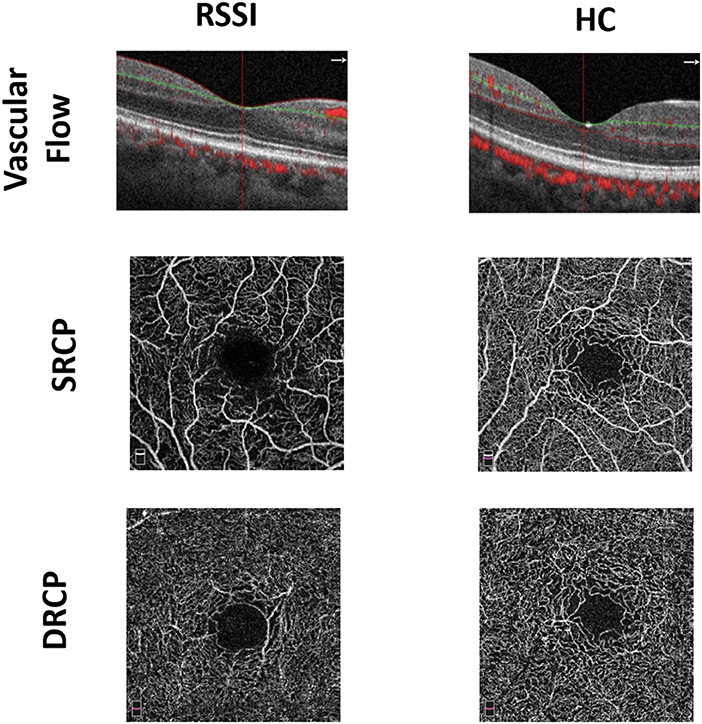
Figure 1. Representative en-face optical coherence tomography (OCT) angiogram of recent small subcortical infarct (RSSI) patients and healthy controls (HCs). The first row shows the vascular flow in the macula between the two groups. The second and third rows show superficial retinal capillary plexus (SRCP) and deep retinal capillary plexus (DRCP) plexus of RSSI and HCs, respectively.
The SQ of the ONH and macula images was significantly lower (P < 0.001, Table 2) in RSSI patients when compared with the healthy controls.
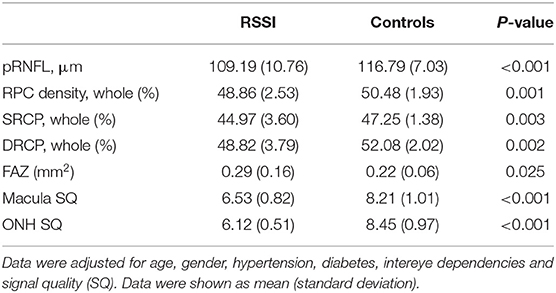
Table 2. Comparison of the macular microvascular densities between RSSI patients and healthy controls.
Densities in the RPC (P = 0.001, Table 2), SRCP (P = 0.003, Table 2), and DRCP (P = 0.002, Table 2) were significantly reduced in RSSI patients when compared with healthy controls. In the RSSI group, the SRCP significantly correlated with the DRCP (rho = 0.398, P = 0.015). The FAZ was significantly larger (P = 0.025) in RSSI patients when compared with the healthy controls.
The pRNFL thickness was thinner (P < 0.001, Table 2) in RSSI patients when compared with healthy controls. The pRNFL thickness displayed a significant correlation with the RPC density (rho = 0.401, P = 0.014) in the RSSI group.
pRNFL thickness in RSSI patients inversely correlated with their VA (rho = −0.313, P = 0.039).
Reports have suggested the eye as a route to the brain (18, 19); moreover, retinal microvasculature has been reported to be associated with the cerebral microcirculation because both tissues share similar physiological, embryological, and anatomical features (5, 20). Our report utilized the OCTA to assess the microvasculature of the macula in RSSI patients and found that the macular vascular density is significantly reduced in the RPC, SRCP, and DRCP. Additionally, we showed that RSSI patients have significantly reduced pRNFL thickness and have a larger FAZ area than the healthy controls.
Previous reports used fundus photography to evaluate the retinal vasculature in ischemic stroke (9, 14). Nonetheless, using these imaging modalities limits the resolution and depth of imaging; moreover, these imaging modalities cannot visualize the deeper retinal layers in the retina and cannot give additional information on the capillary structure in the retina. OCTA is an imaging tool that offers a detailed non-invasive image of the retinal microvasculature; it allows a three-dimensional view of the retinal microvasculature in different layers of the retina without dye injection; it also provides perfusion of the retinal blood flow. Additionally, the OCTA provides imaging and perfusion of the blood flow in and around the ONH, which has been reported to serve as additional diagnostic parameters in systemic disorders such as neuromyelitis optica spectrum disorders.
Our current report showed that RSSI patients have significantly reduced SRCP density than healthy controls. Previous reports (7, 9, 21) used different algorithms to evaluate the photography in patients with ischemic stroke; these reports showed that patients with ischemic stroke have significant arteriolar and venular changes than healthy controls. Sprodhuber et al. (9) also showed that patients with ischemic stroke have significantly reduced retinal vascular density than healthy controls. The SRCP is within the retinal nerve fiber layer (RNFL) and superficial portion of the ganglion cell complex (GCC), which consists of both large vessels and microvessels (22) as seen via the fundus photography. Thus, reduced SRCP density in RSSI patients in our current study is congruent with previous studies, echoing the importance of retinal imaging in ischemic stroke.
A novel finding in our current report is the reduced microvascular density in the DRCP of RSSI patients when compared with healthy controls. Reports on OCTA studies have suggested that the DRCP makes up the end of the macula capillary plexus in which blood flows from the superficial capillary layers (retinal peripapillary capillaries and SRCP) and flows into the deep venules through the DRCP (22–24). Interestingly, our report showed that the SRCP density is significantly associated with the DRCP density; thus, reduced microvascular density in the DRCP of RSSI patients found in our current study may be due to the injury caused in the SRCP, which leads to the damage in the DRCP. The DRCP is positioned at the border of the inner plexiform layer (IPL) and outer plexiform layer (OPL), an area in the macular where the level of oxygen is significantly lower. As such, hypoperfusion may be easily prone to this area and maybe another reason for the reduced density in the DRCP of RSSI patients. Furthermore, deep capillary plexus has been reported to consist of capillaries (23) (more capillaries than superficial capillary plexus), which are responsible for oxygen diffusion in the tissue (25). The microvasculature in the deep capillary plexus is thinner and has a small cross section, making it sensitive to any disease that affects the retina; as such, any injury or insult to the capillary plexus leads to the tissue receiving less oxygen. Additionally, pericytes have been reported to play a pivotal role in the pathogenesis of lacunar ischemic stroke. Reports have shown pericyte alterations in the retinal capillaries of ischemic patients (26, 27). The reduced microvascular density in the deep capillary plexus of RSSI patients could also be as result of the pericyte alteration during the disease cascade.
RPCs are situated around the ONH and constitute a unique vascular network within pRNFL. The RPCs are also associated with metabolism of the RNFL and ganglion cell layer (GCL) (28–30). Because of their thin capillary anastomoses, RPCs are suggested to be susceptible to changes that occur in the ONH and macula (31, 32). Previous reports showed reduction of the pRNFL thickness in ischemic stroke patients when compared with healthy controls (33, 34); another report also showed optic nerve atrophy in patients with minor stroke (6). Furthermore, a previous report used the fundus photography to characterize the density of vessels around the ONH in ischemic stroke patients and found significantly lower values of the vessel area than in healthy controls (9). The authors suggested that retinal imaging could serve as a quantitative marker for cerebrovascular events. To the best of our knowledge, this is the first study to evaluate the microvascular density around the ONH in RSSI patients using the OCTA. Our report showed that RSSI patients had significantly reduced pRNFL thickness and RPC density than the healthy controls; our data also showed a significant association between the pRNFL thickness and RPC density in RSSI patients. The optic nerve has been reported associated with the brain (35); furthermore, the optic nerve is a boundary between the brain and the retina; thus, changes in and around the optic nerve mirror the changes that occur in the brain and retina. Besides, neural activity has been reported to be correlated with local blood flow (36); thus, the changes in the microvascular perfusion could be a result of the neurodegeneration or vice versa. We suggest that microvascular changes around the optic nerve mirror the neural and microcirculation in the brain of these patients. Longitudinal studies are needed to validate our hypothesis.
With the interaction between the neural activity and microvasculature, we suggest that the microvascular changes seen in our report may be due to the neurodegeneration associated with the disease cascade (37). Moreover, it has been reported that retinal vascular changes precede apparent cerebral changes seen on neuroimaging (38). With the connection between the activity of neurons and the flow of blood (39, 40), changes in the thickness of the pRNFL could lead to a secondary decrease in the density of microvessels seen in our current study.
It has been reported that about 30% of all stroke patients suffer from visual impairment (41). Our report found a significant correlation between the decreased pRNFL thickness and VA in RSSI patients. Although it has been suggested that patients with stroke have reduced RNFL thickness than have healthy controls (33, 34), an association between the reduced thickness and visual function has not been reported yet. The pRNFL, which contains axons (42), has been reported to play an important role in vision because of its proximity to the optic nerve (cranial nerve II). The association between pRNFL thickness and VA suggests that insult to the pRNFL thickness have a significant effect on the VA of RSSI patients.
Some limitations were seen in our current study. Our participants were Chinese; thus, our data cannot be translated across other ethnicities. Secondly, because of the observational, cross-sectional plan of our study, our study did not investigate the causative mechanism involved in our results. The OCT-A imaging technique requires that patients focus and work together with the examiner; therein, some of the images obtained were unsuitable for imaging because some patients could not focus and cooperate. Another limitation in our study is not evaluating other significant layers of the macula, which are associated with the neurodegeneration associated with the disease cascade such as the GCC. Nonetheless, our current study used the OCTA with an inbuilt software to assess the macula structure, which limited our macula structure thickness to the retinal sublayer thickness (RNFL) only. Reports with a segmentation algorithm of the retina are needed to elucidate the association between the macular thickness and microvascular densities. Another limitation is that our current study did not assess the association among the MRI parameters, OCTA measures, and clinical variables.
In conclusion, RSSI patients showed interrupted capillary plexuses leading to its significant impairment and neurodegeneration. Our report sheds light on the role of macula microvasculature and shows the global changes in macula microvasculature in RSSI. We also suggest that the OCTA could be useful to evaluate and monitor the microvasculature of the macula in patients with RSSI; nonetheless, longitudinal studies are needed to validate our report before the OCTA could be implemented as a screening tool.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The study was approved by the Ethics committee of Second Affiliated Hospital and Yuying Childrened Hospital and followed the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YC, JY, YL, and ZH: main authors and assisted with distribution as well as data collation and analysis. YC and JY: formal analysis. YC and ZZ: data curation. YC: writing—original draft. ZH: writing—review and editing. YL: supervision. All authors: contributed to the article and approved the submitted version.
This study was funded by the Clinical Scientific Research Fund of the Second Affiliated Hospital of Wenzhou Medical University (SAHoWMU-CR2017-01-212) and the National Natural Science Foundation of China (Grant Nos. 81571114 and 81771267).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
pRNFL, peripapillary retinal nerve fiber layer; RPC, radial peripapillary capillary; SRCP, superficial retinal capillary plexus; DRCP, deep retinal capillary plexus; FAZ, foveal avascular zone; RSSI, recent small subcortical infarct; OCTA, optical coherence tomography angiography; SD-OCT, spectral domain optical coherence tomography; SQ, signal quality; IOP, intraocular pressure.
1. Kawasaki R, Xie J, Cheung N, Lamoureux E, Klein R, Klein BE, et al. Retinal microvascular signs and risk of stroke: the Multi-Ethnic Study of Atherosclerosis. (MESA) Stroke. (2012) 43:3245–51. doi: 10.1161/STROKEAHA.112.673335
2. Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. (2017) 120:449–71. doi: 10.1161/CIRCRESAHA.116.308427
3. Mok VC, Lam BY, Wong A, Ko H, Markus HS, Wong LK. Early-onset and delayed-onset poststroke dementia - revisiting the mechanisms. Nat Rev Neurol. (2017) 13:148–59. doi: 10.1038/nrneurol.2017.16
4. Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, et al. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. (2013) 44:525–7. doi: 10.1161/STROKEAHA.112.669994
5. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. (2013) 9:44–53. doi: 10.1038/nrneurol.2012.227
6. Wolz J, Audebert H, Laumeier I, Ahmadi M, Steinicke M, Ferse C, et al. Telemedical assessment of optic nerve head and retina in patients after recent minor stroke or TIA. Int Ophthalmol. (2017) 37:39–46. doi: 10.1007/s10792-016-0222-7
7. Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, et al. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. (2013) 44:2121–7. doi: 10.1161/STROKEAHA.113.001741
8. Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res. (2017) 57:89–107. doi: 10.1016/j.preteyeres.2017.01.001
9. Sprodhuber A, Wolz J, Budai A, Laumeier I, Audebert HJ, Michelson G. The role of retinal vascular density as a screening tool for ageing and stroke. Ophthalmic Res. (2018) 60:1–8. doi: 10.1159/000488491
10. Baker ML, Hand PJ, Wang JJ, Wong TY. Retinal signs and stroke: revisiting the link between the eye and brain. Stroke. (2008) 39:1371–9. doi: 10.1161/STROKEAHA.107.496091
11. Moss HE. Retinal vascular changes are a marker for cerebral vascular diseases. Curr Neurol Neurosci Rep. (2015) 15:40. doi: 10.1007/s11910-015-0561-1
12. Jia Y, Bailey ST, Hwang TS, Mcclintic SM, Gao SS, Pennesi ME, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci USA. (2015) 112:E2395–402. doi: 10.1073/pnas.1500185112
13. Huang Y, Zhou L, Zhangbao J, Cai T, Wang B, Li X, et al. Peripapillary and parafoveal vascular network assessment by optical coherence tomography angiography in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders. Br J Ophthalmol. (2019) 103:789–96. doi: 10.1136/bjophthalmol-2018-312231
14. Doubal FN, Macgillivray TJ, Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology. (2009) 72:1773–8. doi: 10.1212/WNL.0b013e3181a60a71
15. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-O
16. Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
17. Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PloS ONE. (2012) 7:e34823. doi: 10.1371/journal.pone.0034823
18. Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. (2013) 34:1270–8. doi: 10.1093/eurheartj/eht023
19. Hansen MH, Li XQ, Larsen M, Olsen EM, Skovgaard AM, Kessel L, et al. Five-year change in choroidal thickness in relation to body development and axial eye elongation: the CCC2000 eye study. Invest Ophthalmol Vis Sci. (2019) 60:3930–6. doi: 10.1167/iovs.19-26807
20. Erskine L, Herrera E. Connecting the retina to the brain. ASN Neuro. (2014) 6:1759091414562107. doi: 10.1177/1759091414562107
21. Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. (2002) 12:358–70. doi: 10.1111/j.1750-3639.2002.tb00450.x
22. Bonnin S, Mane V, Couturier A, Julien M, Paques M, Tadayoni R, et al. New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina J Retinal Vitreous Dis. (2015) 35:2347–52. doi: 10.1097/IAE.0000000000000839
23. Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. (2017) 7:42201. doi: 10.1038/srep42201
24. Garrity ST, Paques M, Gaudric A, Freund KB, Sarraf D. Considerations in the Understanding of Venous Outflow in the Retinal Capillary Plexus. Retina J Retinal Vitreous Dis. (2017) 37:1809–12. doi: 10.1097/IAE.0000000000001784
25. Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc Res. (1996) 32:632–43. doi: 10.1016/S0008-6363(96)00110-1
26. Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, et al. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. (2017) 8:107–21. doi: 10.1007/s12975-016-0504-4
27. Erdener SE, Dalkara T. Small vessels are a big problem in neurodegeneration and neuroprotection. Front Neurol. (2019) 10:889. doi: 10.3389/fneur.2019.00889
28. Yu PK, Cringle SJ, Yu DY. Correlation between the radial peripapillary capillaries and the retinal nerve fibre layer in the normal human retina. Exp Eye Res. (2014) 129:83–92. doi: 10.1016/j.exer.2014.10.020
29. Bonini Filho MA, Adhi M, De Carlo TE, Ferrara D, Baumal CR, Witkin AJ, et al. Optical coherence tomography angiography in retinal artery occlusion. Retina. (2015) 35:2339–46. doi: 10.1097/IAE.0000000000000850
30. Yu PK, Balaratnasingam C, Xu J, Morgan WH, Mammo Z, Han S, et al. Label-free density measurements of radial peripapillary capillaries in the human retina. PLoS ONE. (2015) 10:e0135151. doi: 10.1371/journal.pone.0135151
31. Alterman M, Henkind P. Radial peripapillary capillaries of the retina. II Possible role in Bjerrum scotoma. Br J Ophthalmol. (1968) 52:26–31. doi: 10.1136/bjo.52.1.26
32. Kornzweig AL, Eliasoph I, Feldstein M. Selective atrophy of the radial peripapillary capillaries in chronic glaucoma. Arch Ophthalmol. (1968) 80:696–702. doi: 10.1001/archopht.1968.00980050698002
33. Wang D, Li Y, Wang C, Xu L, You QS, Wang YX, et al. Localized retinal nerve fiber layer defects and stroke. Stroke. (2014) 45:1651–6. doi: 10.1161/STROKEAHA.113.004629
34. Anjos R, Vieira L, Costa L, Vicente A, Santos A, Alves N, et al. Macular ganglion cell layer and peripapillary retinal nerve fibre layer thickness in patients with unilateral posterior cerebral artery ischaemic lesion: an optical coherence tomography study. Neuroophthalmology. (2016) 40:8–15. doi: 10.3109/01658107.2015.1122814
35. Gala F. Magnetic resonance imaging of optic nerve. Indian J Radiol Imaging. (2015) 25:421–38. doi: 10.4103/0971-3026.169462
36. O'herron P, Chhatbar PY, Levy M, Shen Z, Schramm AE, Lu Z, et al. Neural correlates of single-vessel haemodynamic responses in vivo. Nature. (2016) 534:378–82. doi: 10.1038/nature17965
37. Brown WR, Thore CR. Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. (2011) 37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x
38. Colligris P, De Lara MJP, Colligris B, Pintor J. Ocular manifestations of alzheimer's and other neurodegenerative diseases: the prospect of the eye as a tool for the early diagnosis of Alzheimer's disease. J Ophthalmol. (2018) 2018:8538573. doi: 10.1155/2018/8538573
39. Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Transac R Soc B Biol Sci. (2015) 370:20140195. doi: 10.1098/rstb.2014.0195
40. Palkovits S, Lasta M, Told R, Schmidl D, Werkmeister R, Cherecheanu AP, et al. Relation of retinal blood flow and retinal oxygen extraction during stimulation with diffuse luminance flicker. Sci Rep. (2015) 5:18291. doi: 10.1038/srep18291
41. Rowe F, Brand D, Jackson CA, Price A, Walker L, Harrison S, et al. Visual impairment following stroke: do stroke patients require vision assessment? Age Ageing. (2009) 38:188–93. doi: 10.1093/ageing/afn230
Keywords: recent small subcortical infarcts, macular capillaries, retina, capillary densities, optical coherence tomography angiography
Citation: Cao Y, Yan J, Zhan Z, Liang Y and Han Z (2021) Macula Structure and Microvascular Changes in Recent Small Subcortical Infarct Patients. Front. Neurol. 11:615252. doi: 10.3389/fneur.2020.615252
Received: 08 October 2020; Accepted: 23 November 2020;
Published: 07 January 2021.
Edited by:
Silvia Di Angelantonio, Sapienza University of Rome, ItalyReviewed by:
Christian Cordano, University of California, San Francisco, United StatesCopyright © 2021 Cao, Yan, Zhan, Liang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanbo Liang, eXVhbmJvbGlhbmdAMTI2LmNvbQ==; Zhao Han, d3poYW56aGFvQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.