
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 03 February 2021
Sec. Neurorehabilitation
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.611453
This article is part of the Research Topic Tele-NeuroRehabilitation View all 22 articles
 Steven C. Cramer1,2,3*
Steven C. Cramer1,2,3* Lucy Dodakian3
Lucy Dodakian3 Vu Le3
Vu Le3 Alison McKenzie4
Alison McKenzie4 Jill See3
Jill See3 Renee Augsburger3
Renee Augsburger3 Robert J. Zhou3
Robert J. Zhou3 Sophia M. Raefsky3
Sophia M. Raefsky3 Thalia Nguyen3
Thalia Nguyen3 Benjamin Vanderschelden3
Benjamin Vanderschelden3 Gene Wong3
Gene Wong3 Daniel Bandak3
Daniel Bandak3 Laila Nazarzai3
Laila Nazarzai3 Amar Dhand5
Amar Dhand5 Walt Scacchi6
Walt Scacchi6 Jutta Heckhausen7
Jutta Heckhausen7Introduction: High doses of activity-based rehabilitation therapy improve outcomes after stroke, but many patients do not receive this for various reasons such as poor access, transportation difficulties, and low compliance. Home-based telerehabilitation (TR) can address these issues. The current study evaluated the feasibility of an expanded TR program.
Methods: Under the supervision of a licensed therapist, adults with stroke and limb weakness received home-based TR (1 h/day, 6 days/week) delivered using games and exercises. New features examined include extending therapy to 12 weeks duration, treating both arm and leg motor deficits, patient assessments performed with no therapist supervision, adding sensors to real objects, ingesting a daily experimental (placebo) pill, and generating automated actionable reports.
Results: Enrollees (n = 13) were median age 61 (IQR 52–65.5), and 129 (52–486) days post-stroke. Patients initiated therapy on 79.9% of assigned days and completed therapy on 65.7% of days; median therapy dose was 50.4 (33.3–56.7) h. Non-compliance doubled during weeks 7–12. Modified Rankin scores improved in 6/13 patients, 3 of whom were >3 months post-stroke. Fugl-Meyer motor scores increased by 6 (2.5–12.5) points in the arm and 1 (−0.5 to 5) point in the leg. Assessments spanning numerous dimensions of stroke outcomes were successfully implemented; some, including a weekly measure that documented a decline in fatigue (p = 0.004), were successfully scored without therapist supervision. Using data from an attached sensor, real objects could be used to drive game play. The experimental pill was taken on 90.9% of therapy days. Automatic actionable reports reliably notified study personnel when critical values were reached.
Conclusions: Several new features performed well, and useful insights were obtained for those that did not. A home-based telehealth system supports a holistic approach to rehabilitation care, including intensive rehabilitation therapy, secondary stroke prevention, screening for complications of stroke, and daily ingestion of a pill. This feasibility study informs future efforts to expand stroke TR.
Clinical Trial Registration: Clinicaltrials.gov, # NCT03460587.
Stroke is perennially among the leading causes of human disability (1, 2) and the leading neurological cause of lost disability-adjusted life years (3). The number of affected people has doubled over the past two decades (4), partly because of the aging population (5) and partly because advances in stroke medicine have increased the fraction of patients surviving acute stroke (6). Motor deficits, present in >80% of patients with stroke acutely, are a major contributor to this disability. Few patients recover completely, with 55–75% having enduring motor deficits (7, 8). At 6 months post-stroke, 65% of patients are unable to incorporate the paretic hand effectively into daily activities (9). Persistent arm impairment is linked with greater activity limitations, higher participation restrictions, poorer quality of life, and reduced well-being (10–12).
There is strong evidence that higher doses of rehabilitation therapy are associated with greater behavioral gains, especially for paretic arm function after stroke (13–18), even with variability in treatment content and definition of dose (19). This remains true when higher therapy doses are delivered in the home (14, 20). However, patients generally do not receive high doses of rehabilitation therapies, due to cost, traveling difficulties, and regional shortages of rehabilitation providers–factors that are exacerbated in the COVID-19 era. Quality of rehabilitation therapy is also important and can increase the extent to which clinical neuroplasticity is harnessed (21): effects are higher when therapy is challenging, motivating, and engaging (22–25).
Telehealth might be able to help by increasing access to high quality therapy (26). Telerehabilitation (TR) has been defined as the delivery of rehabilitation services via communication technologies and encompasses a range of rehabilitation and habilitation services that include evaluation, assessment, monitoring, prevention, intervention, supervision, education, consultation and coaching (27). This is similar to the holistic framework outlined by Demiris et al. (28), who suggested that home-based post-stroke TR should include support that spans an array of medical, mental health, and social services. Compared to traditional in-clinic therapy, TR uses the same principles of individualized care by a licensed therapist. This telehealth approach provides enhanced options compared to delivery of rehabilitation services using a brick-and-mortar approach (29–33), potentially decreasing transportation needs for patients with functional limitations, boosting physical activity, and expanding access to care.
Telehealth can also help by increasing motivation and compliance. The technological underpinnings of TR can facilitate a personalized approach to upper extremity (UE) motor rehabilitation (34). Telehealth can deliver therapy in the form of games, an approach known to promote patient participation in health care (35–39). Games motivate patients to engage in enjoyable play behavior that involves therapeutically relevant movements (40, 41), which is important because patient compliance with stroke rehabilitation is often limited (42–44).
The overall experience with motor TR after stroke is mixed. While one review found that all 18 studies of post-stroke motor TR improved disability (32), a recent meta-analysis concluded that drawing general conclusions about the effects of stroke TR is difficult, as interventions and comparators varied greatly across studies (45). We have completed three trials of TR targeting arm motor deficits after stroke. The first was a pilot study (46) that provided 12 patients with chronic stroke with 4 weeks of home-based, therapist-supervised TR. Findings included that patients were highly compliant (97.9% of assigned days), videoconferences supported regular communication between the patient at home and therapists in the clinic, arm motor status improved significantly based on the UE Fugl-Meyer (UE-FM) motor scale, and no computer skills were needed, as computer literacy was not related to usage or treatment gains. With 60 min/day of TR, patients averaged 879 arm repetitions/day. A second study found that eight sessions of visuomotor training in the home improved visuomotor tracking by the UE (47).
More recently we led an 11-site, randomized, assessor-blind trial of TR (48). A total of 124 patients with stroke were randomized to receive 36 sessions of 70-min duration, either in-clinic or in the home via TR. In the 62 patients randomized to TR, UE-FM scores increased by 7.9 ± 6.7 points, and TR was found to be non-inferior to in-clinic therapy. Motor gains remained significant when patients enrolled >90 days post-stroke were examined separately. Gains were also significant when examining change in Box & Blocks score, a measure of arm function (activities limitation). In a separate manuscript under review, we found that 39.5% of patients randomized to TR and enrolled >90 days post-stroke showed reduced global disability (improved mRS score); in contrast, natural history data indicate that mRS scores generally plateau by day 90 (49, 50), suggesting that TR benefits might generalize to improved global functional outcomes.
The purpose of the current study was to evaluate the feasibility of several expansions to our prior TR program, in two main ways, treatment and assessment. Treatment topics were extension of daily TR from 6 to 12 weeks; incorporation of therapy targeting the lower extremity (LE), in addition to UE therapy; incorporation of augmented reality (AR) into this TR system; introduction of games that use a real object to train instrumental activities of daily living (iADLs); and addition of a daily study pill to be taken at the start of TR, a feature that might improve secondary stroke prevention and also might facilitate clinical trials of restorative therapies that are administered in pill form. Assessment topics included addition of tests performed by the patient using the TR system independently, with no therapist present; validation of telehealth screening for depression and aphasia; and generation of actionable email reports to clinicians whenever a critical finding occurred. The feasibility of each of these expansions was examined.
In this prospective, single-group, therapeutic feasibility trial, patients underwent live assessment at the UC Irvine clinic twice at baseline, after which a telehealth system was delivered to the patient's home. Patients then received 12 weeks of TR therapy, 6 days/week, with a live clinic assessment at the end of week 6 and week 12. Patients were free to call the lab with questions. This study was approved by the UC Irvine IRB, and was registered as clinicaltrials.gov ID # NCT03460587.
Patients were recruited from the community through local advertisements. In sum, enrollees were adults with arm paresis due to stroke and no limiting cognitive deficits. Full entry criteria appear in Table 1. Patients signed informed consent (no surrogate consent) and were evaluated for eligibility at the first two visits.
After all eligibility criteria were confirmed, the patient signed a behavioral contract (51) that listed a personal treatment goal and the time when therapy would begin each day. An initial treatment plan was created by a licensed occupational therapist (OT) or physical therapist (PT), standardized by use of an algorithm that uses the 33 UE-FM sub-scores to identify the three greatest UE impairments. The algorithm suggests games and exercises that are matched to these three impairments and so calibrates initial TR games and exercises to each patient's impairment level.
Patients were provided 72 treatment sessions, 6/week for 12 weeks. Each session was 60 min in duration and consisted of least 15 min of functional games, at least 15 min of exercises, and 5 min of stroke education using a Jeopardy style game.
There were 12 input devices used by patients to interact with the TR system: a PlayStation Eye camera, motion game controller (PlayStation Move, Sony; Tokyo, Japan), joystick, small buttons (10), large buttons (4), toy pistol holding a Wii remote (Nintendo; Kyoto, Japan) with corresponding IR sensor bar, trackpad (Logitech; Newark, CA), grip force cylinder, pinch force cube, rotating shuttle wheel (Powermate, Griffin Technology; Nashville, TN), steering wheel with gas/brake, and a 9-DOF IMU containing a 3-axis accelerometer, gyroscope, and magnetometer.
A total of 114 exercises were available, targeting UE, LE, and trunk. Each was 1–5 min long and consisted of a video showing the assigned movement. Patients were instructed to move as in the video. Therapists had the option to incorporate standard equipment (e.g., resistance bands; Theraband; Akron OH) provided to patients at the time the TR system was delivered to the home, to be used while watching the exercise videos.
A total of 33 functional games were also available, each 1–5 min long. These stress motor control features, e.g., varying movement speed, range of motion, target size, extent of visuomotor tracking, or level of cognitive demand. Game features were selected and adjusted by the therapist. For example, during the whack-a-mole game, higher difficulty level means a broader area where targets can appear on the tabletop and less time to successfully hit the target. Therapists also select which input device the patient will use for game play, based on UE status, e.g., the flappy-bird game can be played using the grip force cylinder, pinch force cube, or trackpad.
Therapists also decided whether five photographs would be taken at random time points during a given game, to gain insights into how the patient was playing the game. After the day's 1-h of assignments were completed, patients were allowed to free play, i.e., to use the system to play functional games ad libitum.
Stroke education targeted five categories (Stroke Risk Factors, Stroke Prevention, Effects of Stroke, Diet, and Exercise) focused on secondary prevention. Patients made arm movements to enter their answers to multiple-choice questions, delivered via a video Jeopardy game format [an approach known to foster learning (52, 53)], and then received feedback on their answers.
To build each day's treatment session, therapists used a graphical interface to drag treatment elements into a 60-min planner for each day's session; they then adjusted the challenge level (games) and the duration (games and exercises), and selected which input device would be used to drive gameplay (games). The daily treatment plan was regularly updated by a therapist based on findings from videoconferences and from review of TR-based data. Four types of TR-based patient data were automatically transmitted from home to lab, in real time: system usage (time TR was used), patient performance (game scores), behavioral status (assessment scores), and photographs (during games and pill consumption).
Patients had 18 HIPAA-compliant videoconferences (VSee software; VSee; Sunnyvale, CA) with a licensed therapist: three times/week during weeks 1–2, two times/week during weeks 3–4, and one time/week during weeks 5–12. During videoconferences, questions were answered, feedback was provided, progress was reviewed, and on some days remote assessments were made.
During the 30 min prior to the TR session, the computer alerted the subject that the start time was coming soon. The subject hit a large tabletop button to begin the day's session and to start subsequent games/exercises after each one is completed. In this way, patients could take a break between games/exercises. Unsupervised sessions had the same treatment content as supervised sessions, but no therapist contact.
Key novel features added to the TR system and evaluated included the following:
(1) Lower extremity games and exercises: Our prior three TR studies (46–48) were focused exclusively on UE therapy. Here we also targeted the paretic LE, introducing LE exercise videos, LE driving games, and the AR “virtual varmint” game. In the driving games, patients used a steering wheel and gas/brake pedals to navigate a virtual terrain.
(2) Augmented reality (AR) gaming: With an AR-based approach, subjects interact in the real-world workspace with virtual computer-generated objects (47, 54, 55). This was used in the Virtual Varmint game, where subjects looked at a tabletop monitor that showed a real-time video display of their paretic foot; a virtual gopher was projected into this display, and when the subject's foot overlapped with the gopher, points were earned. A camera was placed under the table and pointed at the paretic foot. The TR computer displayed camera output on the tabletop monitor along with a computer-generated varmint (a gopher). Patients looking at the tabletop monitor thus used real-time images of their foot movements to manipulate a virtual varmint.
(3) Use of real objects to drive gameplay targeting Instrumental Activities of Daily Living (iADL): The TR accelerometer had a magnet and was attached to a lemonade pitcher by the patient prior to starting the game. Accelerometer data were sent to the TR computer. As the subject used the paretic arm to rotate the pitcher, a figure of a pitcher on the video screen moved synchronously, allowing the subject to use a real object to play a game where the goal was to fill empty cups to the correct level.
(4) Daily study pill consumption: Each day, patients were also asked to consume a study pill. This pill was an unblinded placebo (small sugar-free mint). The computer screen guided patients through a series of steps to open the pill container, put the lid on the TR table, put a pill in their hand, ingest the pill, and then replace the lid; the TR camera took a picture when the patient hit a button to indicate that each step was completed, and these pictures were later used to confirmed compliance with pill intake. Pills were kept in a yellow container, clipped to the TR table, and had a lid (DoseSmart; RxCap; Boston, MA) that sent a Bluetooth signal to the computer each time the container was opened.
(5) Expanded assessments, as below.
(6) E-mail actionable reports for critical findings: The study coordinator and lead investigator were automatically sent an email (with a suggested response) if either of two conditions arose: (1) sharp increase in pain, defined as increase in the shoulder pain score by ≥20/100, with the suggested response being to contact the patient same day; (2) non-compliance with therapy, defined as the patient failing to initiate TR for 3 days in a row, with the suggested response being to contact the patient same day.
(7) Reliance on home WiFi: In addition, we sought to evaluate the performance of each patient's home WiFi network. In each case, the home-based TR system was connected to the internet using the patient's personal wireless network rather than a study-provided wireless cellular modem.
To fully characterize enrollees, a broad range of assessments was evaluated, including measures of impairment, activities limitation, quality of life, and patient-reported measures. In addition to assessments at the four in-clinic visits, patients underwent assessments at home via the TR system, some of which were supervised by therapists and some scored with no therapist present.
The primary endpoint was the UE-FM scale (56, 57), which ranges from 0 to 66, with higher scores indicating less UE impairment. The main secondary endpoint for UE was the Box & Blocks (B&B) score (58), which counts the number of blocks a subject can lift and move across the table in 60 s. The two main LE secondary endpoints 10 meter walk test of gait velocity (59) (measured as the mean of two trials) and the LE-FM motor scale (56, 57), which ranges from 0 to 34, which higher scores indicating less LE impairment. Demographic data, medical history, and handedness (60) were obtained on study entry. The presence of aphasia was assessed using Philadelphia Naming Test (Form A) (61). The presence of neglect was assessed using the Line Cancellation Test (62).
A social network survey (PERSNET) was assessed during the live visit 6 weeks after enrollment. The results of these social network studies are presented in a separate companion paper (63).
Several additional dimensions of stroke outcome were measured at baseline and after 12 weeks of therapy: Optimization in Primary and Secondary Control (OPS) scale (64), which measures dedication to treatment goals across 12 questions, with scores ranging from 1 to 7 and higher scores reflecting greater motivation; Nottingham sensory scale (65), which assesses a range of sensory modalities in the distal UE, with maximum score of 11 and higher scores reflecting better sensory function; Geriatric Depression Scale (GDS) (66), which measures depression across 15 questions, with a maximum score of 15 and higher scores reflecting greater depression; Montreal Cognitive Assessment (MoCA) (67), which measures cognitive function, with maximum score of 30 and higher scores reflecting less cognitive impairment; modified Rankin Scale (mRS) (68), which measures global function (disability and dependence), with a maximum score of 6 and higher scores reflecting poorer function; EuroQol visual analog scale (EQ-VAS) (69), in which a subject rates his/her own health from 0 to 100 and higher scores reflect better quality of life; and modified Ashworth Spasticity (mAS) scale (70), which measures spasticity at the elbow flexor, with a maximum score of 4 and higher scores reflecting greater spasticity.
Some assessments were made by the study therapist using the TR system. Patient-reported outcomes, which are well aligned with scoring via videoconference, were examined. Hand function was measured using the Stroke Impact Scale (71) (SIS)-hand subsection, with a maximum score of 5 and higher scores reflecting better hand usage. This patient-reported outcome was measured during videoconferences in weeks 1 and in 12. Functional status was measured using the SIS-activity of daily living (ADL) subsection (71) during videoconferences in weeks 2 and in 12; the maximum score is 5, and higher scores reflect less difficulty with ADLs.
Other assessments were made by the patient, with no therapist present, using the TR system. To maximize the likelihood that the unsupervised patient at home would be successfully assessed, the focus here was on Likert scales and visual analog scales. The MOS Social Support Survey (72) (MOS-SSS) was scored via the TR system during week 2; scores range from 19 to 95, with higher scores reflecting stronger social support. The Brief Resilience Scale (73) was also scored via the TR system during week 4; maximum score is 30, with higher scores indicating better resilience. The Generalized Anxiety Disorder-7 (74) (GAD-7) scale was also scored via the TR system in week 3; maximum score is 21, with higher scores reflecting greater anxiety. Finally, shoulder pain and fatigue were assessed weekly with a focus on the first 6 weeks, using a visual analog scale (0–100) where higher numbers indicate greater pain and fatigue, respectively.
Some assessments were scored by the therapist at both a live visit and during a TR videoconference, in order to validate telehealth screening. Measures of mood and language were selected given the expectation that patients would likely be stable in these domains across the 1–3 weeks when serial testing was performed. The GDS score was scored during a week 9 videoconference and a week 12 in-clinic visit. The Philadelphia Naming Test (61) short form was scored twice; the maximum score is 30, and higher scores reflect less aphasia. Form A was scored during the live week 6 visit; Form B, which assesses 30 different objects, was scored by the therapist 1 week later, during a videoconference.
Data analysis used non-parametric statistical testing (JMP 13, SAS; Cary, NC). Statistical moments are presented as median (IQR). All analyses were two-tailed, with statistical significance set at p < 0.05 and no corrections made for multiple comparisons in this feasibility study. Within-subject changes in performance over time were analyzed using the Wilcoxon Signed Rank Test. Comparisons of subject values in weeks 1–6 vs. weeks 7–12 were analyzed using the Wilcoxon Rank Sums Test. Comparisons of two continuous variable were performed using the Spearman Rank Correlation Coefficient. For some telerehab-based assessments, data were missing for 1 subject; missing data were not imputed.
A total of 15 subjects were screened, of whom 13 were enrolled. Each was assigned 72 treatment sessions and 18 videoconferences with a study therapist, distributed over 12 weeks. For all subjects, the home WiFi network consistently supported TR data uploads and downloads as well as videoconferences. Of the 13 patients, nine received concomitant therapy outside of study procedures at some point during the study: seven at baseline; seven at 6-weeks, and eight at 12 weeks. There were no adverse events.
Subjects were a median of 61 years old and 4 months post-stroke at study entry (Table 2); 5 patients were <90 days post-stroke (range, 37–67 days), 8 patients were ≥90 days (range, 119–1,682), and 4 patients were >1 year post-stroke (range 16–56 months). Nine subjects were White, 3 Asian, and 1 African-American. One subject was Hispanic. All subjects had completed high school, with a median of 2 additional years of education. No patient had aphasia or spatial neglect.
Patients completed 50.4 h (33.3–56.7) of TR over the 12 weeks, and attended a median of 16 (14–18) videoconferences. Patients initiated the daily TR session (did >5% of assigned minutes) on 79.9% of days, and completed most of the session (did >50% of assigned minutes) on 65.7% of days. Common reasons for missed therapy sessions were vacation (55), demands from the patient's job (40), scheduling conflicts (32), and illness (22).
Compliance declined across the 12 weeks of therapy. Comparing weeks 1–6 with weeks 7–12: session initiation decreased from 86.4 to 73.5% (p < 0.0001; Figure 1A); and session completion decreased from 76.9 to 54.6% (p < 0.0001; Figure 1B), i.e., non-compliance doubled in the second 6-week block. The rate of session completion across the 12 weeks did not vary in relation to time post-stroke (r = 0.28, p = 0.36), age (r = 0.33, p = 0.27), or baseline scores on the GDS (r = −0.38, p = 0.2), MoCA (r = −0.28, p = 0.35), or UE-FM (r = −0.41, p = 0.17). Although the content and quantity of assigned therapy was constant over time, subjects completed a median of 61.5 (IQR = 33–65.8) min/day of therapy during weeks 1–6 vs. 43.6 (2–63.3) min/day during weeks 7–12 (p < 0.0001). Subjects engaged in free play after finishing their assigned therapy on 1/5 days during weeks 1–6 but only 1/16 days during weeks 7–12 (p < 0.0001).
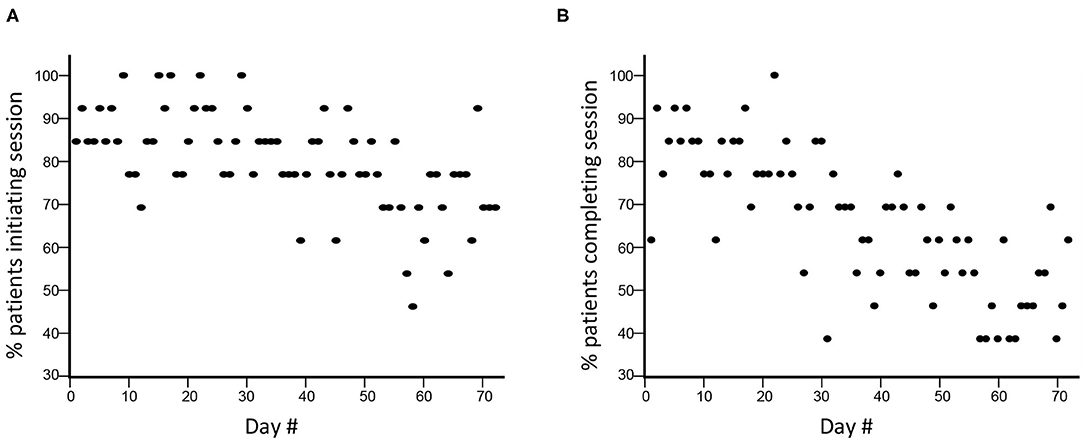
Figure 1. Compliance with TR assignments declined during the 12 weeks. (A) During TR weeks 1–6 (days 1–36), there was a decline in TR session initiation compared to weeks 7–12 (days 37–72), from 86.4 to 73.5% (p < 0.0001). (B) There was a similar decline in in TR session completion, from 76.9 to 54.6% (p < 0.0001).
TR was associated with significant UE motor gains. From baseline pre-therapy to follow-up after 12 weeks of TR therapy, the primary endpoint, UE-FM score, changed by 6 (2.5–12.5) points (p = 0.0005). Most of this change was achieved in the first 6 weeks, as UE-FM score change from baseline to week 6 was 6 (2–9.5) points (p = 0.0007). From week 6 to week 12, median change was 1 (−0.5 to 2) point (p = 0.19). The extent of 12-week gains on the UE-FM scale declined with increasing time post-stroke at enrollment (r = −0.63, p = 0.02). Similar gains were seen from baseline to week 12 for change in affected arm Box & Blocks score, with median change of 9 blocks (3.5–17.5) (p = 0.0005). Median change in unaffected arm Box & Blocks score during this interval was non-significant [2 blocks (−5 to 4.5) (p = 0.69)].
Findings were similar for the LE. Gait velocity improved by a median of 0.15 (0.07–0.22) m/s from baseline to week 12 (p = 0.0007). Most of this change was achieved in the first 6 weeks, where change from baseline was .08 (0.02–0.20) m/s (p = 0.007). Results were more modest for the LE-FM score change, which was 1 (−0.5 to 5) point (p = 0.065) over 12 weeks.
Several other classes of outcome measure also showed improvement. Scores on the mRS ranged from 2 to 3 at baseline and from 1 to 2 at week 12 (change over time, p = 0.03). This change was accounted for by improved mRS score in 6/13 patients (five with initial score = 3 and one with initial score = 2), 3 of whom were <90 days post-stroke (37–67 days) and 3 of whom were >90 days post-stroke (4, 5.5, and 56 months post-stroke) at study enrollment. In addition, the EQ-VAS increased from baseline to week 12 by a median of 15 (2.5–31), indicating improved self-rating of health state. Mood (GDS) improved over time (p = 0.05); note that a GDS score > 5, suggesting depression, was present in 3 subjects at week 1 and 0 subjects at week 12.
There were three types of remote assessments (Table 3). First, therapist-directed measures during videoconferences captured behavioral gains. Hand usage, measured using the SIS-hand scale, showed significant gains (p = 0.002). Functional status, measured using the SIS-ADL scale, showed improvement over time that narrowly missed significance (p = 0.06). Second, therapist-independent measures (no therapist online when scored) reliably assessed patient status. Median score on the MOS-SSS was 83 (69–92), indicating strong social support on average. Median score on the Brief Resilience Scale was 23.5 (22.25–26), indicating overall good resilience. Median score on the GAD-7 scale was 3 (0–8.5), indicating low anxiety on average. Scores for shoulder pain were stable from week 1 to week 6, rising slightly, from 0 (0–31) to 9 (0–25) (p = 0.46) on this 100-point visual analog scale. Fatigue, however, declined significantly from week 1 to week 6, from 36 (8–61) to 16 (0–43) (p = 0.004). Third, for two assessments, therapist scores obtained in-clinic were compared to those obtained during TR. The two sets of GDS scores obtained 3 weeks apart were closely related (r = 0.89, p < 0.0001; Figure 5) and showed an intraclass correlation coefficient of 0.66; note that one subject was not available for the week-9 videoconference. Findings on the Philadelphia Naming Test had a ceiling effect that limited comparisons, as scores on Form A in-clinic were perfect in all but two patients and scores on Form B via TR were perfect in all but three patients.
Augmented reality was successfully incorporated into home-based TR. The equipment was installed in the home, the AR game assigned by the therapist, and these were used by patients during TR (Figure 2). Similarly, an accelerometer could be applied to various objects by the patient at home (Figure 3), allowing movement of a real object in a functional way to play a game that emphasized iADLs.
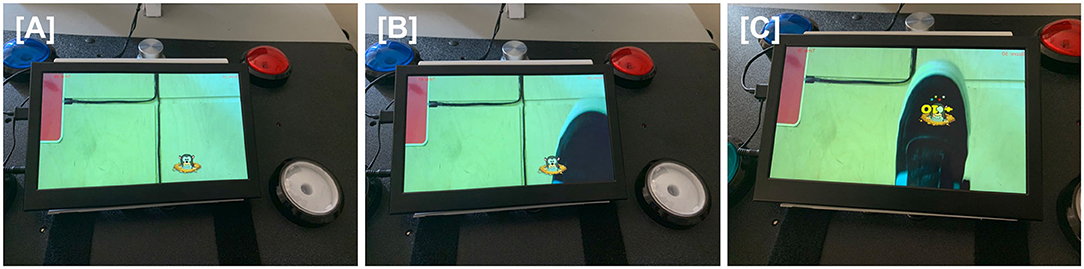
Figure 2. For the virtual varmint game, a camera under the table pointed at the floor captured live images, including the patient's paretic foot, that were projected onto the screen of a tabletop tablet. When patients directed their gaze at the tabletop, they were thus able to see real-time images of their foot movements. A virtual varmint was introduced into the tablet image, which the patient was able to manipulate with their foot. (A) A virtual image of a varmint is introduced onto the screen of the tabletop tablet. (B) The patient moves his foot toward the virtual varmint. (C) The patient swats the virtual varmint with his foot, scoring points.

Figure 3. Sensors were attached to real objects to play a game that drives an iADL. The patient grasped an actual water pitcher onto which an accelerometer (green arrow) was attached magnetically. Accelerometer data were sent to the computer running the TR program. As the subject rotated the hand-held pitcher, the figure of a pitcher on the video screen moved synchronously. In this way, the subject used a real object to play a game, displayed on the TR computer screen, where the goal was to fill empty cups to the correct level.
Consumption of a study pill once/day (Figure 4) was successfully incorporated into TR sessions. One subject requested not to have any photos taken. The remaining 12 subjects used their TR system on 681 days, and took their pill on 619 of these days, resulting in 90.9% compliance with daily study pill consumption. The Bluetooth-enabled pill bottle cap worked properly but there were difficulties keeping its software running at all times in the background of the TR program.

Figure 4. Photographs were used to confirm that the study pill was taken each day as instructed. (A) The patient is seated; arrow indicates cap on the bottle holding study pills. (B) The patient has removed the pill bottle lid and placed onto the trackpad; arrow indicates the lid. (C) The patient has removed one pill and placed into her palm; arrow indicates the pill. (D) The patient has taken the pill. (E) The lid has been replaced; arrow indicates the lid.
Email-alerts were sent to the PI and to the study coordinator reliably and with specificity whenever there was (1) a substantial increase in body or shoulder pain or (2) non-compliance with therapy for 3 days. During the study, reportable incidents only occurred in relation to non-compliance.
High dose rehabilitation therapy can improve outcomes after stroke, but this is not provided to many patients. Telehealth methods have the potential to overcome many of the barriers to high dose therapy, such as transportation limitations or limited regional access. In an effort to improve our approach to home-based TR, the current study aimed to evaluate the feasibility of several system expansions related to assessment and to treatment.
Enrollees were recruited at wide-ranging times post-stroke, had overall moderate motor deficits and little sensory deficits, were highly dedicated to treatment goals, lacked cognitive deficits, had low anxiety and depression symptoms, had good social support, and reported high resiliency (Tables 2, 3). In our prior studies, we relied on a Verizon wireless modem to connect the patient's home-based TR system to the internet and thereby enable communication between the home and the clinic. The current study found that we can instead rely on the patient's personal wireless network, an approach that, when available, has advantages such as connection speed.
Across the 12 weeks of TR, UE and LE motor status, functional status, and quality of life all improved significantly (Table 3), particularly during the first 6 weeks. These gains occurred as patients completed a median of 50.4 h/subject of TR. Some of this improvement might be related to spontaneous post-stroke recovery. Five of the 13 enrollees were <90 days post-stroke at enrollment, and so some of their behavioral improvement is likely attributable to spontaneous recovery; consistent with this, UE-FM gains declined with greater time post-stroke.
Our prior trial (48) evaluated 6 weeks of therapy provided 6 days/week (70-min sessions, 42 h total). Here we aimed to evaluate a course of TR lasting for twice as long: 12 weeks of therapy (60-min sessions, 72 h total). This was driven in part by our review of home-based technologies for stroke rehabilitation (75), which noted that across 31 studies, most technologies were evaluated for short time periods. In addition, larger doses of TR have been reported to result in greater benefit (76).
The rate with which subjects initiated (Figure 1A) and completed (Figure 1B) TR assignments declined significantly across the 12 weeks of therapy. During TR weeks 1–6, TR session completion was 76.9%, lower than the 98.3% value seen during a 6-week course of telerehabilitation in our 11-site study (48). Compliance was not related to time post-stroke, age, depression, cognitive status, or arm motor impairments at baseline, although the sample size is limited for examining these issues. Several reasons might account for lower compliance over time seen in the current study. Functional gains during the first 6 weeks might have reduced motivation to perform TR thereafter. Patients might have become bored with some games. During weeks 7–12, videoconferences were reduced from 3x/week to 1x/week, due to budgetary constraints, which might have contributed to the doubling of non-compliance during this period. These videoconferences were a stimulus for patient accountability, and so a reduction in their frequency might have adversely affected compliance. In addition to driving accountability, videoconferences also foster a relationship between patient and therapist that might be important to sustained compliance. In a qualitative study (77) of 13 patients randomized to TR at one site in our national trial, regular videoconferences with a therapist were highly rated. Fewer interactions during videoconferences might produce weaker patient-therapist bonds, contributing to non-compliance.
This study also examined the feasibility of treating both UE and LE motor deficits. While not all stroke survivors have motor deficits in both UE and LE, involvement of both is more common than is paresis in either alone (78). Despite this, only 2 of the 22 stroke TR trials have targeted both UE and LE deficits (45). The current study found that exercises and games targeting LE motor deficits were readily incorporated alongside those targeting UE, and were associated with significant gains in gait velocity.
Practice of real life tasks with real objects can increase object affordance and task ecology and is often incorporated into constraint induced therapy (51). The TR system is well suited to adopt this strategy. The current study found that a sensor could be attached to real objects, providing data that are used to drive game play that targeted pouring liquids (Figure 3), which is part of meal preparation, an important iADL.
An additional way to expand the functional relevance of TR therapy is to incorporate virtual objects that may be impractical or unsafe in the patient's home. AR integrates virtual elements into the real world (54) and was successfully incorporated into the TR system (Figure 2). AR introduces an additional form of human-computer interface that can be used to modulate a task's cognitive demand (55).
Daily ingestion of a study pill was integrated into the home-based TR system. Patients were prompted to take a study pill at the start of each session and did so 90.9% of the days that they initiated a TR session (Figure 4). Driving patient compliance with pill consumption each day might be useful in clinical practice, e.g., to improve secondary stroke prevention or in clinical research, e.g., when studying an orally ingested drug that might promote recovery, particularly since TR enables careful pairing of behavioral training with pill consumption (79–81).
TR not only provides an opportunity for remote therapy but also provides a platform for remotely measuring, both passively and actively (82), a broad range of human activities (83) and behavioral and psychological symptoms (84). This can promote greater independence and quicker access to healthcare professionals (85). The current results support the feasibility of using TR to measure hand usage (SIS-hand) and functional status (SIS-ADL).The GDS was validated for depression telescreening (Figure 5); interestingly, average scores at home were higher compared to when the same scale was administered in the clinic, in contradistinction to prior results obtained in a non-stroke population (86).
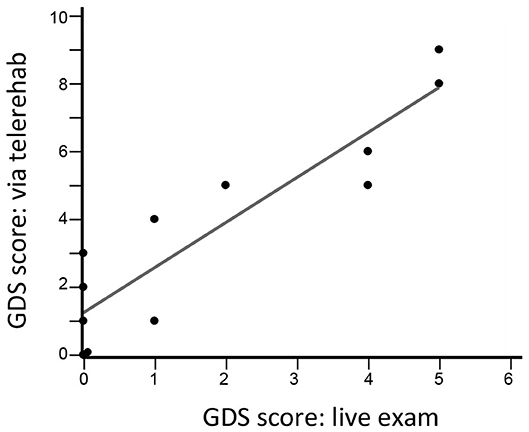
Figure 5. GDS scores during a live visit 12 weeks after study entry are closely related to GDS scores obtained during a TR videoconference 9 weeks after study entry (r = 0.89, p < 0.0001, n = 12). The intraclass correlation coefficient was 0.66.
We evaluated four assessments that were scored asynchronously, i.e., by the patient with no therapist supervision, and all were successfully collected. These include the MOS-SSS, which indicated strong social support; the Brief Resilience Scale, which showed overall good resilience; and the GAD-7, which showed low anxiety scores. In addition, shoulder pain and fatigue were assessed weekly. Shoulder pain, the most common adverse event in patients randomized to TR in our prior national trial (48), was mild and stable over 6 weeks. Patients reported a significant decline in fatigue over time.
A very large amount of data is generated by the TR system. Efficient approaches are needed to bring the most critically important findings to the attention of busy clinicians. We incorporated actionable reports, whereby a clinician is notified electronically of a critical finding, along with a suggested response. Electronic notification of critical results has advantages that include decreased workflow interruptions and more timely closed-loop communications of key patient data (87). Such reports are most effective when recommendations presented to clinicians are clear, explicit, and actionable (88). Such reports should focus on high quality observations that present critical new knowledge (89). Communication of critical results is a national patient safety goal emphasized by the Joint Commission, and is no less important in stroke recovery. The current pilot study provides support for actionable results to transmit critical findings in two categories, pain and treatment compliance.
Strengths of this feasibility study include successful evaluation of several new expanded TR features related to treatment and to assessment, including longer-term therapy, addition of therapy targeting the LE, increased dimensions of assessments, incorporation of real objects and AR, and introduction of a daily study pill. There were several key weaknesses, as well. The sample size was limited. As this was a feasibility study, there was a single treatment arm and no control group. Some patients might not have completed spontaneous recovery at study entry, although the goal was to evaluate new TR features rather than establish efficacy. The total number of daily limb movements during TR was not measured, as in our prior studies. No qualitative study was performed to better understand the perspectives of patients and caregivers. Current results incompletely generalize, as enrollees lacked substantial aphasia, neglect, sensory deficits, depression, and anxiety.
The current study examined the feasibility of adding new modules to a home-based TR system for patients with stroke. Some modules were therapy-focused, such as longer duration of therapy and ingestion of daily study medication, while others were diagnostic, such as assessments performed by the patient with no therapist supervision. These results inform future efforts to develop TR approaches to address the many aspects of treating patients with stroke.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by UC Irvine IRB. The patients/participants provided their written informed consent to participate in this study.
The study was designed by SC, LD, AM, JS, RA, RZ, AD, WS, and JH. The study was conducted, the manuscript was written, and critically revised by all authors.
This work was supported by 17IRG33460393 (American Heart Association/American Stroke Association) and UL1-TR001414 (NIH).
SC has served as a consultant for Constant Therapeutics, MicroTransponder, Neurolutions, SanBio, Stemedica, Fujifilm Toyama Chemical Co., NeuExcell, Medtronic, and TRCare. AD is an expert witness for Neuro Consults, LLC. LD, VL, JS, and RA are consultants for TRCare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. (2003) 2:43–53. doi: 10.1016/S1474-4422(03)00266-7
2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
3. Johnston SC, Hauser SL. Neurological disease on the global agenda. Ann Neurol. (2008) 64:A11–2. doi: 10.1002/ana.21477
4. Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. (2020) 54:171–9. doi: 10.1159/000506396
5. Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. (2004) 3:391–3. doi: 10.1016/S1474-4422(04)00800-2
6. Goldstein L, Adams R, Becker K, Furberg C, Gorelick P, Hademenos G, et al. Primary prevention of ischemic stroke: a statement for healthcare professionals from the stroke council of the American Heart Association. Stroke. (2001) 32:280–99. doi: 10.1161/01.STR.32.1.280
7. Rathore S, Hinn A, Cooper L, Tyroler H, Rosamond W. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke. (2002) 33:2718–21. doi: 10.1161/01.STR.0000035286.87503.31
8. Gresham G, Duncan P, Stason W, Adams H, Adelman A, Alexander D, et al. Post-Stroke Rehabilitation. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research (1995).
9. Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, et al. Interdisciplinary comprehensive arm rehabilitation evaluation (ICARE): a randomized controlled trial protocol. BMC Neurol. (2013) 13:5. doi: 10.1186/1471-2377-13-5
10. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98–169. doi: 10.1161/STR.0000000000000098
11. Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. (2013) 44:1111–6. doi: 10.1161/STROKEAHA.111.674671
12. Wyller TB, Sveen U, Sodring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil. (1997) 11:139–45. doi: 10.1177/026921559701100207
13. Galvin R, Murphy B, Cusack T, Stokes E. The impact of increased duration of exercise therapy on functional recovery following stroke–what is the evidence? Top Stroke Rehabil. (2008) 15:365–77. doi: 10.1310/tsr1504-365
14. Galvin R, Cusack T, O'Grady E, Murphy TB, Stokes E. Family-mediated exercise intervention (FAME): evaluation of a novel form of exercise delivery after stroke. Stroke. (2011) 42:681–6. doi: 10.1161/STROKEAHA.110.594689
15. Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke. (2004) 35:2529–39. doi: 10.1161/01.STR.0000143153.76460.7d
16. Cauraugh JH, Naik SK, Lodha N, Coombes SA, Summers JJ. Long-term rehabilitation for chronic stroke arm movements: a randomized controlled trial. Clin Rehabil. (2011) 25:1086–96. doi: 10.1177/0269215511410580
17. Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE. (2014) 9:e87987. doi: 10.1371/journal.pone.0087987
18. Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. (2014) 45:2053–8. doi: 10.1161/STROKEAHA.114.004695
19. Van Stan JH, Dijkers MP, Whyte J, Hart T, Turkstra LS, Zanca JM, et al. The rehabilitation treatment specification system: implications for improvements in research design, reporting, replication, and synthesis. Arch Phys Med Rehabil. (2019) 100:146–55. doi: 10.1016/j.apmr.2018.09.112
20. Outpatient Service Trialists. Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. Lancet. (2004) 363:352–6. doi: 10.1016/S0140-6736(04)15434-2
21. Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. (2011) 134:1591–609. doi: 10.1093/brain/awr039
22. Woldag H, Hummelsheim H. Evidence-based physiotherapeutic concepts for improving arm and hand function in stroke patients: a review. J Neurol. (2002) 249:518–28. doi: 10.1007/s004150200058
23. Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. (2008) 131:425–37. doi: 10.1093/brain/awm311
24. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. (2008) 51:S225–39. doi: 10.1044/1092-4388(2008/018)
25. Cramer SC, Riley JD. Neuroplasticity and brain repair after stroke. Curr Opin Neurol. (2008) 21:76–82. doi: 10.1097/WCO.0b013e3282f36cb6
26. Bloem BR, Dorsey ER, Okun MS. The coronavirus disease 2019 crisis as catalyst for telemedicine for chronic neurological disorders. Jama Neurol. (2020) 77:927–8. doi: 10.1001/jamaneurol.2020.1452
27. Richmond T, Peterson C, Cason J, Billings M, Terrell EA, Lee ACW, et al. American Telemedicine Association's principles for delivering telerehabilitation services. Int J Telerehabil. (2017) 9:63–8. doi: 10.5195/IJT.2017.6232
28. Demiris G, Shigaki CL, Schopp LH. An evaluation framework for a rural home-based telerehabilitation network. J Med Syst. (2005) 29:595–603. doi: 10.1007/s10916-005-6127-z
29. Coleman JJ, Frymark T, Franceschini NM, Theodoros DG. Assessment and treatment of cognition and communication skills in adults with acquired brain injury via telepractice: a systematic review. Am J Speech Lang Pathol. (2015) 24:295–315. doi: 10.1044/2015_AJSLP-14-0028
30. Theodoros DG. Telerehabilitation for service delivery in speech-language pathology. J Telemed Telecare. (2008) 14:221–4. doi: 10.1258/jtt.2007.007044
31. Hill AJ, Theodoros D, Russell T, Ward E. Using telerehabilitation to assess apraxia of speech in adults. Int J Lang Commun Disord. (2009) 44:731–47. doi: 10.1080/13682820802350537
32. Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. Tele-rehabilitation after stroke: an updated systematic review of the literature. J Stroke Cerebrovasc Dis. (2018) 27:2306–18. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.013
33. Hillsdon M, Thorogood M. A systematic review of physical activity promotion strategies. Br J Sports Med. (1996) 30:84–9. doi: 10.1136/bjsm.30.2.84
34. Coscia M, Wessel MJ, Chaudary U, Millan JDR, Micera S, Guggisberg A, et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain. (2019) 142:2182–97. doi: 10.1093/brain/awz181
35. Baranowski T, Buday R, Thompson DI, Baranowski J. Playing for real: video games and stories for health-related behavior change. Am J Prev Med. (2008) 34:74–82. doi: 10.1016/j.amepre.2007.09.027
36. Brox E, Fernandez-Luque L, Tøllefsen T. Healthy gaming – video game design to promote health. Appl Clin Inform. (2011) 2:128–42. doi: 10.4338/ACI-2010-10-R-0060
37. Hansen MM. Versatile, immersive, creative and dynamic virtual 3-D healthcare learning environments: a review of the literature. J Med Internet Res. (2008) 10:e26. doi: 10.2196/jmir.1051
38. Lieberman D. Designing serious games for learning and health in informal and formal settings. In: Ritterfeld M, Vorderer P, editors. Serious Games: Mechanisms and Effects. New York, NY: Routledge (2009). p. 117–30.
39. Thompson D, Baranowski T, Buday R, Baranowski J, Thompson V, Jago R, et al. Serious video games for health how behavioral science guided the development of a serious video game. Simul Gaming. (2010) 41:587–606. doi: 10.1177/1046878108328087
40. Robert Wood Johnson Foundation. Advancing the Field of Health Games: A Progress Report on Health Games Research. Robert wood johnson foundation (2011). Available online at: http://www.rwjf.org/content/rwjf/en/research-publications/find-rwjf-research/2011/03/advancing-the-field-of-health-games.html (accssed November 21, 2020).
41. Przybylski A, Rigby C, Ryan R. A motivational model of video game engagement. Rev Gen Psychol. (2010) 14:154–66. doi: 10.1037/a0019440
42. Duncan PW, Horner RD, Reker DM, Samsa GP, Hoenig H, Hamilton B, et al. Adherence to postacute rehabilitation guidelines is associated with functional recovery in stroke. Stroke. (2002) 33:167–77. doi: 10.1161/hs0102.101014
43. Jurkiewicz MT, Marzolini S, Oh P. Adherence to a home-based exercise program for individuals after stroke. Top Stroke Rehabil. (2011) 18:277–84. doi: 10.1310/tsr1803-277
44. Touillet A, Guesdon H, Bosser G, Beis JM, Paysant J. Assessment of compliance with prescribed activity by hemiplegic stroke patients after an exercise programme and physical activity education. Ann Phys Rehabil Med. (2010) 53:250–7, 257–65. doi: 10.1016/j.rehab.2010.03.005
45. Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev. (2020) 1:CD010255. doi: 10.1002/14651858.CD010255.pub3
46. Dodakian L, McKenzie AL, Le V, See J, Pearson-Fuhrhop K, Burke Quinlan E, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. (2017) 31:923–33. doi: 10.1177/1545968317733818
47. Zhou RJ, Hondori HM, Khademi M, Cassidy JM, Wu KM, Yang DZ, et al. Predicting gains with visuospatial training after stroke using an EEG measure of frontoparietal circuit function. Front Neurol. (2018) 9:597. doi: 10.3389/fneur.2018.00597
48. Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol. (2019) 76:1079–87. doi: 10.1001/jamaneurol.2019.1604
49. Jorgensen H, Nakayama H, Raaschou H, Vive-Larsen J, Stoier M, Olsen T. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen stroke study. Arch Phys Med Rehabil. (1995) 76:406–12. doi: 10.1016/S0003-9993(95)80568-0
50. de Havenon A, Tirschwell D, Heitsch L, Cramer S, Braun R, Cole J, et al. Variability of the modified Rankin scale score between day 90 and 1 year after ischemic stroke. Neurol Clin Pract. (2020).
51. Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. (2006) 296:2095–104. doi: 10.1001/jama.296.17.2095
52. Wirth LA, Breiner J. Jeopardy: using a familiar game to teach health. J Sch Health. (1997) 67:71–4. doi: 10.1111/j.1746-1561.1997.tb06304.x
53. Webb TP, Simpson D, Denson S, Duthie E Jr. Gaming used as an informal instructional technique: effects on learner knowledge and satisfaction. J Surg Educ. (2012) 69:330–4. doi: 10.1016/j.jsurg.2011.10.002
54. Baus O, Bouchard S. Moving from virtual reality exposure-based therapy to augmented reality exposure-based therapy: a review. Front Hum Neurosci. (2014) 8:112. doi: 10.3389/fnhum.2014.00112
55. Mousavi Hondori H, Khademi M, Dodakian L, McKenzie A, Lopes CV, Cramer SC. Choice of human-computer interaction mode in stroke rehabilitation. Neurorehabil Neural Repair. (2016) 30:258–65. doi: 10.1177/1545968315593805
56. Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, S S. The post-stroke hemiplegic patient: a method for evaluation of physical performance. Scand J Rehabil Med. (1975) 7:13–31.
57. See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer DJ, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. (2013) 27:732–41. doi: 10.1177/1545968313491000
58. Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. (1985) 39:386–91. doi: 10.5014/ajot.39.6.386
59. Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. (1997) 26:15–9. doi: 10.1093/ageing/26.1.15
60. Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. (1971) 9:97–113. doi: 10.1016/0028-3932(71)90067-4
61. Roach A, Schwartz MF, Martin N, Grewal R, Brecher A. The Philadelphia naming test: scoring and rationale. Clin Aphasiol. (1996) 24:121–33. doi: 10.1037/t56477-000
62. Albert ML. A simple test of visual neglect. Neurology. (1973) 23:658–64. doi: 10.1212/WNL.23.6.658
63. Podury A, Raefsky S, Dodakian L, McCafferty L, Le V, McKenzie A, et al. Social network structure is related to functional improvement from home-based telerehabilitation after stroke. Front Neurol. (in press).
64. Heckhausen J, Schulz R, Wrosch C. Developmental regulation in adulthood: optimization in primary and secondary control–a multiscale questionnaire (OPS-scales). In: Heckhausen J, Dweck C, editors. Motivation and Self-Regulation Across the Life Span. New York, NY: Cambridge University (1998). p. 50–77.
65. Lincoln N, Jackson J, Adams S. Reliability and revision of the Nottingham sensory assessment for stroke patients. Physiotherapy. (1998) 84:358–65. doi: 10.1016/S0031-9406(05)61454-X
66. Agrell B, Dehlin O. Comparison of six depression rating scales in geriatric stroke patients. Stroke. (1989) 20:1190–4. doi: 10.1161/01.STR.20.9.1190
67. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
68. Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. (2017) 48:2007–12. doi: 10.1161/STROKEAHA.117.017866
69. Rabin R, de Charro F. Eq-5d: a measure of health status from the EuroQoL group. Ann Med. (2001) 33:337–43. doi: 10.3109/07853890109002087
70. Bohannon R, Smith M. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. (1987) 67:206–7. doi: 10.1093/ptj/67.2.206
71. Duncan P, Wallace D, Lai S, Johnson D, Embretson S, Laster L. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. (1999) 30:2131–40. doi: 10.1161/01.STR.30.10.2131
72. Sherbourne CD, Stewart AL. The mos social support survey. Soc Sci Med. (1991) 32:705–14. doi: 10.1016/0277-9536(91)90150-B
73. Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. (2008) 15:194–200. doi: 10.1080/10705500802222972
74. Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder - the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
75. Chen Y, Abel KT, Janecek JT, Chen Y, Zheng K, Cramer SC. Home-based technologies for stroke rehabilitation: a systematic review. Int J Med Inform. (2019) 123:11–22. doi: 10.1016/j.ijmedinf.2018.12.001
76. Jansen-Kosterink S, In 't Veld RH, Hermens H, Vollenbroek-Hutten M. A telemedicine service as partial replacement of face-to-face physical rehabilitation: the relevance of use. Telemed J E Health. (2015) 21:808–13. doi: 10.1089/tmj.2014.0173
77. Chen Y, Chen Y, Zheng K, Dodakian L, See J, Zhou R, et al. A qualitative study on user acceptance of a home-based stroke telerehabilitation system. Top Stroke Rehabil. (2020) 27:81–92. doi: 10.1080/10749357.2019.1683792
78. de Freitas GR, Devuyst G, van Melle G, Bogousslavsky J. Motor strokes sparing the leg: different lesions and causes. Arch Neurol Chicago. (2000) 57:513–8. doi: 10.1001/archneur.57.4.513
79. Feeney D, Gonzalez A, Law W. Amphetamine, halperidol, and experience interact to affect the rate of recovery after motor cortex injury. Science. (1982) 217:855–7. doi: 10.1126/science.7100929
80. Fang PC, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1-40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. (2009) 41:544–9. doi: 10.1161/STROKEAHA.109.572073
81. Jeffers MS, Corbett D. Synergistic effects of enriched environment and task-specific reach training on poststroke recovery of motor function. Stroke. (2018) 49:1496–503. doi: 10.1161/STROKEAHA.118.020814
82. Choi YK, Lazar A, Demiris G, Thompson HJ. Emerging smart home technologies to facilitate engaging with aging. J Gerontol Nurs. (2019) 45:41–8. doi: 10.3928/00989134-20191105-06
83. Sanchez-Comas A, Synnes K, Hallberg J. Hardware for recognition of human activities: a review of smart home and aal related technologies. Sensors. (2020) 20:4227. doi: 10.3390/s20154227
84. Husebo BS, Heintz HL, Berge LI, Owoyemi P, Rahman AT, Vahia IV. Sensing technology to monitor behavioral and psychological symptoms and to assess treatment response in people with dementia. A systematic review. Front Pharmacol. (2019) 10:1699. doi: 10.3389/fphar.2019.01699
85. Sapci AH, Sapci HA. Innovative assisted living tools, remote monitoring technologies, artificial intelligence-driven solutions, and robotic systems for aging societies: systematic review. JMIR Aging. (2019) 2:e15429. doi: 10.2196/15429
86. Hansen CH, Walker J, Thekkumpurath P, Kleiboer A, Beale C, Sawhney A, et al. Screening medical patients for distress and depression: does measurement in the clinic prior to the consultation overestimate distress measured at home? Psychol Med. (2013) 43:2121–8. doi: 10.1017/S0033291712002930
87. Lacson R, Prevedello LM, Andriole KP, O'Connor SD, Roy C, Gandhi T, et al. Four-year impact of an alert notification system on closed-loop communication of critical test results. AJR Am J Roentgenol. (2014) 203:933–8. doi: 10.2214/AJR.14.13064
88. Sahni VA, Khorasani R. The actionable imaging report. Abdom Radiol. (2016) 41:429–43. doi: 10.1007/s00261-016-0679-x
Keywords: stroke, telehealth, recovery, rehabilitation, holistic
Citation: Cramer SC, Dodakian L, Le V, McKenzie A, See J, Augsburger R, Zhou RJ, Raefsky SM, Nguyen T, Vanderschelden B, Wong G, Bandak D, Nazarzai L, Dhand A, Scacchi W and Heckhausen J (2021) A Feasibility Study of Expanded Home-Based Telerehabilitation After Stroke. Front. Neurol. 11:611453. doi: 10.3389/fneur.2020.611453
Received: 29 September 2020; Accepted: 04 December 2020;
Published: 03 February 2021.
Edited by:
Swathi Kiran, Boston University, United StatesReviewed by:
Han Gil Seo, Seoul National University Hospital, South KoreaCopyright © 2021 Cramer, Dodakian, Le, McKenzie, See, Augsburger, Zhou, Raefsky, Nguyen, Vanderschelden, Wong, Bandak, Nazarzai, Dhand, Scacchi and Heckhausen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven C. Cramer, c2NjcmFtZXJAbWVkbmV0LnVjbGEuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.