
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 21 December 2020
Sec. Endovascular and Interventional Neurology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.609607
This article is part of the Research Topic Intracranial Atherosclerotic Disease: Epidemiology, Imaging, Treatment and Prognosis View all 17 articles
 Haiyan Liu1,2†
Haiyan Liu1,2† Yuehua Pu1†
Yuehua Pu1† Yilong Wang1,3,4,5
Yilong Wang1,3,4,5 Xinying Zou1
Xinying Zou1 Yuesong Pan3,4,5
Yuesong Pan3,4,5 Changqing Zhang1
Changqing Zhang1 Yannie O. Y. Soo6
Yannie O. Y. Soo6 Thomas W. H. Leung6
Thomas W. H. Leung6 Xingquan Zhao1,3,4,5
Xingquan Zhao1,3,4,5 Ka Sing Lawrence Wong6
Ka Sing Lawrence Wong6 Yongjun Wang1,3,4,5
Yongjun Wang1,3,4,5 Liping Liu1,3,4,5* on behalf of the Chinese IntraCranial AtheroSclerosis (CICAS) Study Group
Liping Liu1,3,4,5* on behalf of the Chinese IntraCranial AtheroSclerosis (CICAS) Study GroupBackground and Purpose: This study aimed to assess the effect of baseline white matter hyperintensities (WMH) on 1-year stroke recurrence and the functional outcome for patients with intracranial atherosclerosis (ICAS).
Methods: We analyzed 2,076 patients who were enrolled in the Chinese IntraCranial AtheroSclerosis (CICAS) study. ICAS and WMH were diagnosed by baseline magnetic resonance angiography. The primary outcomes were stroke recurrence and unfavorable functional outcome (modified Rankin Scale score 3–6) at 1 year.
Results: Of the 2,076 patients included in this study, 1,370 (65.99%) were men, and the mean age was 61.70 years. In total, 224 (10.79%) patients had no WMH and no ICAS, 922 (44.41%) patients had WMH and no ICAS, 157 (7.56%) patients had ICAS and no WMH, and 773 (37.24%) had both WMH and ICAS. During the follow-up period, 87 patients had a recurrent stroke and 333 had unfavorable outcomes at 1 year. Compared to WMH (–) ICAS (–) group, the adjusted odd ratios and 95% confidence interval for unfavorable functional outcome were 0.791 (0.470–1.332; p = 0.3779) in the WMH (+) ICAS (–) group, 1.920 (1.024–3.600; p = 0.0421) in the WMH (–) ICAS (+) group, and 2.046 (1.230–3.403; p = 0.0058) in the WMH (+) ICAS (+) group. There was no significant difference in stroke recurrence risk among the four groups.
Conclusion: ICAS coexisting with WMH may predict an unfavorable functional outcome at 1 year, but not stroke recurrence.
Intracranial atherosclerosis (ICAS) is likely to be the most common stroke subtype worldwide (1). It accounts for about 15% of Caucasian patients with ischemic attack or stroke (2) and nearly 50% of ischemic strokes in Asia (1, 3, 4). Cerebral small vessel disease (SVD) is another kind of common cerebrovascular disease, which manifests as recent small subcortical infarcts, lacunes, white matter hyperintensities (WMH), perivascular spaces, microbleeds, and brain atrophy on neuroimaging (5). SVD may also have racial differences, and an observational study found that Han Chinese had a higher prevalence of confluent WMH than white Australians, but had a similar prevalence of lacunes and microbleeds (6). Some studies have suggested that patients with ICAS may be particularly prone to having coexistent SVD (7–9). Patients with multiple ICAS lesions, occlusive lesions, and atherosclerotic lesions in the posterior circulation were more likely to coexist with WMH (10). Previous studies have found that WMHs were associated with risk of incident stroke, ischemic stroke, intracerebral hemorrhage, dementia, Alzheimer's Disease, and death (11). A study from the PICASSO (Prevention of Cardiovascular Events in Ischemic Stroke Patients with High Risk of Cerebral Hemorrhage) trial showed that the severity of WMH on baseline brain magnetic resonance imaging scans may be associated with a 2.15-fold risk of stroke, 2.11-fold risk of ischemic stroke, and 3.72-fold risk of hemorrhagic stroke (12).
It is still uncertain whether the presence of SVD on baseline magnetic resonance imaging could affect the stroke recurrent risk and functional outcome of patients with ICAS. A subgroup analysis of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) showed that the SVD image markers are not independently associated with an increased risk of stroke in patients with ICAS (13). Lau et al. validated a total small vessel disease score in two independent prospective studies and found that a higher score was associated with an increased risk of recurrent ischemic stroke and intracerebral hemorrhage (14). A study from the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial, indicated that SVD and ICAS may have different levels of risk for future strokes. SVD was associated with more disability and bleeding events, and ICAS was associated with an increased risk of stroke and disability in patients with minor stroke and TIA at 3 months (15).
This study hypothesized that ICAS and WMH may interact with each other, resulting in an increased risk of unfavorable functional outcome and stroke recurrence. The study aimed to assess the effect of baseline WMH on 1-year stroke recurrence and the functional outcome for patients with acute cerebral ischemia in the Chinese Intracranial Atherosclerosis (CICAS) study database.
From October 2007 to June 2009, a total of 2,864 patients were recruited into the CICAS study (4). We excluded 444 patients who did not have interpretable images for the presence of white matter changes and 344 patients with extracranial large artery stenosis or occlusion. Finally, 2,076 patients were included in this study. The CICAS study was a multicenter, hospital-based cohort study that included 22 hospitals in mainland China and the Hong Kong Special Administrative Region. The study was approved by the Institutional Review Boards of the participating hospitals. Details of the CICAS study design and the definition of baseline characteristics have been published previously (4). This study recruited patients with cerebral ischemia, aged from 18 to 80, admitted within 7 days of symptom onset. We excluded patients who were clinically unstable and those that required close monitoring or were disabled before admission, physically or subjectively unable to comply with magnetic resonance (MR) examination or had severe comorbidity, and those who were presumed to have had a cardioembolic stroke such as atrial fibrillation.
All MR images were stored in digital format and were read by two readers blinded to the clinical information of subjects (4). Intracranial stenosis or occlusion was estimated by 3-dimensional time-of-flight MR angiography (3D TOF MRA). ICAS was defined as stenosis more than or equal to 50% on MRA for the main intracranial arteries. Intracranial arterial segments included the distal internal carotid artery (ICA), middle cerebral artery (MCA) (M1 and M2 segment), anterior cerebral artery (ACA) (A1 and A2 segment), posterior cerebral artery (PCA) (P1 and P2 segment), and basilar artery (BA). Duplex color Doppler ultrasound or contrast-enhanced MRA were used for extracranial carotid vessels. WMH was defined as a hyperintense lesion on both T2-weighted imaging and FLAIR but was usually not seen on T1-weighted imaging or showed faint hypointensity (5). DWI was used to differentiate acute ischemic stroke lesions from WMH. The severity of WMH was assessed according to the Fazekas scale (16). Scores in periventricular white matter hyperintensities (PWMH) and deep white matter hyperintensities (DWMH) were evaluated separately and summed together as Fazekas scores. The total Fazekas score was classified into two categories: 0–3 and 4–6. According to the presence of ICAS or WMH, the patients were classified into four groups: WMH (–) ICAS (–), WMH (+) ICAS (–), WMH (–) ICAS (+), and WMH (+) ICAS (+). WMH (–) was defined as a Fazekas score equal to 0.
We monitored the included patients for 1 year through telephone or face to face consultations with trained research personnel from the follow-up center of the Beijing Tiantan Hospital and the Hong Kong Prince of Wales Hospital. The primary outcomes were stroke recurrence and unfavorable functional outcome (modified Rankin Scale score 3–6). Stroke recurrence was defined as sudden functional deterioration in neurological status with a decrease in the National Institutes of Health Stroke Scale (NIHSS) score of four or more, or a new focal neurological deficit of vascular origin lasting >24 h, including recurrent ischemic or hemorrhagic stroke (4).
All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). Two-sided P-values <0.05 were considered statistically significant. Continuous variables were summarized as median (interquartile range) or mean (SD). Categorical variables were presented as numbers (percentages). The χ2 test (or Fisher exact test, when appropriate) was used to test differences in proportions for categorical variables. A Wilcoxon signed rank test was used to test differences in median for the continuous variables. The cumulative probabilities of stroke recurrence over time were estimated by the Kaplan–Meier product-limit method and were compared among WMH (–) ICAS (–), WMH (+) ICAS (–), WMH (–) ICAS (+), and WMH (+) ICAS (+) group using the log-rank test. Cox proportional hazards regression analyses were used to estimate the hazards ratio of each group for stroke recurrence adjusted by potential confounders. For the unfavorable functional outcome at 1 year, odds ratio (OR), and 95% confidence interval (CI) were given and logistic regression was used for adjusting confounders. All tests were two-sided with a significance level fixed at 5%.
Among the 2,076 patients included in this study, 1,370 (65.99%) were men, and the mean (SD) of age was 61.70 (11.32) years. Two hundred and twenty-four (10.79%) patients had no WMH and no ICAS, 922 (44.41%) patients had WMH and no ICAS, 157 (7.56%) patients had ICAS and no WMH, and 773 (37.24%) had both WMH and ICAS. The baseline characteristics for each group are shown in Table 1. Patients with both WMH and ICAS were older and more likely to have diabetes, hypertension, hyperhomocysteinemia, and a history of stroke. The median (interquartile range) of baseline Fazekas score in the WMH (+) group was 3 (2–4).
During the follow-up period, 87 patients had a recurrent stroke and 333 had unfavorable outcomes at 1 year. The predictors of recurrent stroke in 2,076 patients are shown in Table 2. Patients with recurrent stroke were older and a higher percentage had hypertension, history of stroke, heart disease, multiple infarction, and ICAS. The stroke recurrence rate was 6 (2.68%) in the WMH (–) ICAS (–) group, 30 (3.25%) in the WMH (+) ICAS (–) group, 5 (3.18%) in the WMH (–) ICAS (+) group, 46 (5.95%) in the WMH (+) ICAS (+) group. Compared to the WMH (–) ICAS (–) group, the hazard ratio (HR) and 95% confidence interval (CI) was 0.682 (0.270–1.721), 1.124 (0.340–3.719), and 1.263 (0.510–3.131) in each group, respectively, after adjustment by age, sex, diabetes, hypertension, hyperhomocysteinemia, family history of stroke, current smoker, heavy drink, history of stroke, and heart disease. There was no significant difference between groups for recurrent risk of stroke at 1 year. For patients with ICAS, the stroke recurrent rate had no significant difference between Fazekas score 0–3 and 4–6. The HR (95%CI) of stroke recurrence in the patients in each group are shown in Table 3. Kaplan-Meier curves of recurrent stroke showed in Figure 1.
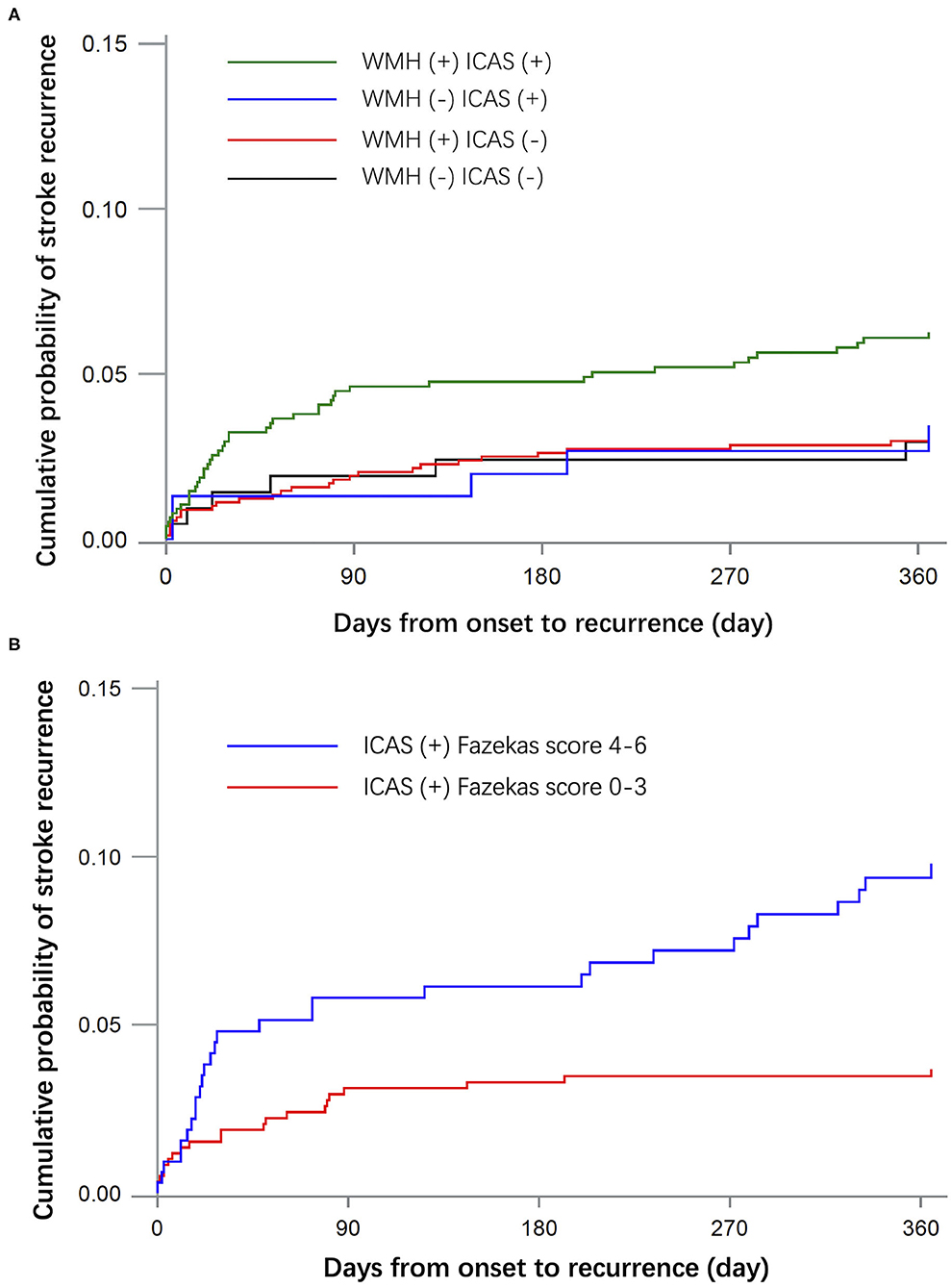
Figure 1. Kaplan-Meier curves of recurrent stroke within 1 year. (A) Shows the cumulative incidence of recurrent stroke in groups WMH (–) ICAS (–), WMH (+) ICAS (–), WMH (–) ICAS (+) and WMH (+) ICAS (+). (B) Shows the cumulative incidence of recurrent stroke in patients with ICAS by different severity of WMH.
For unfavorable functional outcome at 1 year, there were 21 (10.05%) patients in the WMH (–) ICAS (–) group, 96 (28.83%) in the WMH (+) ICAS (–) group, 26 (7.81%) in the WMH (–) ICAS (+) group, and 190 (26.46%) in the WMH (+) ICAS (+) group. Compared to the WMH (–) ICAS (–) group, the adjusted odd ratios (OR) and 95% CI were 0.791 (0.470–1.332), 1.920 (1.024–3.600), 2.046 (1.230–3.403) in the other three groups, respectively. The OR (95%CI) of unfavorable outcomes in patients from each group are shown in Table 4. The distribution of Functional Scores at 1 year are shown in Figure 2.
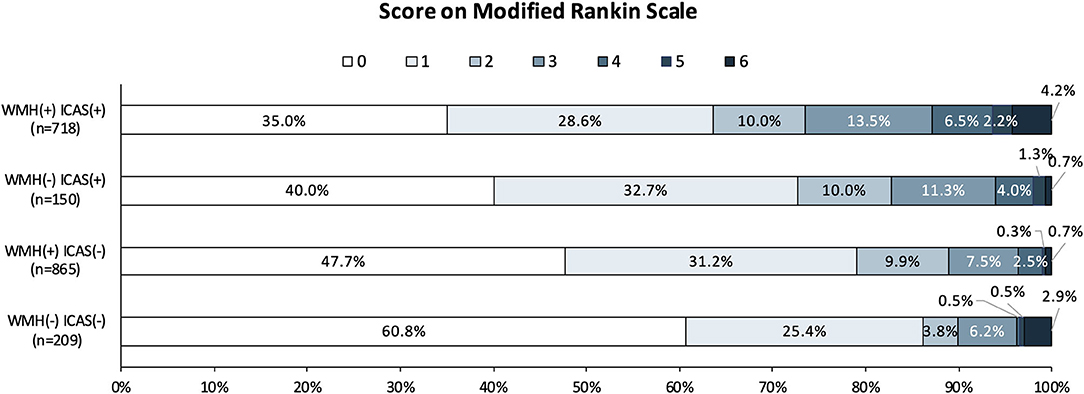
Figure 2. Distribution of mRS score at 1 year in the four groups. Scores on the modified Rankin scale range from 0 to 6, with 0 indicating no symptoms; 1, no clinically significant disability; 2, slight disability (able to handle own affairs without assistance but unable to carry out all previous activities); 3, moderate disability requiring some help, but able to walk unassisted; 4, moderately severe disability (unable to attend body needs and unable to walk); 5, severe disability (requiring constant nursing care and attention); and 6, death. mRS scores at 1 year were missing in 134 cases.
The findings of this study are consistent with those of other recently reported trials (13, 15), which indicated that the presence of WMH on baseline magnetic resonance imaging is not independently associated with an increased risk of stroke recurrence in patients with ICAS. The coexistence of intracranial atherosclerosis with changes in white matter may predict an unfavorable functional outcome at 1 year. Although the 1-year stroke recurrence rate in the ICAS and WMH group was higher than that of other groups, there was no significant difference after adjusting by confounders, suggesting that age, baseline NIHSS score, the number of acute infarctions, and medical history may be more relevant in stroke recurrence than WMH alone. For patients with ICAS, the severity of WMH had no significant correlation with the risk of stroke recurrence and functional outcome.
WMH is a common imaging feature that is associated with small-vessel disease (SVD) and related to stroke incidence, dementia, or death (11). SVD is common in patients with ICAS. Kwon et al. (13) observed that nearly half of ICAS patients had SVD on a baseline MRI. Lee et al. (9) reported a significant association between WMH and stroke subtypes. The large-artery-disease group in this study had a higher prevalence of WMH than other groups (55.4% in the large-artery-disease group, 30.3% in the lacunar group, and 14.3% in the cardioembolic). In a CICAS study, 41.45% of acute ischemic stroke or TIA patients had WMH at baseline MRI and patients with ICAS had a higher percentage of WMH than those without (45.77% vs. 37.67%) (4). However, there are limited data on the effect of WMH on stroke recurrence or functional outcome in patients with acute cerebral ischemia. A study from the SAMMPRIS trial showed that the presence of SVD on baseline magnetic resonance imaging was not independently associated with an increased risk of stroke in patients with ICAS (13). Data from the CHANCE trial found that SVD was associated with more disability and bleeding events and that ICAS is associated with an increased risk of stroke and disability in patients with minor stroke and TIA at 3 months, which implies that SVD and ICAS may represent different vascular pathologies and play distinct roles in stroke outcomes (15).
How to explain the results by the pathogenesis? The mechanisms of ICAS-related stroke include parent artery atherosclerosis occluding penetrating artery, artery to artery embolism, hypoperfusion, and mixed mechanisms). Different pathogenesis has different recurrence risk. Previous reports showed that ICAS with multiple infarctions (indicated by an artery to artery mechanism) (17), borderzone infarcts and impaired collateral flow (hemodynamic markers) (18), or a mixed mechanism of artery to artery embolism and hypoperfusion (19) were more likely to have recurrent stroke. WMHs are often considered to be a consequence of chronic hypoperfusion (20). Patients with hemodynamically more severe ICAS are more likely to have more severe ipsilateral WMH (21). Some researchers believe that impaired cerebral blood flow is one of the physiopathology mechanisms of WMH (22). However, the relationship between WMH and ICAS is still not clear. A meta-analysis study reviewed available published (and unpublished) research on cerebral blood flow (CBF) in small vessel disease, and data showed that a high WMH load is associated with lower CBF, but they are not causally related. In cross-sectional studies, low CBF was observed in most patients with more WMHs. The association was less pronounced after removing non-age matched subjects and those with dementia, which suggests that the underlying association is between reduced CBF and age or dementia rather than just WMH (20). Therefore, ICAS and WMH represent different types of pathophysiology. The ICAS-related recurrence of stroke correlates with plaque stability, hemodynamics, and collateral circulation, while the mechanisms of stroke recurrence related to WMH are more complex and involve arteriolar tortuosity, reduced vessel density, and occlusive venous collagenosis (23). WMH is usually associated with brain dysfunction, and ICAS is associated with stroke recurrence.
This study had several limitations. First, the included data was, in some cases, older, which could cause bias due to the advancement of medical treatment strategies such as dual anti platelet and high intensity statin. This was also a hospital-based study involving upper first-class hospitals, and most of the enrolled patients had a minor stroke, meaning that selective bias affects the included population. Second, the study included patients within seven days of onset, the rate of stroke recurrence might have been underestimated. Third, we did not distinguish the subtypes of recurrent stroke. Finally, other manifestations of cerebral small vessel disease (recent small subcortical infarcts, lacunes, perivascular spaces, microbleeds, and brain atrophy on neuroimaging) were not analyzed in this study.
In conclusion, the presence of WMH on baseline magnetic resonance imaging is not an independent predictor of stroke recurrence for patients with acute cerebral ischemia. The co-existence of intracranial atherosclerosis with changes in white matter may predict an unfavorable functional outcome at 1 year. For patients with ICAS, the severity of WMH had no significant correlation with the risk of stroke recurrence and functional outcome. The manifestations of WMH with different pathophysiological mechanisms may be different in images and the visual features of ICAS-related WMH need to be further explored in future studies.
Requests for access to the data reported in this paper will be considered by the corresponding author.
The studies involving human participants were reviewed and approved by Beijing Tiantan Hospital of Capital Medical University Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
HL and YPu undertook the literature search, data analysis and interpretation, figures, and wrote the manuscript. LL, XZh, YiW, YoW, TL, and KW contributed to the study design, data analysis, data interpretation, and provided comments on the manuscript. XZo and YS contributed to data collection and image analysis and interpretation. CZ contributed to the image interpretation of white matter lesions. YPa contributed to data analysis and interpretation and figures. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81870913 and 81820108012) and the National Key R&D Program of China (2016YFC1307301 and 2018YFC1312402).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Hongyi Yan for their work on statistical analysis, the study quality coordinators for their meticulous data quality control, and the investigators and participants in the CICAS study.
1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107505776
2. Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol. (2020) 19:413–21. doi: 10.1016/S1474-4422(20)30079-X
3. Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045x
4. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
5. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
6. Mok V, Srikanth V, Xiong Y, Phan TG, Moran C, Chu S, et al. Race-ethnicity and cerebral small vessel disease–comparison between Chinese and White populations. Int J Stroke. (2014) 9:36–42. doi: 10.1111/ijs12270
7. Chutinet A, Biffi A, Kanakis A, Fitzpatrick KM, Furie KL, Rost NS. Severity of leukoaraiosis in large vessel atherosclerotic disease. AJNR Am J Neuroradiol. (2012) 33:1591–5. doi: 10.3174/ajnrA3015
8. Park JH, Kwon HM, Lee J, Kim DS, Ovbiagele B. Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur J Neurol. (2015) 22:44–52. doi: 10.1111/ene12431
9. Lee SJ, Kim JS, Lee KS, An JY, Kim W, Kim YI, et al. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol. (2008) 8:31. doi: 10.1186/1471-2377-8-31
10. Duan W, Pu Y, Liu H, Jing J, Pan Y, Zou X, et al. Association between leukoaraiosis and symptomatic intracranial large artery stenoses and occlusions: the Chinese Intracranial Atherosclerosis (CICAS) study. Aging Dis. (2018) 9:1074–83. doi: 10.14336/AD.20180118
11. Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:81–94. doi: 10.1001/jamaneurol.20183122
12. Park JH, Heo SH, Lee MH, Kwon HS, Kwon SU, Lee JS, et al. White matter hyperintensities and recurrent stroke risk in patients with stroke with small-vessel disease. Eur J Neurol. (2019) 26:911–8. doi: 10.1111/ene13908
13. Kwon H-M, Lynn MJ, Turan TN, Derdeyn CP, Fiorella D, Lane BF, et al. Frequency, risk factors, and outcome of coexistent small vessel disease and intracranial arterial stenosis. JAMA Neurol. (2016) 73:36–42. doi: 10.1001/jamaneurol.20153145
14. Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology. (2017) 88:2260–7. doi: 10.1212/WNL0000000000004042
15. Chen H, Pan Y, Zong L, Jing J, Meng X, Xu Y, et al. Cerebral small vessel disease or intracranial large vessel atherosclerosis may carry different risk for future strokes. Stroke Vasc Neurol. (2020) 5:128–37. doi: 10.1136/svn-2019-000305
16. Fazekas F. Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2351
17. Pan Y, Meng X, Jing J, Li H, Zhao X, Liu L, et al. Association of multiple infarctions and ICAS with outcomes of minor stroke and TIA. Neurology. (2017) 88:1081–8. doi: 10.1212/WNL0000000000003719
18. Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. (2018) 50: 143–7. doi: 10.1161/STROKEAHA.118.020840
19. Feng X, Chan KL, Lan L, Abrigo J, Liu J, Fang H, et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: classification and clinical implications. Stroke. (2019) 50:2692–9. doi: 10.1161/STROKEAHA.119025732
20. Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJM, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab. (2016) 36:1653–67. doi: 10.1177/0271678X16662891
21. Fang H, Leng X, Pu Y, Zou X, Pan Y, Song B, et al. Hemodynamic significance of middle cerebral artery stenosis associated with the severity of ipsilateral white matter changes. Front Neurol. (2020) 11:214. doi: 10.3389/fneur.202000214
22. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. (2019) 18:684–96. doi: 10.1016/S1474-4422(19)30079-1
Keywords: white matter hyperintensities, intracranial atherosclerosis (ICAS), stroke recurrence, small vessel disease (SVD), outcome
Citation: Liu H, Pu Y, Wang Y, Zou X, Pan Y, Zhang C, Soo YOY, Leung TWH, Zhao X, Wong KSL, Wang Y and Liu L (2020) Intracranial Atherosclerosis Coexisting With White Matter Hyperintensities May Predict Unfavorable Functional Outcome in Patients With Acute Cerebral Ischemia. Front. Neurol. 11:609607. doi: 10.3389/fneur.2020.609607
Received: 23 September 2020; Accepted: 27 November 2020;
Published: 21 December 2020.
Edited by:
Shyam Prabhakaran, University of Chicago, United StatesReviewed by:
Yang-Ha Hwang, Kyungpook National University, South KoreaCopyright © 2020 Liu, Pu,Wang, Zou, Pan, Zhang, Soo, Leung, Zhao, Wong, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Liu, bGlwaW5nX3Npc3RlckAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.