
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 21 December 2020
Sec. Movement Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.606925
 Marian L. Dale1*
Marian L. Dale1* Barbara H. Brumbach2
Barbara H. Brumbach2 Adam L. Boxer3
Adam L. Boxer3 Amie L. Hiller1, on behalf of the AL-108-231 investigators
Amie L. Hiller1, on behalf of the AL-108-231 investigatorsIntroduction: Amantadine anecdotally improves gait in progressive supranuclear palsy (PSP) but definitive data is lacking. We investigated associations between amantadine usage, gait, cognition, and activities of daily living in 310 subjects with PSP using data from the davunetide trial.
Method: We compared baseline demographics, PSP Rating Scale (PSPRS), Repeat Battery for the Assessment of Neuropsychological Status (RBANS), and Schwab and England Activities of Daily Living (SEADL) scores between subjects taking vs. not taking amantadine using chi-square tests for categorical variables and independent sample t-tests for continuous variables. Using the general linear model (GLM), we tested whether group status predicted total PSPRS, PSPRS-gait and midline, total RBANS, RBANS-attention, and SEADL before and after the 52-weeks follow-up.
Results: Subjects taking vs. not taking amantadine were similar at baseline, except subjects taking amantadine had a higher Clinical Global Impression (CGI) Score (p = 0.01). However, the CGI change score did not differ between groups at week 52 (p = 0.10). Using GLM models (controlling for covariates), we found that subjects taking vs. not taking amantadine did not significantly predict total PSPRS, PSPRS-gait and midline, total RBANS, RBANS-attention, or SEADL at baseline, week 52, or the change score between baseline and week 52.
Discussion: This post-hoc analysis of the davunetide trial did not find an association between amantadine and gait or cognitive measures in PSP, but was not powered to find such a difference. Future studies should still examine amantadine for symptomatic benefit in multiple PSP subtypes.
There are limited pharmacological options available for gait impairment and falls in progressive supranuclear palsy (PSP), but movement disorder clinicians anecdotally note that patients with PSP report fewer falls when taking amantadine. Some clinicians report improved freezing of gait in patients with PSP on amantadine; others suspect improved attention may reduce falls. Other than anecdotal use of amantadine, there are no established pharmacological interventions for postural instability or falls PSP. Levodopa can be helpful for gait to the degree that it improves concurrent bradykinesia (particularly in the PSP-parkinsonism subtype) (1), but it does not improve postural instability (2, 3).
Several retrospective studies have examined amantadine as a treatment for PSP, but none specifically assessed changes in gait or balance. In a retrospective case review of 14 patients with PSP, Rajput found 43% showed improved bradykinesia or rigidity on amantadine, but gait and balance were not specifically assessed (4). In a retrospective review of 16 patients with PSP, Jackson et al. found that of seven patients treated with amantadine, only one displayed improved parkinsonian symptoms overall, and six either showed no change or worsened (5). Kompoliti et al. examined clinical records of 12 autopsy-confirmed PSP cases and found that five of 12 patients received amantadine (6). Two of those five demonstrated modest improvement of parkinsonism (both patients) and neck dystonia (one patient). Three of the five amantadine-treated patients complained of overall deterioration (reported side effects included orthostasis and hallucinations or delusions) (6). In a larger and more promising retrospective review, Niefort and Golbe calculated risk/benefit ratios for various pharmacologic treatments in 87 patients PSP and found that carbidopa-levodopa and amantadine gave the best risk/benefit ratios as monotherapy (0.61 and 0.80, respectively) (7). Other agents studied included MAO-B inhibitors, TCAs, and SSRIs. Overall, existing retrospective reviews of amantadine in PSP are mixed and find that a subset of patients' parkinsonian symptoms respond to amantadine.
Two small studies have prospectively examined the role of amantadine for freezing of gait in PSP. Using a crossover design, Kondo found a significant reduction in freezing of gait on the Freezing of Gait Questionnaire in two subjects with PSP when taking amantadine (8). In another prospective study of 200 mg amantadine IV given twice daily for 2 days, 1 PSP subject showed a mild improvement in freezing of gait, but the other did not respond (9). The effect of amantadine on gait and balance in PSP has not been systematically examined in a large, prospective trial.
As an initial step toward more definitively answering this clinically relevant question, the davunetide trial offers a large, longitudinal dataset in which the relationship between amantadine usage, gait, and cognition in PSP can be examined. The davunetide trial was a randomized, double-blind, placebo-controlled phase 2/3 study of a microtubule stabilizer and reducer of tau phosphorylation (10). It did not achieve its primary endpoints on the PSP Rating Scale (PSPRS) or the Schwab and England Activities of Daily Living (SEADL), but provided the opportunity to examine the natural history of PSP, including motoric, cognitive, imaging, and CSF biomarkers. Information regarding subjects' amantadine status (taking or not taking and average dose at baseline) is also available within the dataset. We hypothesized that PSP subjects taking amantadine during the davunetide trial may have increased attention and improved gait and balance compared to subjects not on amantadine, and sought to analyze this in a post-hoc review of the davunetide dataset.
We obtained deidentified longitudinal participant data from baseline through week 52 in 313 randomized PSP subjects in the davunetide trial. We excluded three subjects who were not on amantadine for the duration of the trial or subjects who didn't have data for both baseline and week 52 timepoints, leaving 310 subjects for this analysis. We calculated the average daily total dose of amantadine for the group at baseline. Subjects met PSP criteria from the national Neuroprotection and Natural History in Parkinson Plus Syndromes (“NNIPPS”) Study (11), as the 2017 MDS PSP criteria were not available at the time of recruitment. This analysis was approved by the Oregon Health and Science University Investigational Review Board and was not classified as human research due to the deidentified nature of the data (IRB # 20472).
For taking and not taking amantadine groups, baseline descriptive data analyzed included age, sex, Schwab and England Activities of Daily Living Scale (SEADL) (12), Clinical Global Impression (CGI) (13), Geriatric Depression Scale (GDS) (14), PSP Rating Scale (PSPRS) (15), and the Repeatable Battery for Neuropsychological Status (RBANS) (16). All variables used in analyses were approximately normal for both groups. To determine if there were group differences at baseline, chi-square tests were used for categorical variables and independent sample t-tests for continuous variables.
We then analyzed group differences in SEADL, CGI, Clinical Global Impression-Change (CGI-C), GDS, PSPRS, and RBANS outcomes as well as component scores from the RBANS and PSPRS over the 52-weeks study, including change scores. The RBANS is a cognitive battery that includes with five domains: immediate memory, attention, visuospatial/constructional, language, and delayed memory (16). Total scores range from 40 to 160, and lower scores indicate more severe cognitive deficit. The PSPRS is a global scale of PSP severity and includes subsections for history, mentation, bulbar exam, oculomotor exam, limb motor exam, and gait and midline exam (15). Higher scores on the PSPRS correspond to more severe PSP symptoms (maximum score 100). Within the RBANS we extracted the attention sub-score for further analysis, and within the PSPRS we extracted the gait and midline sub-score. The attention score comprises digit span and coding and the gait and midline sub-score tests the following five measures: neck rigidity and dystonia, arising from a chair, gait, postural stability, and sitting down. Finally, we used the general linear model (GLM) to assess whether there were differences between amantadine groups in RBANS total, RBANS-attention, PSPRS total, PSPRS-gait and midline, and SEADL scores while accounting for possible covariates. Covariates included age, disease duration, davunetide vs. placebo group status, baseline CGI scores, and baseline scores of the respective dependent variable for the 52 weeks and change scores.
Of 310 subjects analyzed, 43 patients were on amantadine; 267 were not. Among the 43 patients who were on amantadine at the start of the study the average daily dose was 227.9 mg (SD 95.3). Eight of the 43 subjects on amantadine were on a baseline dose of 100 mg or less. Only 10 of the subjects on amantadine were noted to have PSP with parkinsonian features; the majority were considered to have classic Richardson syndrome.
At baseline, taking vs. not taking amantadine groups did not significantly differ on demographics such as age, sex, activities of daily living (SEADL), and depression scores (GDS). The only notable group difference was that the group on amantadine had a higher CGI score at baseline, indicative of more severe illness (Table 1). Taking and not taking amantadine group differences within the davunetide and placebo groups were also examined at week 52 for SEADL, CGI, CGI-C, PSPRS total, RBANS total, PSPRS total change score, and RBANS total change score (Table 1). There were no statistically significant group differences.
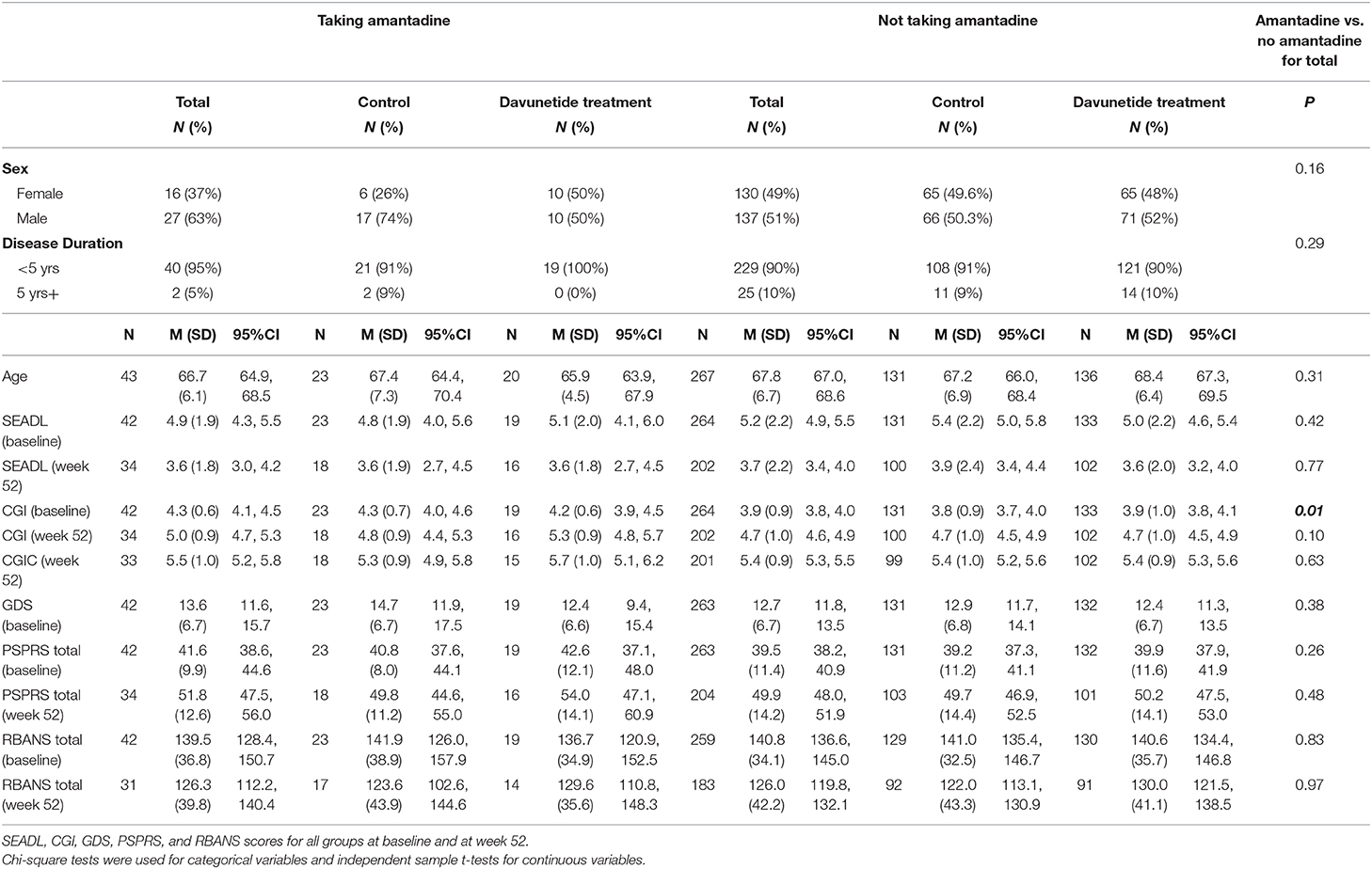
Table 1. Baseline demographics of subjects in the davunetide trial in taking and not taking amantadine groups.
Using the GLM we then assessed the following outcome variables: RBANS total, RBANS-attention, PSPRS total, PSPRS-gait and midline, and SEADL scores while controlling for covariates (age, disease duration, davunetide vs. placebo group status, baseline CGI scores, and baseline scores of the respective dependent variable for the 52 weeks and change scores). The primary predictor variable was whether or not someone was taking amantadine. Models were evaluated for each outcome variable at baseline, week 52, and the change score between baseline and week 52. Whether or not someone was taking amantadine did not significantly predict any of the outcome variables (RBANS total, RBANS-attention, PSPRS total, PSPRS-gait and midline scores, or SEADL), at baseline, week 52, or in the change score.
This post-hoc analysis of the davunetide trial failed to show significant associations between amantadine use and indices of gait and cognitive impairment in PSP. Nonetheless, this is the first analysis of the role of amantadine in a large, longitudinal dataset in PSP. Prior studies examining amantadine and freezing of gait in PSP showed mixed results and were limited to small sample sizes (8, 9). No large studies focusing on the potential role of amantadine for gait and cognition in PSP have been conducted.
The davunetide trial was not powered to find a group difference by amantadine status, and the dataset has several other important limitations. We lacked statistical power to analyze outcomes by baseline or cumulative amantadine dosage. The subjects taking amantadine may have had more severe disease at baseline as reflected in their CGI ratings. We did not have information on fall frequency or the geographic distribution of subjects taking vs. not taking amantadine. Also, the available cognitive and gait data lacked certain important elements. There was no measure of impulsivity in the cognitive data, a feature that clinicians know increases fall risk in PSP. There is also no specific measure of freezing of gait in the PSPRS, and this is relevant because some clinicians note that freezing of gait responds to amantadine in a subset of patients with PSP. Because this trial was conducted prior to establishment of the 2017 Movement Disorders Society PSP Criteria, the dataset lacks sufficient information for statistical analysis of the potential utility of amantadine in various PSP-subtypes.
Based on its purported mechanism of action, there may still be reason to power a larger study to explore the role of amantadine in gait and cognition in PSP. In Parkinson's disease, amantadine may modulate dopamine neurotransmission by promoting dopamine release and inhibiting uptake (17). This dopaminergic effect may benefit the subset of PSP patients with bradykinesia, such as in PSP-parkinsonism. Amantadine's NMDA-receptor antagonist properties account for its role in awareness and attention in diseases such as traumatic brain injury (18–20), ADHD, (21–24), catatonia, (25–28) multiple sclerosis (29), and reduced consciousness (30–34). Reduction of bradykinesia and improvement in awareness would both conceptually improve gait and balance. Finally, there is evidence that amantadine inhibits microglial activation and decreases neural inflammation (35), potentially improving the overall disease course in neurodegenerative disorders such as PSP.
This first analysis of amantadine in a large, longitudinal dataset in PSP failed to show significant associations between amantadine use and indices of gait and cognitive impairment in PSP, but was constrained by the methodological limitations of a post-hoc analysis. This analysis did not specifically compare subjects before and after the initiation of amantadine. It is possible that amantadine provides a symptomatic benefit after initiation that later wanes with chronic treatment. Additionally, the majority of subjects in the davunetide trial met 2009 criteria for PSP-Richardson syndrome. Amantadine may still be a beneficial symptomatic treatment for the other subtypes of PSP delineated in the 2017 MDS criteria. Future studies should compare symptomatic benefit before and after amantadine initiation in multiple PSP subtypes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Oregon Health & Science University Investigational Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Australia: David Williams; Canada: Anne Louise Lafontaine, Connie Marras, Mandar Jog, Michael Panisset, Anthony Lang, Lesley Parker, Alistair J. Stewart; France: Jean-Christophe Corvol, Jean-Philippe Azulay, Philippe Couratier; Germany: Brit Mollenhauer, Stefan Lorenzl, Albert Ludolph, Reiner Benecke, Gunter Hoglinger, Axel Lipp, Heinz Reichmann, Dirk Woitalla; United Kingdom: Dennis Chan, Adam Zermansky, David Burn, Andrew Lees; Israel: Illana Gozes, United States: Adam Boxer, Bruce L. Miller, Iryna V. Lobach, Erik Roberson, Lawrence Honig, Edward Zamrini, Rajesh Pahwa, Yvette Bordelon, Erika Driver-Dunkley, Stephanie Lessig, Mark Lew, Kyle Womack, Brad Boeve, Joseph Ferrara, Argyle Hillis, Daniel Kaufer, Rajeev Kumar, Tao Xie, Steven Gunzler, Theresa Zesiewicz, Praveen Dayalu, Lawrence Golbe, Murray Grossman, Joseph Jankovic, Scott McGinnis, Anthony Santiago, Paul Tuite, Stuart Isaacson, Julie Leegwater-Kim, Irene Litvan, David S. Knopman, Lon S. Schneider, Rachelle S. Doody, Lawrence I. Golbe, Erik D. Roberson, Mary Koestler, Clifford R. Jack, Jr., Viviana Van Deerlin, Christopher Randolph, Steve Whitaker, Joe Hirman, Michael Gold, Bruce H. Morimoto.
MD: organization and execution of the research project and writing of the first draft of the manuscript. BB: design, execution, review and critique of the statistical analysis, and review and critique of the manuscript preparation. AB and AH: conception of the research project and review and critique of the manuscript preparation. All authors contributed to the article and approved the submitted version.
MD received a pilot grant from the Parkinson Center of Oregon, an OCTRI KL2 Scholarship Award (KL2TR002370), a Collins Medical Trust Pilot Project Award, and a Loan Repayment Program (LRP) award from NIH-NINDS for projects examining cerebellar TMS in progressive supranuclear palsy. AB receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Association for Frontotemporal Degeneration, and the Alzheimer's Association. He has served as a consultant for Abbvie, AGTC, Alector, Arkuda, Arvinas, Asceneuron, AZTherapeutics, Bioage, Eisai, Ionis, Lundbeck, Passage BIO, Pinteon, Regeneron, Samumed, Third Rock,Transposon and UCB, and received research support from Biogen, Eisai, Eli Lilly, Genentech, Novartis, Roche, and TauRx. AH received a pilot grant from the Huntington's Disease Society of America examining psychological stress and salivary cortisol in Huntington's disease and a grant from Adamas Pharmaceuticals to examine the effects of extended release amantadine on gait and balance in Parkinson's disease.
AH received funding from Adamas Pharmaceuticals for an investigator-initiated study looking at the effects of extended release amantadine (Gocovri) on falls and gait measures in persons with Parkinson's disease.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CGI, Clinical Global Impression; CGI-C, Clinical Global Impression-Change; GDS, Geriatric Depression Scale; PSPRS, PSP Rating Scale; PSPRS-gait and midline, PSP Rating Scale gait and midline subscore; RBANS, Repeat Battery for the Assessment of Neuropsychological Status; RBANS-attention, RBANS attention subscore; SEADL, Schwab and England Activities of Daily Living.
1. Stamelou M, Hoglinger G. A review of treatment options for progressive supranuclear palsy. CNS Drugs. (2016) 30:629–36. doi: 10.1007/s40263-016-0347-2
2. Van der Heeden JF, Marinus J, Martinez-Marin P, Van Hilten JJ. Importance of non-dopaminergic features in evaluating disease severity of Parkinson's disease. Neurology. (2014) 82:412–8. doi: 10.1212/WNL.0000000000000087
3. Di Giulio I, St George RJ, Kalliolia E, Peters AL, Limousin P, Day BL. Maintaining balance against force perturbations: impaired mechanisms unresponsive to levodopa in Parkinson's disease. J Neurophysiol. (2016) 116:493–502. doi: 10.1152/jn.00996.2015
4. Rajput AH, Uitti RJ, Fenton ME, Georges D. Amantadine effectiveness in multiple system atrophy and progressive supranuclear palsy. Parkinsonism Relat Disord. (1998) 3:211–4. doi: 10.1016/S1353-8020(97)00022-9
5. Jackson JA, Jankovic J, Ford J. Progressive supranuclear palsy: clinical features and response to treatment in 16 patients. Ann Neurol. (1983) 13:273–8. doi: 10.1002/ana.410130308
6. Kompoliti K, Goetz CG, Litvan I, Jellinger K, Verny M. Pharmacological therapy in progressive supranuclear palsy. Arch Neurol. (1998) 55:1099–102. doi: 10.1001/archneur.55.8.1099
7. Nieforth KA, Golbe LI. Retrospective study of drug response in 87 patients with progressive supranuclear palsy. Clin Neuropharmacol. (1993) 16:338–46. doi: 10.1097/00002826-199308000-00006
8. Kondo T. Drug intervention for freezing of gait to dopaminergic therapy: a pilot resistant study. Parkinsonism Relat Disord. (2006) 12:S63–6. doi: 10.1016/j.parkreldis.2006.05.018
9. Kim YE, Yun JY, Jeon BS. Effect of intravenous amantadine on dopaminergic drug-resistant freezing of gait. Parkinsonism Relat Disord. (2011) 17:491–2. doi: 10.1016/j.parkreldis.2011.03.010
10. Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomized, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. (2014) 13:676–85. doi: 10.1016/S1474-4422(14)70088-2
11. Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders:the NNIPPS study. Brain. (2009) 132:156–71. doi: 10.1093/brain/awn291
12. Schwab R, England A. Projecton technique for evaluating surgery in Parkinson's disease. In: Gillingham F, Donaldson M, editor. Third Symposium on Parkinson's Disease Research. Edinburgh, Scotland: ES Livingstone (1969).
13. Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. (1997) 11(Suppl.2):S22–32. doi: 10.1097/00002093-199700112-00004
14. Yesavage JA, Brink TL, Rolse TL, Lum O, Huang V, Adey M, et al. Development and validity of a Geriatric Depression Scale: a preliminary report. J Psychiatric Res. (1983) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
15. Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. (2007) 130:1552–65. doi: 10.1093/brain/awm032
16. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. (1998) 20:310–9. doi: 10.1076/jcen.20.3.310.823
17. Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson's disease. Neurology. (2012) 78:1096–9. doi: 10.1212/WNL.0b013e31824e8f0d
18. Stelmaschuk S, Will MC, Meyers T. Amantadine to treat cognitive dysfunction in moderate to severe traumatic brain injury. J Trauma Nurs. (2015) 22:194–203. doi: 10.1097/JTN.0000000000000138
19. Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil. (2013) 28:260–5. doi: 10.1097/HTR.0b013e318257fbc6
20. Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. (2012) 366:819–26. doi: 10.1056/NEJMoa1102609
21. Donfrancesco R, Calderoni D, Vitiello B. Open-label amantadine in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. (2007) 17:657–64. doi: 10.1089/cap.2006.0128
22. Mohammadi M-R, Kazemi M-R, Zia E, Rezazadeh S-A, Tabrizi M, Akhondzadeh S. Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double-blind trial. Hum Psychopharmacol. (2010) 25:560–5. doi: 10.1002/hup.1154
23. Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. (2013) 22:55–60. doi: 10.1007/s00787-012-0362-x
24. McGrane IR, Loveland JG, Zaluski HJ. Adjunctive amantadine treatment for aggressive behavior in children: a series of eight cases. J Child Adolesc Psychopharmacol. (2016) 26:935–8. doi: 10.1089/cap.2016.0042
25. Ellul P, Rotge JY, Choucha W. Resistant catatonia in a high-functioning autism spectrum disorder patient successfully treated with amantadine. J Child Adolesc Psychopharmacol. (2015) 25:726. doi: 10.1089/cap.2015.0064
26. De Lucena DF, Pinto JP, Hallak JE, Crippa JA, Gama CS. Short-term treatment of catatonia with amantadine in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol. (2012) 32:569–72. doi: 10.1097/JCP.0b013e31825ebf6e
27. Ene-Stroescu V, Nguyen T, Waiblinger BE. Excellent response to amantadine in a patient with bipolar disorder and catatonia. J Neuropsychiatry Clin Neurosci. (2014) 26:E43. doi: 10.1176/appi.neuropsych.13020038
28. Goetz M, Kitzlerova E, Hrdlicka M, Dhossche D. Combined use of electroconvulsive therapy and amantadine in adolescent catatonia precipitated by cyber-bullying. J Child Adolesc Psychopharmacol. (2013) 23:228–31. doi: 10.1089/cap.2012.0045
29. Yang TT, Wang L, Deng XY, Yu G. Pharmacological treatments for fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. (2017) 15:256–61. doi: 10.1016/j.jns.2017.07.042
30. Schnakers C, Hustinx R, Vandewalle G, Majerus S, Moonen G, Boly M, et al. Measuring the effect of amantadine in chronic anoxic minimally conscious state. J Neurol Neurosurg Psychiatry. (2008) 79:225–7. doi: 10.1136/jnnp.2007.124099
31. Saniova B, Drobny M, Kneslova L, Minarik M. The outcome of patients with severe head injuries treated with amantadine sulphate. J Neural Transm. (2004) 111:511–4. doi: 10.1007/s00702-004-0112-4
32. Patrick PD, Blackman JA, Mabry JL, Buck ML, Gurka MJ, Conaway MR. Dopamine agonist therapy in low-response children following traumatic brain injury. J Child Neurol. (2006) 21:879–85. doi: 10.1177/08830738060210100901
33. Lehnerer SM, Scheibe F, Buchert R, Kliesch S, Meisel A. Awakening with amantadine from a persistent vegetative state after subarachnoid haemorrhage. BMJ Case Rep. (2017) 2017:bcr2017220305. doi: 10.1136/bcr-2017-220305
34. Reynolds JC, Rittenberger JC, Callway CW. Methylphenidate and amantadine to stimulate reawakening in comatose patients resuscitated from cardiac arrest. Resuscitation. (2013) 84:818–24. doi: 10.1016/j.resuscitation.2012.11.014
Keywords: progressive supranuclear palsy, amantadine, gait, balance, cognition
Citation: Dale ML, Brumbach BH, Boxer AL and Hiller AL (2020) Associations Between Amantadine Usage, Gait, and Cognition in PSP: A post-hoc Analysis of the Davunetide Trial. Front. Neurol. 11:606925. doi: 10.3389/fneur.2020.606925
Received: 16 September 2020; Accepted: 30 November 2020;
Published: 21 December 2020.
Edited by:
Pedro J. Garcia-Ruiz, University Hospital Fundación Jiménez Díaz, SpainReviewed by:
Stefan Lorenzl, Agatharied Hospital GmbH, GermanyCopyright © 2020 Dale, Brumbach, Boxer and Hiller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marian L. Dale, ZGFsZW1Ab2hzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.