
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 03 December 2020
Sec. Pediatric Neurology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.597505
This article is part of the Research Topic Personalized Precision Medicine in Autism Spectrum Related Disorders View all 15 articles
Introduction: Previous research suggests children diagnosed with autism spectrum disorder (ASD or “autism”) born extremely and very preterm face substantially delayed development than their peers born full-term. Further, children born preterm are proposed to show a unique behavioral phenotype, which may overlap with characteristics of autism, making it difficult to disentangle their clinical presentation. To clarify the presentation of autism in children born preterm, this study examined differences in key indicators of child development (expressive language, receptive language, fine motor, and visual reception) and characteristics of autism (social affect and repetitive, restricted behaviors).
Materials and Methods: One fifty-eight children (136 full-term, twenty-two preterm) diagnosed with autism, aged 22–34 months, were identified prospectively using the Social Attention and Communication Surveillance tools during community-based, developmental surveillance checks in the second year of life. Those identified at “high likelihood” of an autism diagnosis were administered the Mullen Scales of Early Learning and the Autism Diagnostic Observation Schedule.
Results: The children born preterm and full-term did not differ significantly in their fine motor, visual reception, expressive language, or receptive language skills. No significant differences in social affect and repetitive and restrictive behavior traits were found.
Discussion: The findings of this study differs from previous research where children diagnosed with autism born very or extremely preterm were developmentally delayed and had greater autistic traits than their term-born peers. These null findings may relate to the large proportion of children born moderate to late preterm in this sample. This study was unique in its use of a community-based, prospectively identified sample of children diagnosed with autism at an early age. It may be that children in these groups differ from clinic- and hospital-based samples, that potential differences emerge later in development, or that within the autism spectrum, children born preterm and full-term develop similarly. It was concluded that within the current sample, at 2 years of age, children diagnosed with autism born preterm are similar to their peers born full-term. Thus, when clinicians identify characteristics of autism in children born preterm, it is important to refer the child for a diagnostic assessment for autism.
Two key areas of development characterize a diagnosis of autism spectrum disorder (ASD), hereafter “autism”: differences in social-communication (e.g., eye contact and interest in peers) and restricted, repetitive patterns of behavior (RRBs; e.g., fixated interests and stereotyped motor movements) (1). For children born preterm, there is a risk of early markers of autism, such as atypical eye gaze and protodeclarative pointing (2), being misattributed to the long-term effects from their preterm birth (3), as these can also be observed in children born preterm who do not go on to be diagnosed with autism (4, 5), despite the higher than expected prevalence of autism in children born preterm (6). This has the potential to delay diagnosis and appropriate support.
As survival rates following preterm birth increase with medical advances, more becomes known about the developmental outcomes of children born preterm (7). Children are considered preterm if they were born prior to or during the thirty-sixth week of gestation and full-term if they were born between the thirty-seventh and forty-second weeks of gestation (8). In Australia, 8.50% of children are born preterm (9). This is comparable to the estimated rate of 8.60% for developed regions and lower than the world-wide average estimated rate of 11.10% (10). There are several classifications for preterm birth based on gestational age: extremely preterm (<28 weeks gestation), very preterm (28–32 weeks gestation), and moderate to late preterm (32–36 weeks gestation) (11). Moderate to late preterm births account for 84.70% of preterm births across the world (12). Across the classifications for prematurity, children born preterm have a higher likelihood for developmental difficulties, such as having a neurodevelopmental disability (13) or meeting Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for any mental health disorder (6, 14). Although there is no single known cause, several factors may increase the likelihood for preterm birth, such as multiple gestation, maternal ethnicity, and maternal age (15). A gene by environment interaction process is likely in the etiology of preterm birth (3).
By comparing children born preterm to children born full-term, Johnson and Marlow (16) identified and described a “preterm phenotype” characterized by a distinct pattern of behaviors. The preterm phenotype is believed to result in higher rates of attentional, cognitive, and socio-emotional difficulties that can be evident across the lifespan (16, 17). These difficulties have been attributed to reduced intrauterine development of the nervous system and complications typically associated with preterm birth (3). Atypical early life experiences [e.g., over-stimulation from the neonatal intensive care unit (NICU) environment] associated with preterm birth may contribute to differences in early brain development (18). While those born preterm are often described as needing to “catch up” to those born full-term, these structural differences typically continue throughout childhood development into adulthood (19). This suggests that, rather than being delayed, brain development after preterm birth has its own trajectory (16, 19).
Research on development after preterm birth largely focuses on extreme and very preterm birth; however, a “dose effect” (17) can be observed across prematurity, in which likelihood for developmental concerns is inversely associated with gestational age. The effects of moderate to late preterm birth, where the impact of dose effect would be weakest, can be observed throughout childhood and adolescence. For example, at 2 years of age, children born moderate to late preterm were twice as likely to have a neuromotor or sensory impairment when compared to children born full-term (13). In a meta-analysis of seventy-four studies, children and adolescents born preterm had significantly lower full scale and performance intelligence quotients than their full-term counterparts (20). A dose effect was observed, with effect sizes ranging from medium and large for children born extremely preterm, reducing to small effects for children born moderate to late preterm (20). Interestingly, children born moderate to late preterm were not significantly different than their peers born full-term on verbal intelligence (20), indicating that some differences in development from preterm birth are less clear, or even undetectable, for children born moderate to late preterm.
The association between autism and preterm birth has been investigated to better understand the potential implications of preterm birth on social development. For children under the age of three, there is an estimated prevalence of 7.00% for autism in children born preterm (21). This is substantially higher than population estimates of autism for children in that age group with an estimated prevalence in the United States at 0.02% (22) or Sweden at 0.80% (23). It has been suggested that, as preterm brain development has its own trajectory (16, 19), autism may manifest differently in children born preterm (3, 24). Some evidence for this hypothesis can be found when examining cerebellum development in children on the spectrum born preterm (25–27). Further, many risk factors for autism are common characteristics of preterm births, such as low birth weight (28, 29), birth complications, more days in spent in hospital following birth (30–32), maternal infection (33), and being born small for gestational age (29). There is emerging evidence for a relationship between preterm birth and autism diagnoses, with prevalence rates of autism having an inverse relationship with gestation age (6). This suggests the importance of examining autism in preterm populations across the different categories for preterm birth to understand the dose effect across development. The relationship between preterm birth and autism is further complicated by the hypothesis that children born preterm with subtle autism traits are misdiagnosed, as their atypical behavior is attributed to their preterm birth rather than a neurodevelopmental condition (3, 16). Common markers for autism include gaze aversion and inconsistent or lack of social smiling; both behaviors that can be observed in infants born preterm with and without autism (34–36). Furthermore, children born preterm are more likely to have visual and/or hearing impairments (37, 38), which may result in atypical eye contact or response to name and subsequently a false positive screen for autism (39). Thus, developmental difficulties related to preterm birth further entangle the presentation of development after preterm birth with autism.
Attempts have been made to identify early markers for autism specific to preterm populations with mixed results. One study found that 9 month old (corrected age) infants born preterm who showed typical eye contact and gaze behavior were more likely to be screened as “high likelihood” for autism using screening measures (40). Corrected aged is used for children born preterm to account for their expected development had they been born full-term and is calculated subtracting the number of weeks born preterm from the child's chronological age. This finding was surprising as atypical eye contact and gaze are normally reliable markers for autism in young children (41–43). However, another study using a matched sample of NICU infants on the autism spectrum and not on the autism spectrum found infants later diagnosed with autism displayed expected patterns of atypical eye contact as early as 1 month (corrected age) when compared to infants not on the autism spectrum (44). Given the inconsistent results, it is unclear if children on the spectrum born preterm show the same key early markers as children on the spectrum born full-term and how these markers relate to children not on the spectrum born preterm.
Currently, there are no clear indicators of autism specific to children born preterm. However, retrospective (45) and prospective (42) studies have reliably identified early markers of autism in infants and toddlers in the general population. Specifically, atypical social-communication behavior can accurately differentiate between children on the spectrum and not on the spectrum in the first years of life (41, 43), whereas the presence of RRBs does not clearly distinguish autism from other developmental differences, such as global developmental delay (41, 43). As a result, developmental surveillance with an emphasis on early social-communication behaviors, rather than RRBs, has been shown to be effective in identifying children at an increased likelihood for autism (2). This is beneficial, as a reliable diagnosis can be made by 2 years of age (46) with early identification and diagnosis having positive impact on future development as compared to later diagnosis (47–49).
Barbaro et al. (2, 42, 50–53) used social-communication markers to develop a universal tool to monitor infants and toddlers for autism, the Social Attention and Communication Surveillance (SACS) tool. The SACS tool and its revised version (SACS-R) (53), were developed for use in community settings to prospectively identify children between 12 and 30 months of age who display a pattern of atypical behaviors indicating a higher likelihood of autism. Development is monitored at 6-month intervals based on age-expected behaviors. A strength of the SACS tool is in its positive predictive value (the probability that children with a positive screening test truly have autism) of 81.00–83.00% (42, 53) between 12 and 24 months of age, which is higher than other commonly used autism screening tools for young children, such as the Modified Checklist for Autism in Toddlers (M-CHAT) (54) with a positive predictive value of 6.00% (55) and the Ages and Stages Questionnaire with a positive predictive value between 26.70 and 30.30% (56). While there have been previous studies in preterm children using the M-CHAT, discussed in further detail below, thus far no studies have targeted infants and toddlers on the spectrum born preterm who were identified using the SACS.
In addition, few studies have explicitly examined differences between preterm and full-term groups with an autism diagnosis. Two studies considering the impact of preterm birth on social-communication presentation found greater autism behaviors for children born very or moderate to late preterm (57, 58). Five year old children on the spectrum were identified to have a specific weakness in social reciprocity compared to their peers born full-term on the Autism Diagnostic Interview-Revised (ADI-R) (59). However, no differences were found for the same domain on the clinical observation measure of the Autism Diagnostic Observation Schedule (ADOS) (57, 60). An additional study using the Revised Behavior Summarized Evaluation Scale (61), another observational measure, found no significant differences for social-communication behaviors in young children on the spectrum born moderate to late preterm (62). Differences in the other key criteria for autism diagnosis, RRBs, has not been well researched between preterm and full-term groups. Using the ADOS and ADI-R, no differences were found in RRB presentation for 5 year old children on the spectrum born preterm and full-term on either measure (57). Another study focusing on children born moderate to late preterm at 5 years of age used the Repetitive and Restrictive Behavior Scale (63), which includes four subscales: sensorimotor stereotypies, reaction to chance, restricted behaviors, and modulation insufficiency. No significant differences were found on any of the subscales between the preterm and full-term groups (62). While there is consistency in the findings of these two studies, presentation of RRBs at earlier stages of development for children on the spectrum born preterm has yet to be examined.
As with research on non-autistic children born preterm, previous research on children on the spectrum born preterm indicates that these children are more likely to have delayed development than their term-born peers. Several studies have compared preterm and full-term groups that were identified at “high likelihood” for autism using autism screening measures. These studies found that children born preterm had lower overall development scores across cognitive, language, and motor developmental profiles than their term-born peers, with medium to large effect sizes (64, 65). When considering older children and adolescents on the spectrum, those born preterm are more likely to be non-verbal compared to those born full-term indicating that differences in cognitive development can be identified by 3 years of age (62, 66). Identifying potential differences in developmental profiles would be useful in identifying additional needs that children born preterm may have as a group, though no known studies thus far have examined this.
Clinical uncertainty pertaining to diagnosis of autism in preterm populations could impact the care provided to these children and, consequently, their development. Concerningly, a meta-analysis found that the median age for diagnosis in children born preterm was 5.7 years of age (21) while the average age for diagnosis in Australia (67) and the United States (68) are both 4 years of age. Potentially, being born preterm may delay assessment, diagnosis, and the opportunity to access early supports that can improve developmental outcomes.
To date, no known studies have compared children on the spectrum born preterm and full-term within a prospectively identified, community-based sample. This study aimed to identify differences in developmental profiles and autistic trait presentation in children on the spectrum born preterm and full-term aged 22–34 months who were identified from a community-based sample. It was hypothesized that children on the spectrum born preterm would have lower developmental quotients than children on the spectrum born full-term for receptive language, expressive language, fine motor, and visual reception. Further, when comparing children on the spectrum, it was hypothesized that those born preterm would have greater autistic presentation than those born full-term for social communication on clinical observation measures. Due to the limited number of studies that have investigated differences in RRB presentation between children on the spectrum born preterm and full-term, no hypotheses were made for this young sample.
Participants were drawn from two existing prospective, community-based, studies: the SACS (42) and SACS-R (53). Between the two studies, 35,732 children from Victoria, Australia were monitored between 11 and 30 months of age using the SACS tools, resulting in 357 children identified at “high likelihood” for autism.
Of these, 218 children underwent diagnostic assessment at 2 years of age. After excluding children whose gestational age or birth weight was unknown (n = 3 preterm, n = 55 full-term), one twin born preterm (to retain independence of observations), one child born preterm with an incomplete assessment, the final sample included twenty-two children born preterm and 136 children born full-term with an autism diagnosis, aged 22–34 months at the time of assessment. Of the children born preterm, one (4.50%) was born very preterm, with twenty-one (95.50%) born moderate to late preterm; no child was born extremely preterm. Approximately half of the children born preterm (n = 11) were born in the thirty-sixth week of gestation (see Table 1).
The SACS (42) and SACS-R (53) are universal, community-based screening tools for monitoring children between 11 and 30 months of age to identify those with a “high likelihood” of autism. Trained raters mark whether a child displays typical or atypical behavior against several items, the number and content of which differs at each age as the items are based on developmental expectations. Each assessment has five “key items” for autism and a number of “non-key” items, as identified in Barbaro and Dissanayake (2). Children who are rated as having atypical behavior on at least three of the “key items” for their age group are deemed at “high likelihood” for autism. The SACS and SACS-R tools both have overall positive predictive values of 81.00–83.00%, negative predictive value of 99.00%, sensitivity of 82.00–84.00%, and specificity of 99.00–99.50% for identifying children on the spectrum between 12 and 24 months of age, and an inter-rater reliability of 0.90 (42, 53).
Parents/caregivers completed a demographic questionnaire, reporting on characteristics of their family, education level, culture, occupation, income, and language/s spoken at home. The demographic questionnaire in the SACS-R study had additional questions on whether siblings or other family members had a diagnosis of autism. Information about the child's birth was recorded via the demographics questionnaire, notes provided by maternal and child health (MCH) nurses, documentation from families during their visit, or in photocopies from the “My Health, Learning and Development Record” birth record books provided to families in Victoria when their child is born. Birth and development information is recorded in these books by hospital and MCH nurses.
The Mullen Scales of Early Learning (MSEL) (69) was used to examine developmental profiles for children. This task-based measure for children aged between 3 and 68 months of age includes subscales of fine motor, visual reception, receptive language, and expressive language skills. In the current study, the MSEL subscales had excellent internal reliability (α = 0.75–0.91). Further, the MSEL has been validated for use with young children with an autism diagnosis with excellent construct validity between 0.84 and 0.92 (70). Per procedure for the MSEL (69), corrected age was used when the child's chronological age was under 24 months and chronological age used thereafter. Developmental quotients for subscales were calculated by dividing scale age equivalents by the child's chronological or corrected age and multiplying by one hundred.
The ADOS is a semi-structured, standardized, play-based assessment with modules administered based on the child's age and language development. Module 1 of the ADOS-Generic (ADOS-G) (71) was used in the SACS study, as appropriate for the children's age and language development. In the SACS-R study, children aged between 12 and 30 months completed the ADOS-Toddler Module (ADOS-T) (72) or the ADOS-2 Modules 1–2 (60) were administered as appropriate for their language level. Items are coded between zero and two, with higher scores indicating greater autism traits. A Cochrane review of the ADOS-G, ADOS-T and ADOS-2 modules found a summary sensitivity of 0.94 and specificity of 0.80 in preschool aged children (73).
To allow for comparisons between the different ADOS versions and modules, algorithms for calibrated severity scores (CSSs) were created for social affect, RRBs, and overall severity, using the method proposed by Hus et al. (74), Gotham et al. (75), and Esler et al. (76). Higher CSSs indicate greater autism traits, ranging from zero to ten. However, the possible range of scores for RRB CSSs is zero or between five and ten, skipping numbers one to four. These algorithms had internal reliability coefficients ranging from acceptable (ADOS-G; α = 0.68) to excellent (ADOS-T and ADOS-2 all modules; α = 0.73–0.91) within this study. The process to calculate CSSs is frequently used in autism research to allow comparisons across editions and modules of the ADOS (57, 77).
Ethics approval from the La Trobe University Human Ethics Committee was obtained for the SACS (Project 06-94) and SACS-R (UHEC13-001) prior to data collection. An application for this secondary data analysis was approved prior to commencement (HEC-19209).
Across both studies, MCH centers from nineteen local government areas (LGAs) across Melbourne, Victoria took part, with five LGAs taking part in both studies. The MCH service provides caregivers with a schedule of free consultations with MCH nurses for ten “key ages and stages” of development in the first 6 years of life (78). Nurses from MCH attended a half-day workshop on identifying early behavioral signs of autism (42, 53). Children attending routine MCH appointments in Victoria, Australia were subsequently screened using the SACS or SACS-R tools at all scheduled 12-, 18-, and 24-month “key ages and stages” appointments between September 2006 to September 2008 for the SACS study (42) and June 2013 to June 2018 for the SACS-R study (53).
All children who were identified at “high likelihood” for an autism diagnosis were invited to attend developmental assessments at the University's Child Development Unit at six-monthly intervals to track their development over time. Parents provided informed consent for their child's assessment, MCH records, and photocopies made from their “My Health, Learning and Development Record” books to be used for the SACS/SACS-R research and future studies. At the developmental assessment for children at 2-years of age, parent/caregiver and child measures were completed in tandem, with one clinician administering the ADOS and MSEL to the child while another clinician interviewed the parent(s) or caregiver(s). An assessment report was provided for the family after each appointment.
Prior to analysis, assumptions were tested. The level of measurement assumption was met as dependent variables were continuous. As children were tested independently and one child from a pair of twins was removed from analysis, the assumption of independence of observations was met. Normality was assessed using visual inspection of histograms, skewness z-scores with magnitude >0.5, and Kolmogorov-Smirnov and Shapiro-Wilks Tests of Normality. The assumption of normality was violated for all MSEL developmental quotients (except receptive language) and ADOS RRB CSSs due to negative skew. When cell sizes are ≥20, multivariate analyses of variances (MANOVAs) are robust against violations to normality, so no transformations were made (79). Two multivariate outliers were detected from the full-term group and were not removed as they accounted for <5.00% of the participants in that group (80).
Assumptions required for MANOVAs were tested to examine the MSEL development quotients and ADOS CSSs. Box's Test was not significant, indicating the assumption of variance-covariance matrices was met. Levene's Test was not significant for any of the MANOVAs, indicating that the assumption of equality of variances was met.
Pearson's correlations were used to identify relationships between gestational age, birth weight, and the dependent measures. To identify differences in birth characteristics between the preterm group and full-term group, t-tests and Fisher's Exact Test were used. Fisher's Exact Test was used instead of chi-squared tests when the assumption of minimum cell frequency was not met, specifically, ≥10 cases per cell for 2x2 tables and ≥5 cases per cell for 2x3 tables. Given Fisher's Exact Test with tables larger than 2x2 is not available within the Statistical Package for Social Sciences (SPSS) (81), Fisher's Exact Test (2-tailed) with Freeman-Halton extension for 2x3 tables was calculated using VassarStats (82).
To examine group differences between the preterm and full-term groups, a MANOVA was used to examine the MSEL developmental quotients and another MANOVA for social affect and RRB ADOS CSSs. To determine whether a difference in age between the preterm and full-term groups affected the main results, an MANCOVA was perform on MSEL developmental quotients and ADOS CSSs.
Correlations were calculated to determine the strength of the relationships between gestational age, birth weight, and the outcome measures (see Table 2). While the correlation between birth weight and gestational age was significant, neither were significantly correlated with any of the outcome measures. Correlations between the MSEL developmental quotients were significant, with weak to large positive correlations (83). The ADOS social affect and RRB CSSs were significantly and positively correlated with moderate strength (see Table 2).
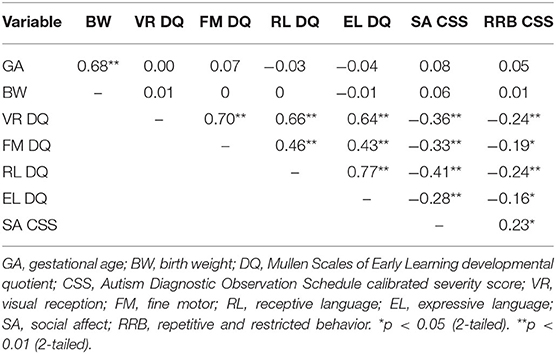
Table 2. Correlations between gestational age, birth weight, Mullen Scales of Early Learning developmental quotients, and Autism Diagnostic Observation Schedule calibrated severity scores.
Children in the preterm group had significantly lower gestational age and birth weight and were significantly more likely to have been born small for gestational age and have a complication at birth than children in the full-term group (see Table 3). Children in the preterm group were significantly older than children in the full-term group in chronological age. After controlling for chronological age, the results of main analyses remain the same (see Supplementary Table 1).
No significant differences were found between the preterm and full-term groups on any of the MSEL development quotients for visual reception, fine motor, receptive language, and expressive language between children on the spectrum born preterm and full-term. Further, there were no significant differences in behavior presentation using the ADOS CSSs for social affect and RRBs (see Table 4).
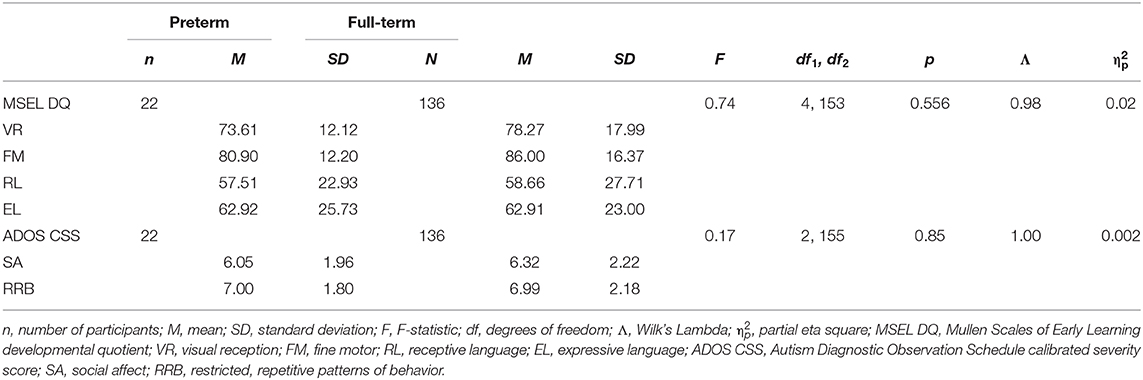
Table 4. MANOVA results for differences between preterm and full-term groups on Mullen Scales of Early Learning developmental quotients and Autism Diagnostic Observation Schedule calibrated severity scores.
Early years presentation of autism was examined in children born preterm and full-term who were prospectively identified at “high likelihood” for autism from the community. The hypothesis that children on the spectrum born preterm would have more delayed development than children on the spectrum born full-term was not supported as no significant differences were identified in visual reception, fine motor, receptive language, and expressive language developmental quotients. Further, the hypothesis that children on the spectrum born preterm would have greater social-communication presentation than children on the spectrum born full-term was not supported, with no significant group differences found.
The non-significant differences across key indicators of child development between children on the spectrum born preterm and full-term were not consistent with previous literature on older children and adolescents between 3 and 18 years of age, where delayed development was observed for those born preterm with a diagnosis of autism as compared to their peers without an autism diagnosis (62, 66). Specifically, previous literature identified verbal development as being delayed and was identified in children as young as 3 years of age (62, 66). In the current study using a sample of 2-year-old children, these differences were not identified. Similar results were found for children born preterm who were identified at “high likelihood” for an autism diagnosis using screening tools (28, 65). Further, previous findings using typically developing samples have suggested that children born preterm have substantially delayed development when compared to children born full-term (20, 84).
The inconsistency of findings regarding child development after preterm birth in the current study with previous literature may be attributed to the young age of the children in this sample (22–34 months) as compared to previous studies that had included children between 3 and 18 years of age (62, 65, 66). As previous research has not yet included children within toddler age with a diagnosis of autism, it is possible that differences in development may not become apparent until the child reaches an older age. While differences were found for young children who had screened at “high likelihood” without a diagnosis of autism in previous studies (28, 65), comparing them to children with a diagnosis of autism may be problematic due to the high rates of false positives when using autism screening tools in preterm groups (56, 85). Extrapolating development of children who have screened at “high likelihood” for autism to children with a diagnosis of autism may be misleading due to the other potential explanations for a child born preterm being identified at “high likelihood” without a full developmental assessment, as was conducted in the current study.
The finding that children on the spectrum born preterm and full-term did not differ on social communication behavior presentation was not consistent with previous studies using parent report measures, where children on the spectrum born preterm were shown to have greater social-communication behavior presentation (57, 58). It is possible that more subtle differences in social-communication behaviors could be unpacked using a measure with subscales within the domains or an item-by-item analysis. Another explanation for the findings of the current study on social-communication for populations of children on the spectrum born preterm and full-term being inconsistent with previous literature may be the young sample. As with developmental profiles, it is possible that differences in behavioral presentation do not emerge until children are at an older age. While Movsas and Paneth (58) included children as young as 4 years of age in their study, the mean age of their participants was 10 years (58)—much older than the current sample's mean age of 26.20 months. Another study found differences between children on the spectrum born preterm and full-term at 5 years of age; however, only children who were born very preterm but not at a low birth weight (<1,500 grams) were excluded from this study (57). The non-significant finding of the current study is consistent with other literature using children born moderate to late preterm (57, 62). When examining samples of 5-year-old children on the spectrum born very (61) and moderate to late preterm (66), no significant differences were found on social-communication behavior presentation. This is not surprising, given 95.50% of the preterm group of the current study were born moderate to late preterm.
This study also examined potential differences in RRB presentation between children on the spectrum born preterm and full-term. The current study builds upon emerging evidence that no differences exist in this domain. Previously, no differences were found for 5 year old children on the spectrum born preterm and full-term on parent report (57) and clinical observation measures (57, 62). The sample in the current study included children who were younger than those used in these previous studies, where the youngest participants were aged 3 years in Brayette et al. (62) and 5 years in Chen et al. (57). Therefore, the findings that children on the spectrum born preterm and full-term do not differ on RRB presentation extend to earlier in development with the current sample.
Instability in patterns of autism screening have been observed in children born preterm between 8 and 18 months of age (86). In that study, half of the children born preterm who were identified at “high likelihood” for autism at 18 months of age using the M-CHAT had not been previously identified at 8 or 12 months of age (86). Further, several children suddenly no longer screened positive at eighteen, despite previously screening positive at 8 and/or 12 months of age (86). When the children were 3 years old, only one child born preterm and no children born full-term was diagnosed with autism (65). These findings may point to some children born preterm having a “sudden onset” of behaviors while others have a sudden decrease at an older age (86). It is possible that instability of RRB presentation could explain the inconsistent findings in the literature at different age groups. The diagnostic inclusion for the current study was based on the child's most recent diagnosis (had they attended subsequent follow up appointments) rather than the diagnosis received at their first appointment. Furthermore, diagnoses were based on gold standard developmental assessments and clinical judgement, rather than the presence or absence of behaviors at the age of diagnosis. Thus, while the trajectory for autistic traits seems to be difficult to predict using screening measures in preterm population, the continued developmental surveillance was advantageous in ensuring their diagnoses were accurate.
Previous studies have largely recruited from clinics and/or university hospitals, where samples typically consist of families who have concerns about their child's development (87). As parents are less likely to be aware of the subtle differences in development that may indicate that a child is at “high likelihood” of an autism diagnosis, children who attend clinics following parental concerns may have more challenging autism traits or other developmental concerns than those identified by trained clinicians. Further, when seeking advice on these subtle differences, clinicians who are unaware of the relationship between preterm birth and autism may assure parents that many of these behaviors, such as atypical eye contact (34) or toe-walking (88), are behaviors common for children born preterm without autism. Thus, autism is not considered as a potential explanation for behavior and the child is not referred to a full developmental assessment. It would not be until a child is showing a pattern of behavior which more clearly indicates autism as an explanation for behavior, that a full developmental assessment is considered. In contrast, the current study was a community-based sample where all children within the community were monitored for autism, rather than only those whose parents have concerns. This difference in sampling could account for the inconsistency between the findings of previous literature and the current study, due to the comparison of children with potentially more subtle developmental differences in both preterm and full-term groups.
Another potential explanation for the inconsistent findings across the literature may be the dose effect in the preterm phenotype, where children with lower gestational ages face more developmental difficulties (17). Previous studies (62) and the current study, which have involved children born preterm with higher gestational ages, have not detected differences in behavioral presentation; similar findings can be observed across preterm phenotype literature (3, 20). Alternatively, it may be that a subset of children born moderate to late preterm are susceptible to developmental difficulties, rather than all children born between moderate to late preterm (89). Further, Sansavini et al. (90) note that many children born extremely preterm, where occurrence of developmental difficulties would be greatest and easiest to detect due to the dose effect, are found to have development within the normal distribution for development (90). As many children born preterm have developmental scores within the range of children born full-term, differences become difficult to detect. The findings of the current study further suggest that even within children who have been diagnosed with autism, their general development is similar to children with an autism diagnosis born full-term—expanding the literature on similarities in development for children born preterm and full-term.
The preterm phenotype suggests that autism in preterm populations may have inherently different etiology than autism in full-term populations (3, 16). Children born preterm were identified at “high likelihood” and diagnosed using criteria based on full-term groups. Potentially, current diagnostic criteria may not accurately represent autism in preterm groups or difficulties might emerge later in life than in full-term groups (86). As a result, the prevalence of autism in preterm populations may be under- or over-estimated. Thus, participants in this study might reflect those with patterns of autistic traits that reflect “typical” autism for full-term populations, which may not accurately represent “typical” autism in preterm populations. While this is a limitation to the study, it is also a limitation to this area of research. Until differences in the presentation of autism in children born preterm and full-term are identified (or ruled out), using the diagnostic criteria based on full-term populations is unavoidable.
This study had several strengths in its unique contributions to an area of research in the preterm phenotype. Firstly, it was the first study to investigate developmental differences and behavioral presentation in children on the spectrum born preterm and full-term, aged 22–34 months. Development of children on the spectrum born preterm has yet to be examined in children at this age, with most studies focusing on children with a diagnosis aged 5 years or older. In younger samples, the children tend to have been identified at “high likelihood” for autism without a confirmed diagnosis, which becomes problematic due to low predictive value and instability of the screening tools used in those studies (55, 56, 86). The use of a young sample is well aligned with the current focus for autism research of early identification and support in autism (91). Secondly, the use of the SACS tools to prospectively identify children on the spectrum in the community may have allowed for more subtle presentations of autism to be detected compared to studies using other screening measures, where these children may have missed. Third, this is one of few studies using a community-based sample of children born preterm and full-term. Use of samples of children born extremely and very preterm from single hospitals are common in this research area, limiting the generalizability of their findings from children born moderate and late preterm, who comprise the majority the preterm population; thus, the use of community-based samples increases the ecological validity of the findings. Finally, as prior research has primarily focused on extremely and very preterm populations, the inclusion of the moderate to late preterm population helps fill the gap for an underserved group of children in the premature phenotype research area.
Although there are notable strengths of this study, it is not without limitations. Firstly, although rates of children born preterm identified with the SACS tools were consistent with population rates of preterm birth, a low number of children born very preterm and no children born extremely preterm were involved in the study. This did not allow for a detailed examination of the dose effect of the preterm phenotype on the outcome measures, as lesser developmental differences from moderate to late preterm birth may obscure larger differences from extreme and very preterm birth. Secondly, while the age range of the current sample was small relative to other studies, very subtle differences in development within this age group may not have been captured. However, measures that account for age and developmental norms were chosen to counteract this, resulting in children being compared based on their expected level of development. Third, children in the preterm group in the current sample were significantly older than children in the full-term group in chronological age. However, when the data were analyzed with age as a covariate, the results remained the same indicating that the difference in age did not significantly affect the results. Lastly, as children who did not have known gestational ages were excluded from the study, fewer preterm children were excluded from the study (n = 3) than children born full-term (n = 55). This discrepancy likely occurred as more care is taken to record gestational age and birth weight for children born preterm than children born full-term. Gestational age and birth weight have a much more relevant impact on development for children born preterm and the accuracy of these figures becomes more important than for a child born full-term. However, one demographic difference (paternal education) was found between the children who were excluded due to missing gestational age or birth weight when compared with children who were included in the current study (see Supplementary Table 2). As no other demographic differences were identified, and there was incomplete data on paternal education, this indicated that those included and excluded were largely similar to each other.
In future, researchers may wish to further explore the differences in RRB presentation and preterm groups using other measures that further break down RRBs into subscales for more detailed analysis. As this study did not examine gross motor performance, it is possible that children on the spectrum born preterm were unable to perform gross motor-based RRBs due to other developmental difficulties, which may have been a confounding factor in the lack of differences between the preterm and full-term groups on the RRB measure. A more detailed examination into other predictors of autism, such as birth weight, and their effect on development and behavioral presentation, may be useful in unpacking inconsistent findings on preterm autism literature. Additionally, no detailed data were available regarding participants experiencing neonatal complications, which could also account for future developmental difficulties. Further, an examination into behaviors and characteristics of children born preterm, with and without autism, would give further insight into the boundary between the preterm phenotype and autism in preterm populations. Lastly, future research using longitudinal study designs could examine the trajectories of behavioral presentation within preterm populations to determine whether, and at which age, differences become apparent.
Previous literature paints a picture of children born preterm having many additional needs due to developmental difficulties and delays. This picture may lead clinicians to expect and look for more challenging characteristics of autism when assessing a child born preterm, overlooking those presenting with more mild developmental differences and behavioral presentation. Using a community-based sample of children identified prospectively, it was found that children on the spectrum born moderate to late preterm did not differ in development and behavioral presentation from their peers born full-term, when assessed at the age of 2 years. As current autism research has largely focused on identifying children at younger ages and providing support as early as possible, the findings of the current study suggest that clinicians should consider autism as a potential explanation for behaviors that are often presumed to be due to preterm birth, particularly for children who were born moderate to late preterm. As these results indicate that the autism phenotype is similar for moderate to late preterm and full-term children, clinicians should not change their clinical approach of diagnosis and treatment for a child presenting with the characteristics of autism simply due to their preterm birth. While further research is required to replicate and extend these findings, it is possible that many 2-year-old children on the spectrum born moderate to late preterm whom clinicians meet in the community will have similar needs to their term-born peers. Still, individual differences in development should not be overlooked, particularly for children born preterm who are more likely to face additional developmental difficulties.
As autism research moves to improving early identification, these findings have practical implications for clinicians who may overlook autism as an explanation for behavior due to expectations for greater developmental differences in children born preterm. Further, these findings may provide reassurance to families, who may have concerns for their child's support needs and outcomes after an autism diagnosis.
The data analyzed in this study are subject to the following licenses/restrictions: the original contributions presented in the study are included in the article/supplementary material. The data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. Requests to access these datasets should be directed to Josephine Barbaro,ai5iYXJiYXJvQGxhdHJvYmUuZWR1LmF1.
The studies involving human participants were reviewed and approved by La Trobe University Human Ethics Committee. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
JL conducted the analyses, contributed to the interpretation of the results, and drafted the initial manuscript. RJ and JB provided supervision to JL, assisted with study design, data analysis and interpretation, and reviewed drafts. MY assisted with the study design and interpretation of results. MG assisted with data analysis and interpretation of results. All authors contributed to the article and approved the submitted version.
Funding from the Telstra Foundation Community Development Fund (for the SACS), the Menzies Foundation (for the SACS and SACS-R), and the Cooperative Research Centre for Living with Autism (Autism CRC; Project 1.005RC), established and supported under the Australian Government's Cooperative Research Centres Program (for the SACS-R), to support the training of MCH nurses who participated in this study is gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge the work of the Maternal and Child Health nurses for their continual efforts to improve early childhood outcomes through the developmental surveillance of infants and toddlers for the early identification of autism. Further, the authors would like to thank the children and families who took part in the studies. The authors would also like to acknowledge ongoing research assistance from Dr. Nancy Sadka and Erin Beattie.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.597505/full#supplementary-material
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC (2013).
2. Barbaro J, Dissanayake C. Early markers of autism spectrum disorders in infants and toddlers prospectively identified in the Social Attention and Communication Study. Autism. (2013) 17:64–86. doi: 10.1177/1362361312442597
3. Yaari M, Eventov-Freidman S, Mankuta D, Bar-Oz B, Yirmiya N. Prematurity and autism spectrum disorders. In: Patel VB, Preedy VR, Martin CR, editors. Comprehensive Guide to Autism. New York, NY: Springer New York (2014). p. 1371–87.
4. Yaari M, Mankuta D, Harel-Gadassi A, Friedlander E, Bar-Oz B, Eventov-Friedman S, et al. Early developmental trajectories of preterm infants. Res Dev Disabil. (2018) 81:12–23. doi: 10.1016/j.ridd.2017.10.018
5. Sansavini A, Guarini A, Zuccarini M, Lee JZ, Faldella G, Iverson JM. Low rates of pointing in 18-month-olds at risk for autism spectrum disorder and extremely preterm infants: a common index of language delay? Front Psychol. (2019) 10:2131. doi: 10.3389/fpsyg.2019.02131
6. Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry. (2013) 54:772–9. doi: 10.1111/jcpp.12040
7. Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. (2019) 321:1188–99. doi: 10.1001/jama.2019.2021
8. World Health Organisation. International Statistical Classification of Diseases and Related Health Problems (11th Revision), Geneva (2018).
9. Australian Institute of Health and Welfare. Australia's Mothers and Babies 2016 - in Brief. Canberra, ACT: Australian Institute of Health and Welfare (2016).
10. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. doi: 10.1016/S0140-6736(12)60820-4
11. World Health Organisation. Born too soon: The Global Action Report on Preterm Birth, Geneva (2012).
12. Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
13. Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Childh Fetal Neonatal Ed. (2015) 100:301–8. doi: 10.1136/archdischild-2014-307684
14. Yates R, Treyvaud K, Doyle LW, Ure A, Cheong JLY, Lee KJ, et al. Rates and stability of mental health disorders in children born very preterm at 7 and 13 years. Pediatrics. (2020) 145:e20192699. doi: 10.1542/peds.2019-2699
15. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
16. Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. (2011) 69:11–8. doi: 10.1203/PDR.0b013e318212faa0
17. Johnson S, Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin Fetal Neonatal Med. (2014) 19:97–104. doi: 10.1016/j.siny.2013.11.004
18. Fenoglio A, Georgieff MK, Elison JT. Social brain circuitry and social cognition in infants born preterm. J Neurodev Disord. (2017) 9:1–16. doi: 10.1186/s11689-017-9206-9
19. Nosarti C, Nam KW, Walshe M, Murray RM, Cuddy M, Rifkin L, et al. Preterm birth and structural brain alterations in early adulthood. NeuroImage Clin. (2014) 6:180–91. doi: 10.1016/j.nicl.2014.08.005
20. Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: A meta-analysis and systematic review involving 64 061 children. BJOG Int J Obstetr Gynaecol. (2018) 125:16–25. doi: 10.1111/1471-0528.14832
21. Agrawal S, Rao SC, Bulsara MK, Patole SK. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics. (2018) 142:e20180134. doi: 10.1542/peds.2018-0134
22. Christensen DL, Maenner MJ, Bilder D, Constantino JN, Daniels J, Durkin MS, et al. Prevalence and characteristics of autism spectrum disorder among children aged 4 years - early autism and developmental disabilities monitoring network, seven sites, United States, 2010, 2012, and 2014. MMWR Surveill Summ. (2019) 68:1–19. doi: 10.15585/mmwr.ss6802a1
23. Nygren G, Cederlund M, Sandberg E, Gillstedt F, Arvidsson T, Carina Gillberg I, et al. The prevalence of autism spectrum disorders in toddlers: a population study of 2-year-old Swedish children. J Autism Dev Disord. (2012) 42:1491–97. doi: 10.1007/s10803-011-1391-x
24. Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. (2010) 156:525–31. doi: 10.1016/j.jpeds.2009.10.041
25. Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL Jr, et al. Positive screening for autism in ex-preterm infants: prevalence and risks factors. Pediatrics. (2008) 121:758–65. doi: 10.1542/peds.2007-2158
26. Ure AM, Treyvaud K, Thompson DK, Pascoe L, Roberts G, Lee KJ, et al. Neonatal brain abnormalities associated with autism spectrum disorder in children born very preterm. Autism Res. (2016) 9:543–52. doi: 10.1002/aur.1558
27. Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. (2014) 83:518–32. doi: 10.1016/j.neuron.2014.07.016
28. Gray PH, Edwards DM, O'Callaghan MJ, Gibbons K. Screening for autism spectrum disorder in very preterm infants during early childhood. Early Hum Dev. (2015) 91:271–6. doi: 10.1016/j.earlhumdev.2015.02.007
29. Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr. (2012) 161:830–6. doi: 10.1016/j.jpeds.2012.04.058
30. Duan G, Yao M, Ma Y, Zhang W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res. (2014) 220:410–7. doi: 10.1016/j.psychres.2014.05.057
31. Dudova I, Kasparova M, Markova D, Zemankova J, Beranova S, Urbanek T, et al. Screening for autism in preterm children with extremely low and very low birth weight. Neuropsychiatr Dis Treat. (2014) 10:277–82. doi: 10.2147/NDT.S57057
32. Hadjkacem I, Ayadi H, Turki M, Yaich S, Khemekhem K, Walha A, et al. Prenatal, perinatal and postnatal factors associated with autism spectrum disorder. J Pediatr. (2016) 92:595–601. doi: 10.1016/j.jpedp.2016.08.011
33. Guisso DR, Saadeh FS, Saab D, El Deek J, Chamseddine S, El Hassan HA, et al. Association of autism with maternal infections, perinatal and other risk factors: a case-control study. J Autism Dev Disord. (2018) 48:2010–21. doi: 10.1007/s10803-017-3449-x
34. De Schuymer L, De Groote I, Desoete A, Roeyers H. Gaze aversion during social interaction in preterm infants: a function of attention skills? Infant Behav Dev. (2012) 35:129–39. doi: 10.1016/j.infbeh.2011.08.002
35. Imafuku M, Kawai M, Niwa F, Shinya Y, Inagawa M, Myowa-Yamakoshi M. Preference for dynamic human images and gaze-following abilities in preterm infants at 6 and 12 months of age: an eye-tracking study. Infancy. (2017) 22:223–39. doi: 10.1111/infa.12144
36. Yaari M, Rotzak NL, Mankuta D, Harel-Gadassi A, Friedlander E, Eventov-Friedman S, et al. Preterm-infant emotion regulation during the still-face interaction. Infant Behav Dev. (2018) 52:56–65. doi: 10.1016/j.infbeh.2018.05.008
37. Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, et al. Visual and hearing impairments after preterm birth. Pediatrics. (2018) 142:e20173888. doi: 10.1542/peds.2017-3888
38. van Dommelen P, Verkerk PH, van Straaten HLM, Baerts W, von Weissenbruch M, Duijsters C, et al. Hearing loss by week of gestation and birth weight in very preterm neonates. J Pediatr. (2015) 166:840–3. doi: 10.1016/j.jpeds.2014.12.041
39. Moore T, Johnson S, Hennessy E, Marlow N. Screening for autism in extremely preterm infants: problems in interpretation. Dev Med Child Neurol. (2012) 54:514–20. doi: 10.1111/j.1469-8749.2012.04265.x
40. Pineda R, Melchior K, Oberle S, Inder T, Rogers C. Assessment of autism symptoms during the neonatal period: is there early evidence of autism risk? Am J Occup Therapy. (2015) 69: 6904220010p1–11. doi: 10.5014/ajot.2015.015925
41. Barbaro J, Dissanayake C. Autism spectrum disorders in infancy and toddlerhood: a review of the evidence on early signs, early identification tools, early diagnosis. J Dev Behav Pediatr. (2009) 30:447–59. doi: 10.1097/DBP.0b013e3181ba0f9f
42. Barbaro J, Dissanayake C. Prospective identification of autism spectrum disorders in infancy and toddlerhood using developmental surveillance: the social attention and communication study. J Dev Behav Pediatr. (2010) 31:376–85. doi: 10.1097/DBP.0b013e3181df7f3c
43. Mitchell S, Cardy JO, Zwaigenbaum L. Differentiating autism spectrum disorder from other developmental delays in the first two years of life. Dev Disabil Res Rev. (2011) 17:130–40. doi: 10.1002/ddrr.1107
44. Karmel BZ, Gardner JM, Meade LS, Cohen IL, London E, Flory MJ, et al. Early medical and behavioral characteristics of NICU infants later classified with ASD. Pediatrics. (2010) 126:457–67. doi: 10.1542/peds.2009-2680
45. Costanzo V, Chericoni N, Amendola FA, Casula L, Muratori F, Scattoni ML, et al. Early detection of autism spectrum disorders: from retrospective home video studies to prospective ‘high risk’ sibling studies. Neurosci Biobehav Rev. (2015) 55:627–35. doi: 10.1016/j.neubiorev.2015.06.006
46. Woolfenden S, Sarkozy V, Ridley G, Williams K. A systematic review of the diagnostic stability of autism spectrum disorder. Res Autism Spectr Disord. (2012) 6:345–54. doi: 10.1016/j.rasd.2011.06.008
47. Clark MLE, Barbaro J, Dissanayake C. Continuity and change in cognition and autism severity from toddlerhood to school age. J Autism Dev Disord. (2017) 47:328–39. doi: 10.1007/s10803-016-2954-7
48. Clark MLE, Vinen Z, Barbaro J, Dissanayake C. School age outcomes of children diagnosed early and later with autism spectrum disorder. J Autism Dev Disord. (2018) 48:92–102. doi: 10.1007/s10803-017-3279-x
49. Fuller EA, Kaiser AP. The effects of early intervention on social communication outcomes for children with autism spectrum disorder: a meta-analysis. J Autism Dev Disord. (2019) 50:1683–700. doi: 10.1007/s10803-019-03927-z
50. Barbaro J, Ridgway L, Dissanayake C. Developmental surveillance of infants and toddlers by maternal and child health nurses in an Australian community-based setting: promoting the early identification of Autism. J Pediatr Nurs. (2011) 26:334–47. doi: 10.1016/j.pedn.2010.04.007
51. Mozolic-Staunton B, Donelly M, Barbaro J, Yoxall J. Right kids, right time, right services: developmental surveillance for autism spectrum disorder in early childhood education settings. Aust Occup Ther J. (2015) 62:64.
52. Mozolic-Staunton B, Donelly M, Yoxall J, Barbaro J. Interrater reliability of early childhood education professionals involved in developmental surveillance for autism spectrum disorder and related conditions. Aust J Early Childh. (2017) 42:61–8. doi: 10.23965/AJEC.42.2.08
53. Mozolic-Staunton B, Donelly M, Yoxall J, Barbaro J. Early detection for better outcomes: universal developmental surveillance for autism across health and early childhood education settings. Res Autism Spectr Disord. (2020) 71:101496. doi: 10.1016/j.rasd.2019.101496
54. Robins DL, Casagrande K, Barton M C., Chen MA, Dumont-Mathieu T, Fein D. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. (2014) 133:37–45. doi: 10.1542/peds.2013-1813
55. Yuen T, Penner M, Carter MT, Szatmari P, Ungar WJ. Assessing the accuracy of the modified checklist for autism in toddlers: a systematic review and meta-analysis. Dev Med Child Neurol. (2018) 60:1093–100. doi: 10.1111/dmcn.13964
56. Lamsal R, Dutton DJ, Zwicker JD. Using the ages and stages questionnaire in the general population as a measure for identifying children not at risk of a neurodevelopmental disorder. BMC Pediatr. (2018) 18:122. doi: 10.1186/s12887-018-1105-z
57. Chen L-W, Wang S-T, Wang L-W, Kao Y-C, Chu C-L, Wu C-C, et al. Behavioral characteristics of autism spectrum disorder in very preterm birth children. Mol Autism. (2019) 10:1–9. doi: 10.1186/s13229-019-0282-4
58. Movsas TZ, Paneth N. The effect of gestational age on symptom severity in children with autism spectrum disorder. J Autism Dev Disord. (2012) 42:2431–9. doi: 10.1007/s10803-012-1501-4
59. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. (1994) 24:659–85. doi: 10.1007/BF02172145
60. Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule. 2nd ed. Torrance, CA: Western Psycholological Services (2012).
61. Barthélémy C, Roux S, Adrien JL, Hameury L, Guérin P, Garreau B, et al. Validation of the revised behavior summarized evaluation scale. J Autism Dev Disord. (1997) 27:139–53. doi: 10.1023/A:1025887723360
62. Brayette M, Saliba E, Malvy J, Blanc R, Ponson L, Tripi G, et al. Incomplete gestation has an impact on cognitive abilities in autism spectrum disorder. J Autism Dev Disord. (2019) 49:4339–45. doi: 10.1007/s10803-019-04105-x
63. Bourreau Y, Roux S, Gomot M, Bonnet-Brilhault F, Barthélémy C. Validation of the repetitive and restricted behaviour scale in autism spectrum disorders. Eur Child Adolesc Psychiatry. (2009) 18:675–82. doi: 10.1007/s00787-009-0028-5
64. Michalec D. Bayley scales of infant development. 3rd ed. In: Goldstein S, Naglieri JA, editors. Encyclopedia of Child Behavior and Development. Boston, MA: Springer (2011). p. 215
65. Harel-Gadassi A, Friedlander E, Yaari M, Bar-Oz B, Eventov-Friedman S, Mankuta D, et al. Risk for ASD in preterm infants: a three-year follow-up study. Autism Res Treat. (2018) 2018:e8316212. doi: 10.1155/2018/8316212
66. Bowers K, Wink LK, Pottenger A, McDougle CJ, Erickson C. Phenotypic differences in individuals with autism spectrum disorder born preterm and at term gestation. Autism. (2015) 19:758–63. doi: 10.1177/1362361314547366
67. Bent CA, Dissanayake C, Barbaro J. Mapping the diagnosis of autism spectrum disorders in children aged under 7 years in Australia, 2010–2012. Med J Aust. (2015) 202:317–20. doi: 10.5694/mja14.00328
68. Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 Sites, United States, 2016. Surv Summ. (2020) 69:1–12. doi: 10.15585/mmwr.ss6904a1
69. Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc. (1995).
70. Swineford LB, Guthrie W, Thurm A. Convergent and divergent validity of the Mullen Scales Of Early Learning in young children with and without autism spectrum disorder. Psychol Assess. (2015) 27:1364–78. doi: 10.1037/pas0000116
71. Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. (2000) 30:205–23. doi: 10.1023/A:1005592401947
72. Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. The autism diagnostic observation schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord. (2009) 39:1305–20. doi: 10.1007/s10803-009-0746-z
73. Randall M, Egberts KJ, Samtani A R, Scholten JPM, Hooft L, Livingstone N, et al. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochr Database Syst Rev. (2018) 7:CD009044. doi: 10.1002/14651858.CD009044.pub2
74. Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. (2014) 44:2400–12. doi: 10.1007/s10803-012-1719-1
75. Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. (2009) 39:693–705. doi: 10.1007/s10803-008-0674-3
76. Esler AN, Bal VH, Guthrie W, Wetherby A, Weismer SE, Lord C. The autism diagnostic observation schedule, toddler module: standardized severity scores. J Autism Dev Disord. (2015) 45:2704–20. doi: 10.1007/s10803-015-2432-7
77. Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early autism spectrum disorder: subtypes and short-term outcomes. J Child Psychol Psychiatry. (2016) 57:93–102. doi: 10.1111/jcpp.12448
78. Department of Education and Early Childhood Development. Maternal and Child Health Service Guidelines. Melbourne, VIC: Department of Education and Early Childhood Development (2011).
80. Field A. Discovering Statistics Using IBM SPSS Statistics. London: Sage Publications Ltd. (2018).
83. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Saint Louis, MO: Routledge (1977).
84. Kuban KC, Joseph RM, O'Shea TM, Allred EN, Heeren T, Douglass L, et al. Girls and boys born before 28 weeks gestation: risks of cognitive, behavioral, and neurologic outcomes at age 10 years. J Pediatr. New York, NY (2016) 173:69–75.
85. Pritchard MA, de Dassel T, Beller E, Bogossian F, Johnston L, Paynter J, et al. Autism in toddlers born very preterm. Pediatrics. (2016) 137:e20151949. doi: 10.1542/peds.2015-1949
86. Yaari M, Yitzhak N, Harel A, Friedlander E, Bar-Oz B, Eventov-Friedman S, et al. Stability of early risk assessment for autism spectrum disorder in preterm infants. Autism. (2016) 20:856–67. doi: 10.1177/1362361315614758
87. Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, et al. How early do parent concerns predict later autism diagnosis? J Dev Behav Pediatr. (2009) 30:367–75. doi: 10.1097/DBP.0b013e3181ba0fcf
88. Baber S, Michalitsis J, Fahey M, Rawicki B, Haines T, Williams C. A comparison of the birth characteristics of idiopathic toe walking and toe walking gait due to medical reasons. J Pediatr. (2016) 171:290–3. doi: 10.1016/j.jpeds.2015.12.069
89. Jois RS. Neurodevelopmental outcome of late-preterm infants. Aust J Gen Pract. (2018) 47:776–85. doi: 10.31128/AJGP-03-18-4539
90. Sansavini A, Guarini A, Caselli MC. Preterm birth: neuropsychological profiles and atypical developmental pathways. Dev Disabil Res Rev. (2011) 17:102–13. doi: 10.1002/ddrr.1105
Keywords: prematurity, preterm, autism spectrum disorder, child development, social development, restricted repetitive behavior
Citation: Luu J, Jellett R, Yaari M, Gilbert M and Barbaro J (2020) A Comparison of Children Born Preterm and Full-Term on the Autism Spectrum in a Prospective Community Sample. Front. Neurol. 11:597505. doi: 10.3389/fneur.2020.597505
Received: 21 August 2020; Accepted: 12 November 2020;
Published: 03 December 2020.
Edited by:
Carl E. Stafstrom, Johns Hopkins Medicine, United StatesReviewed by:
Thalia Harmony, National Autonomous University of Mexico, MexicoCopyright © 2020 Luu, Jellett, Yaari, Gilbert and Barbaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Jellett, ci5qZWxsZXR0QGxhdHJvYmUuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.