- 1Division of Neurocritical Care & Emergency Neurology, Department of Neurology, Yale School of Medicine, New Haven, CT, United States
- 2Department of Neurology, Medical University of Warsaw, Warsaw, Poland
- 3Department of Bioethics, Medical University of Warsaw, Warsaw, Poland
- 4Child Study Center, Departments of Psychiatry, Pediatrics and Psychology, Yale University, New Haven, CT, United States
Gilles de la Tourette syndrome (GTS) is a childhood onset neuropsychiatric disorder characterized by the presence of motor and vocal tics. The clinical spectrum of GTS is heterogeneous and varies from mild cases that do not require any medical attention to cases that are refractory to standard treatments. One of the unresolved issues is the definition of what constitutes treatment-refractory GTS. While for some other neuropsychiatric disorders, such as obsessive–compulsive disorder (OCD), a clear definition has been established, there is still no consensus with regard to GTS. One important issue is that many individuals with GTS also meet criteria for one or more other neurodevelopmental and neuropsychiatric disorders. In many individuals, the severity of these comorbid conditions contributes to the degree to which GTS is treatment refractory. The scope of this paper is to present the current state-of-the-art regarding refractory GTS and indicate possible approaches to define it. In closing, we discuss promising approaches to the treatment of individuals with refractory GTS.
Introduction
Gilles de la Tourette syndrome (GTS) is a neuropsychiatric disorder in which both motor and vocal tics coexist. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (1), to diagnose GTS the following criteria should be fulfilled: the presence of two or more motor tics and at least one vocal tic, although they might not occur at the same time; tics should be present for at least a year and must begin before the age of 18; finally, the symptoms cannot be attributed to administration of drugs or another medical condition, such as encephalitis, Huntington disease, or seizures. In the majority of cases, psychiatric comorbidities are also present, the most frequent being obsessive–compulsive disorder (OCD), attention-deficit hyperactivity disorder (ADHD), depression, and self-injurious behaviors (SIB) (2). Although many patients experience only mild or moderate symptoms, some cases are severe and treatment refractory. One indicator of severe GTS is the level of tic severity measured with the Yale Global Tic Severity Scale (YGTSS) and other related scales (3, 4). However, many patients with severe tics do not demonstrate high impairment in quality of life; on the other hand, some individuals with mild tics describe their tics as severely impairing. This discrepancy is due to the fact that often one tic can be more impairing than another. For example, isolated coprolalia or self-injurious tic could provoke much more impairment than several tics rated as more severe in the YGTSS. That said, the YGTSS does include an overall impairment scale which directly addresses this concern. However, in many patients, psychiatric comorbidities contribute to the final global impairment. Typical first-line treatment for GTS includes well-established behavioral interventions and pharmacotherapy (5). In those cases where these standard treatments are unsuccessful, a number of experimental approaches such as transcranial magnetic stimulation (TMS), deep brain stimulation (DBS), or cannabis-based medicine (CBM) could be implemented (5–8). Further details of the treatment approach in those cases are discussed in the following sections of the manuscript. Therefore, it remains a matter of debate which criteria are required to diagnose GTS patients as refractory. While a consensus about what constitutes treatment refractoriness for other neuropsychiatric disorders has been established, such as OCD, this is not the case for GTS. The purpose of this paper is to summarize the current evidence regarding what constitutes treatment refractory GTS.
Toward the Definition of Treatment Refractoriness in GTS
Lessons From the Field of Pathophysiology
A number of molecular mechanisms have been shown to be involved in the occurrence of GTS. These include dopaminergic neurotransmission (9, 10) as well as glutaminergic (11, 12), serotoninergic (13), GABA-ergic (14–16), or endocannabinoid (17, 18) neurotransmission. In addition, a significant number of genetic variants have also been identified [SLITRK1 (19–21), DRD2 (22), DRD3 (23), AADAC (24), ADORA (25), BTBD9 (26), CNR1 (18), GDNF (27), KCNJ5 (28), IL1RN (29), PNKD (30), and HDC (31, 32), to give only some examples]. The first genome-wide association study (GWAS) (33) in GTS demonstrated a combined genetic background for GTS and OCD. The authors conducted a GWAS in 2723 cases (1310 with OCD, 834 with GTS, 579 with OCD plus GTS/chronic tics) and 5667 controls. Surprisingly, no individual single-nucleotide polymorphisms (SNPs) reached genome-wide significance. On the other hand, GWAS signals were enriched for SNPs associated with variants in brain gene expression. However, polygenic risk score (PRS) analysis predicted only 0.6% of the phenotypic variance. A recently published genome wide association study (GWAS) (34) conducted in 4,819 GTS case subjects and 9,488 controls demonstrated one genome-wide significant locus within FLT3 on chromosome 13 (rs2504235), although this association was not replicated in the population-based sample. GTS-associated genes were expressed in the dorsolateral prefrontal cortex. Additionally, GTS PRS predicted GTS and tic spectrum disorders. Furthermore, GTS PRS was correlated with worst-ever tic severity and a positive family history of tics. Finally, a number of other factors have also been determined to be involved in GTS pathogenesis, particularly, the presence of autoimmune disorders (35–37) as well perinatal factors (38, 39). It can therefore be concluded that GTS pathophysiology is multifactorial which adds to disease complexity and, in some cases, refractoriness.
Lessons From Other Disorders
As treatment refractoriness in GTS has not been clearly defined yet, one possible approach to determine methodological framework for the criteria could be to adapt the instruments used in similar disorders, especially from the field of psychiatry.
Treatment-Refractory Depression
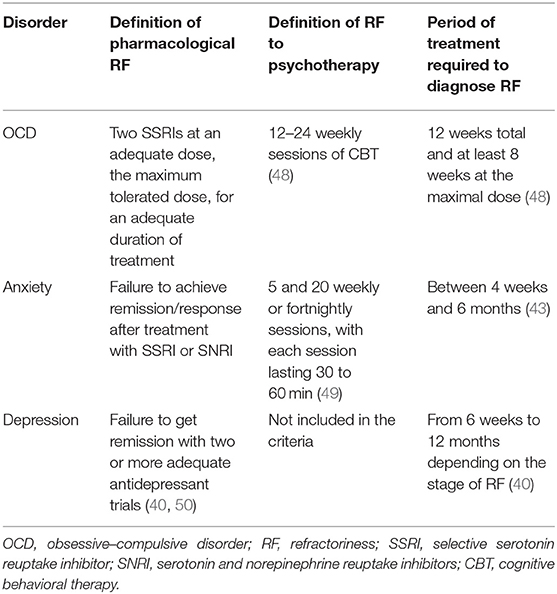
As mentioned by McIntyre et al. (40), there are no clear criteria for treatment-resistant depression (TRD). Nevertheless, it has been suggested that failure to get remission with two or more adequate antidepressant trials defines TRD (41). A recently published systematic review identified five staging models for TRD (42). Each of the staging models defines minimum dosing and duration of therapy, increasing complexity of treatment modality as a function of treatment resistance, and inefficacy with electroconvulsive therapy (ECT) as the most resistant subgroup in major depressive disorder (MDD).
Treatment-Refractory Anxiety
Interestingly, the approach used for TRD follows similar logic to what is used for elaboration of criteria for treatment refractory anxiety. As reviewed by Roy-Berne (43), there are two known causes of treatment resistance: “pseudo-resistance” and true treatment resistance. As the cause of “pseudo-resistance,” the authors indicate lack of sufficient treatment resulting from the selection of the wrong compound, the wrong dosage/treatment regime (44), or lack of compliance. Once pseudo-resistance is excluded, one should then consider the diagnosis of treatment-resistant anxiety. Nevertheless, in a recently published review and meta-analysis by Bokma et al. (44), the authors conclude that there is a lack of consensus on the criteria of treatment-refractory anxiety. In the majority of studies (62.9%), treatment resistance was determined after the first treatment failure. Importantly, in the majority of studies the criterium was failure of pharmacotherapy, while only 29.0% also considered failure of psychotherapy. On the other hand, the exact definition of treatment failure was not provided in the vast majority of the studies. Another issue that should be taken into consideration is the treatment duration, ranging between 4 weeks and 6 months. Upon analysis of the available data, the authors came to the following definition of treatment resistant anxiety: the lack of efficacy of at least one first-line pharmacological treatment and also psychological interventions. What is more, this treatment should be used for an adequate duration of at least 8 weeks.
Treatment-Refractory OCD
Despite the adequate treatment with established therapies such as selective serotonin reuptake inhibitors (SSRIs) and psychotherapy, almost 30% of OCD patients suffer from treatment refractoriness (45). As summarized by Bloch and Storch (46), critically important issues include making an accurate diagnosis; optimal delivery of first-line treatments for OCD; and the presence of moderating factors that have the potential to influence treatment delivery and efficacy. To provide a more detailed evaluation of those criteria, we will analyze each of them separately. Primarily, symptoms of OCD should be differentiated from the following symptoms and diseases: complex tics, stereotypies, some elements of autistic spectrum disorder, and repetitive behaviors being part of normal development or ruminations present in depression and psychosis. The second part of the assessment of OCD refractoriness is the evaluation of response to first-line treatments. Patients should have received two SSRIs at an adequate dose for 12 weeks total and at least 8 weeks at the maximum dose. An important issue is that in some cases, lack of tolerance makes it impossible to implement appropriate treatment; however, these patients should be treated as treatment-intolerant and not treatment-refractory. Another important component of OCD treatment is cognitive–behavioral therapy (CBT), which should be used for an adequate period of time with an adequate amount of sessions. Moreover, when possible, compliance to treatment should also be assessed. Importantly, coexisting conditions that often occur together with OCD, such as tics or hoarding, may influence treatment resistance. Finally, sometimes a watchful, waiting approach could be beneficial. The course of OCD is heterogeneous with two major phenotypes emerging chronic and episodic (47). The chronic course indicates symptom persistence which can be waxing and waning nature, but without complete relief from symptoms. In contrast, the main feature of the episodic course is the presence of periods with complete remission and subsequent relapses. In case of refractory OCD, it is therefore highly recommended to actively inquire about disease history and adapt the intervention to the subtype of OCD.
A summary of all the criteria of treatment resistance anxiety, OCD, and depression is shown in Table 1.
Subtypes of GTS as Avenue to Define Treatment Refractoriness
Pure GTS and GTS Plus
As shown in aforementioned examples of disorders that often coexist and were demonstrated to share common pathophysiology with GTS (51–53), to determine treatment refractoriness it is vital to divide patients into clinical subgroups based on symptom severity. At present, no clear consensus regarding clinical subgroups in GTS has been established. Below, we distinguish between “pure GTS” and GTS plus (54–57). By definition, pure GTS refers to individuals who have motor and phonic tics in the absence of any comorbid conditions. In contrast, GTS plus refers to individuals who have tics coexisting with at least one other neuropsychiatric condition. Below, we present a review of the articles addressing the topic of clinical variability in GTS, particularly, the division in subgroups.
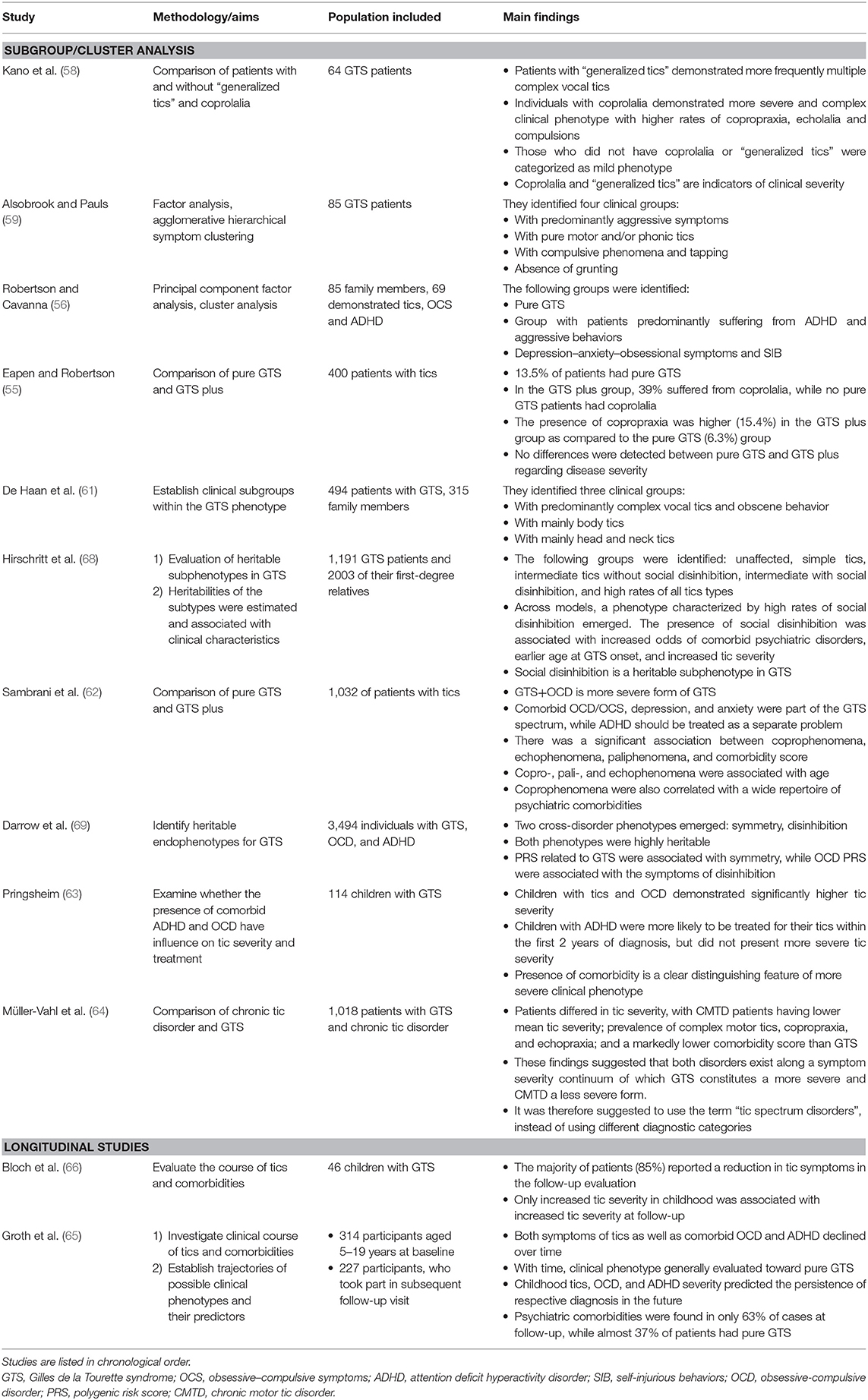
Kano et al. (58) examined 64 Japanese GTS patients and sought to determine the clinical characteristics of individuals with and without so-called generalized tics, which involved the whole body and/or coprolalia. Not surprisingly, patients with “generalized tics” more frequently demonstrated multiple complex vocal tics than those without “generalized tics.” In line with many other studies, individuals with coprolalia demonstrated a more severe and complex clinical phenotype with higher rates of copropraxia, echolalia, and compulsions. As expected, this group was also more socially impaired. On the contrary, those who did not have coprolalia or “generalized tics” were categorized as having a mild phenotype. The authors concluded that coprolalia and “generalized tics” seem to be additional indicators of clinical severity.
Another factor analysis was done by Alsobrook and Pauls (59). They included 85 GTS probands and conducted agglomerative hierarchical symptom clustering. As a result, four clusters were identified: predominantly aggressive symptoms, pure motor and/or phonic tics, compulsive phenomena, and tapping, and the last group characterized by the absence of grunting.
Robertson and Cavanna (56) conducted a principal component factor analysis for members of a large GTS pedigree who had symptoms of the GTS spectrum. They carried out cluster analysis on the pedigree members which consisted of 85 family members, 69 of whom demonstrated the following symptoms: tics, obsessive–compulsive symptoms (OCS) and ADHD. As such, they included a relatively heterogeneous sample, which may have influenced the results. The authors identified three clinical subgroups: the first group, which contained “pure GTS” cases; the second group, comprised of patients chiefly who suffered from ADHD and aggressive behaviors; and the third group, which consisted of patients with “depression–anxiety–obsessional symptoms and self-injurious behaviors.”
In consideration of the previously mentioned findings, it has been also suggested that there is a clinical continuum between pure GTS and GTS plus phenotypes. As mentioned by Grados and Mathews in their review (60), this continuum could be further expanded: GTS with comorbidities would be the most severe and rare form, while transient tics and chronic tic disorders would be the mildest forms. The authors highlight the following phenotypes in the GTS spectrum: pure GTS, GTS+OCD, and GTS+OCD+ADHD.
In 2015, de Haan et al. (61) also sought to establish clinical subgroups within GTS. To carry out this analysis, they analyzed data from 494 patients with GTS from the USA and the Netherlands as well as 351 Dutch family members. As a result of their analysis, they identified three clinical groups: the first group, in which patients predominantly had complex vocal tics and obscene behavior; the second group, in which patients mainly had body tics; and the third group, in which patients mainly had head and neck tics.
Subsequently, Eapen and Robertson (55) compared samples of pure GTS and GTS plus. The authors included in their analysis the following clinical characteristics: disease severity, accompanying clinical features, and family history. To determine symptom severity, they used the following clinical measurements: The National Hospital Interview Schedule (NHIS) for GTS and related behaviors, the Diagnostic Confidence Index (DCI), and the Yale Global Tic Severity Scale (YGTSS). They included 400 patients altogether, but information on psychiatric comorbidities was only available in 222 cases, 13.5% of which had pure GTS. In the GTS plus group, 39% suffered from coprolalia, yet this symptom was not reported by any of the pure GTS patients and this difference was statistically significant. Similarly, the presence of copropraxia was higher (15.4%) in the GTS plus group as compared to the pure GTS (6.3%) group. Interestingly, the only other significant difference between the two groups was that pure GTS was not associated with any family history of OCD, confirming that both groups, pure GTS and GTS plus, may differ specifically when it comes to underlying pathophysiology. Surprisingly, no differences were detected regarding disease severity as measured by the YGTSS and the DCI total score, although results from the DCI approached statistical significance.
In 2016, Sambrani et al. (62) analyzed the clinical characteristics of a large sample (n = 1,032) of German patients with tic disorders. The authors found that GTS+OCD was a more severe form of GTS and that comorbid OCD/OCS, depression, and anxiety were part of the GTS spectrum, while ADHD should be treated as a separate problem. They also compared groups of patients with and without specific complex tics (coprophenomena, echophenomena, and paliphenomena), which was distinguished as “full blown GTS.” Particularly, they identified a significant association between coprophenomena, echophenomena, paliphenomena, and comorbidity score. Furthermore, copro-, pali-, and echophenomena were associated with age. Finally, coprophenomena were also correlated with a wide repertoire of psychiatric comorbidities, such as SIB, depression, or rage attacks. All in all, these findings underline that the presence of copro-, pali-, or echophenomena is an indicator of a more severe and complex GTS plus phenotype. Importantly, the same group repeated the previously mentioned efforts by Robertson and Cavanna (56) and attempted to establish subclassification of GTS. In order to accomplish this, they divided the participants into three clusters, as previously mentioned, but were able to classify only one third of them. The vast majority of participants were included in the third category (GTS and comorbid OCD, OCS, anxiety, depression, and SIB). The authors found significant differences between the groups with respect to copropraxia, being significantly more frequent in cluster 2 than in cluster 3. Moreover, tic suppression was more common in the pure GTS group than in cluster 2 (91.7 vs. 78.2%). Finally, the percentage of patients with premonitory urges was significantly higher in cluster 3 than in cluster 1.
In 2017, Pringsheim evaluated the data of 114 Canadian children with GTS (63). Her work focused on examining whether the presence of comorbid ADHD or OCD has an influence on tic severity and treatment. Children with OCD demonstrated significantly higher tic severity. Although children with ADHD were more likely to be treated for their tics within the first 2 years of diagnosis, they did not present with worse tic severity. As such, the author concluded that the association may be related to greater overall psychosocial impairment in children with this comorbidity. Once again, these findings speak to the idea that the presence of a comorbid psychiatric disorder is a clear distinguishing feature of more severe clinical phenotype in GTS.
In a recent paper by Müller-Vahl et al. (64), the authors point out that although chronic tic disorders and GTS are described separately in classifications, there is no clear genetic and phenotypic background for such distinction. In order to tackle this clinical conundrum, the authors analyzed and compared the clinical data of 1,018 patients with GTS and chronic tic disorder. Patients differed in tic severity, with chronic motor tic disorder (CMTD) patients having lower mean tic severity; prevalence of complex motor tics, copropraxia, and echopraxia; and a markedly lower comorbidity score as compared to GTS patients. These findings suggested that both disorders exist along a symptom severity continuum of which GTS constitutes a more severe and CMTD a less severe form. It was therefore suggested to use the term “tic spectrum disorders,” instead of using distinctive diagnostic categories.
Subtypes of GTS Based on Longitudinal Studies
In this context, it is worth mentioning a number of studies which examine the course of GTS in the long-term follow-up. One important longitudinal analysis was published by Groth et al. (65), in which they investigated the clinical course of tics and comorbidities trying to establish trajectories of possible clinical phenotypes and their predictors. The authors examined a large clinical cohort recruited at the Danish National Tourette Clinic during the periods 2005–2007 and 2011–2013. They included 314 participants aged 5–19 years at baseline and, 6 years later, 227 participants who took part in the subsequent follow-up visit. At each time point, exhaustive clinical evaluation was conducted. As expected, tic symptoms as well as comorbid OCD and ADHD symptoms declined over time. With time, the clinical phenotype generally evaluated toward pure GTS. They also identified several predictors for the clinical course of GTS in adolescence and early adulthood. Particularly, childhood tics, OCD severity, and ADHD severity predicted the persistence of respective diagnoses in the future. Furthermore, psychiatric comorbidities were found in only 63% of cases at follow-up, while almost 37% of patients had pure GTS.
An important contribution evaluating the course of tics and comorbidities in the perspective of clinical variability was published by Bloch et al. (66). The authors examined the adulthood outcome of tics and OCD symptoms in children with GTS. They included forty-six children with GTS, who had received a structured clinical evaluation prior to age 14, and conducted an expert rating of tics and OCD symptoms at baseline and at a follow-up interview after 7.6 years (in average). The majority of cases (85%) reported a reduction in tic symptoms in the follow-up evaluation, although higher levels of tic severity in childhood were associated with higher levels of tic severity at follow-up.
Between Clinics and Genetics—Approach to Refractoriness Based on the Genetic Variability
Taken as background findings from the area of genetics and genomics, it has been established that tics, OCD, and GTS share a common genetic architecture (33, 34, 67). At the same time, the merge between genetic and clinical characteristics may enable the identification of possible subgroups in GTS. Hirschritt et al. (68) showed that social disinhibition is a heritable subphenotype in GTS. The study included 3,494 individuals (1,191 GTS patients and 203 of their first-degree relatives). Heritabilities of the subtypes were estimated and associated with clinical characteristics. The authors conducted exploratory factor analysis as well as latent class model analysis and grouped participants in the following categories: unaffected, simple tics, intermediate tics without social disinhibition, intermediate with social disinhibition, and high rates of all tic types. Across models, a phenotype characterized by high rates of social disinhibition emerged. The presence of social disinhibition was associated with increased odds of comorbid psychiatric disorders, earlier age at GTS onset, and increased tic severity. Darrow et al. (69) identified two heritable endophenotypes for GTS. The authors included 3,494 individuals with GTS, OCD, and ADHD. They carried out symptom-level factor and latent class analyses in GTS families as well as in 882 healthy individuals. The authors were able to identify two cross-disorder phenotypes: symmetry (symmetry, evening up, checking obsessions; ordering, arranging, counting, writing–rewriting compulsions, repetitive writing tics) and disinhibition (uttering syllables/words, echolalia/palilalia, coprolalia/copropraxia, and obsessive urges to offend/mutilate/be destructive). Moreover, both phenotypes were highly heritable. Also, PRS related to GTS were associated with symmetry, while OCD PRS were associated with the symptoms of disinhibition.
A summary of reports investigating different GTS subgroups and longitudinal studies is shown in Table 2.
Definitions of Refractory GTS
The Concept of Pseudo-Refractoriness
Similar to anxiety or OCD, pseudo-refractoriness should also be considered in GTS (70). Kious et al. also describe this term as “apparent refractoriness” (70). The main reasons for this condition are nonadherence, misdiagnosis, preponderance of psychiatric comorbidities, inadequate treatment (low doses or wrong selection of agent by the clinician), incorrect diagnosis, lack of tolerance, or lack of access to all available therapies. Nonadherence in GTS can affect a significant number of patients. Importantly, compliance is highly dependent on the type of medication administered and its adverse events. For typical antipsychotics (71), this rate can be as high as 78%. It is therefore crucial to rigorously assess the accuracy of the diagnosis. In case of treatment refractoriness, misdiagnosis should also be taken into consideration. Tics could be mistaken with functional movement disorder, myoclonus, chorea, paroxysmal movements, epilepsy, stereotypies, or psychiatric symptoms, for example, compulsions or, rarely, hyperactivity in ADHD. Another factor to consider when facing treatment resistant cases is that uncontrolled, coexisting, neuropsychiatric conditions can also exacerbate tics. Consequently, reevaluation of possible comorbid conditions should be considered. Moreover, the majority of medications used for treatment of tics are associated with significant risk of adverse events and, therefore, are not well-tolerated. In the recently published meta-analysis by Yang et al. (72), the authors established that even 95% of patients treated with neuroleptics experience adverse events and between 30 and 80% discontinue treatment due to lack of tolerance.
Refractoriness to Pharmacological Treatment in GTS
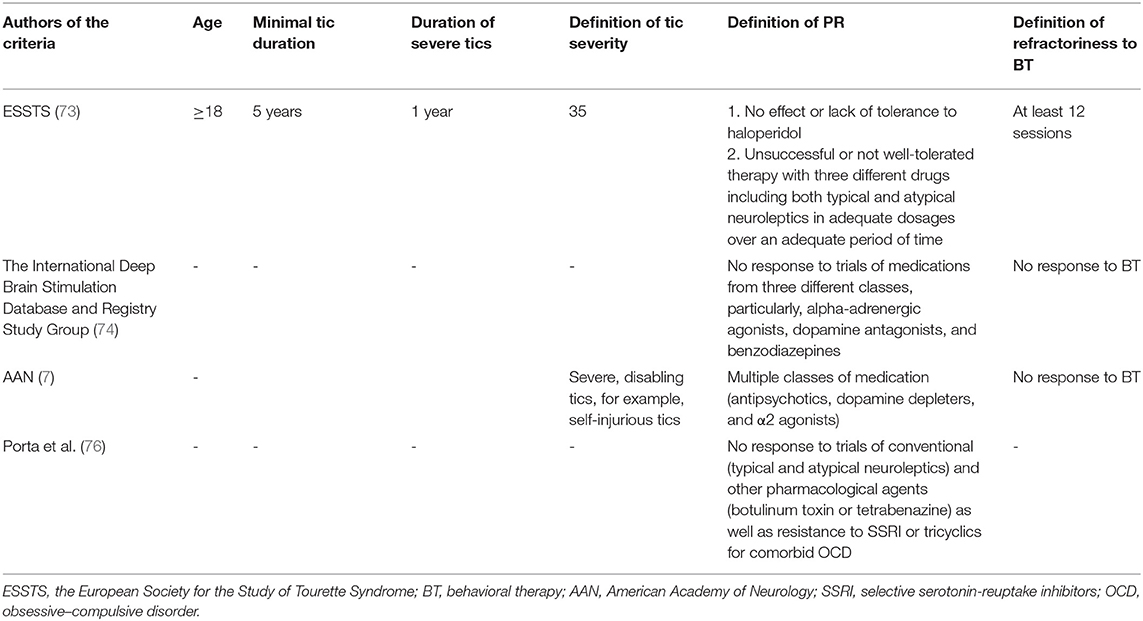
Taking into consideration phenotypic and genetic variability of GTS, the most severe clinical phenotype also could be interpreted as being refractory. Nevertheless, as mentioned previously, there are no widely established criteria for refractory GTS. Discussion about criteria of refractoriness was raised by the European Society for the Study of Tourette Syndrome (ESSTS) (73). In the ESSTS Guidelines (73), it was suggested that the following clinical criteria indicate refractoriness and, as a consequence, could be qualified for experimental treatment with deep brain stimulation (DBS): (a) tics should last at minimum 5 years, (b) tics should be severe for at least 1 year, and (c) tic severity should be rated ≥35 according to YGTSS. Treatment refractoriness could be established after unsuccessful or not well-tolerated therapy with three different drugs including both typical and atypical neuroleptics in adequate dosages over an adequate period of time. If possible, behavioral therapy for at least 12 sessions should also have been implemented. Moreover, the authors indicate that haloperidol, being the only drug licensed for treatment of GTS in the majority of European countries, suggests that failure of this drug already implicates treatment resistance. The International Deep Brain Stimulation Database and Registry Study Group (74) suggested to identify candidates for DBS in GTS based on the following criteria: lack of response to trials of medications from three different classes, particularly, alpha-adrenergic agonists, dopamine antagonists, and benzodiazepines.
The subgroup of refractory GTS was investigated in only few studies. Colquhoun et al. (75) analyzed records of 329 GTS patients and identified refractory cases using the previously mentioned ESSTS criteria. Only 14 individuals (4.3%) were identified as belonging to the refractory group. Porta et al. (76) systematically reviewed the literature with the aim to revise the criteria of treatment refractoriness in GTS and concluded that this term is poorly defined in the scientific literature. As a matter of fact, the only publications regarding this matter were related to efficacy and tolerability of DBS. They also proposed their own definition of treatment refractoriness, based on their clinical experience. Specifically, they suggested that the following factors should be taken into consideration: no significant improvement in health-related quality of life in response to trials of conventional (typical and atypical neuroleptics) and other pharmacological agents (botulinum toxin or tetrabenazine) as well as resistance to selective serotonin-reuptake inhibitors (SSRI) or tricyclics for comorbid OCD.
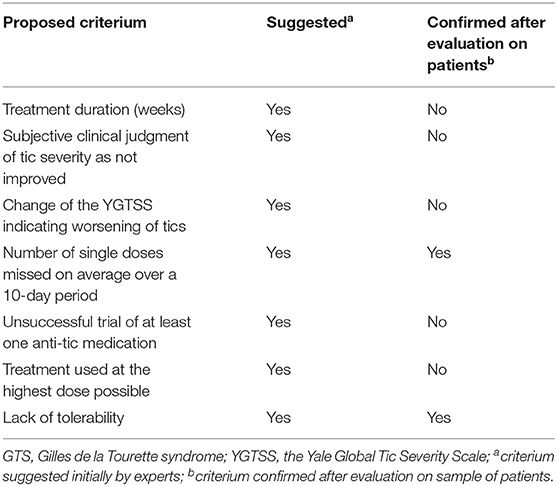
Another study by Macerollo et al. (77) reported the findings of the European audit survey. The main goal was to establish tentative criteria for treatment refractoriness in GTS as well as evaluate how frequently these criteria are taken into consideration in routine clinical practice. The methodological approach used in this case included expert-based interviews conducted by seven clinicians with extensive experience in GTS. Different criteria were proposed and rated according to whether the domain was considered as necessary for the definition of refractory GTS. Those criteria that were rated as necessary were also included in subsequent analyses. As a result, the following final criteria were proposed: adequate treatment duration (for at least 3 months); subjective clinical judgment of tic severity as improved or not; change in the YGTSS indicating worsening of tics; reason for the use of maximal dose; and number of single doses missed on average over a 10-day period. Subsequently, seven other experts were asked to comment on the previously selected criteria. This questionnaire was implemented for patients with an unsuccessful trial of at least one anti-tic medication. Two additional criteria were treatment used at the highest dose possible and lack of tolerability. Their final study was conducted on a group of 68 patients rated as treatment refractory; 45 of them were rated as refractory due to lack of efficacy in spite of treatment at maximal dose, while another 23 were included in the refractory group because of intolerability due to side effects. The median time of treatment was of 39 weeks. The final results of this study demonstrated that even among the selected group of GTS experts there was no consensus regarding the treatment refractoriness. While subjectively clinicians did not see improvement under particular treatment, in 39 out of 68 patients, there was a significant tic reduction according to YGTSS total tic score. As also indicated in this paper, not all experts used the YGTSS to confirm refractoriness. It could therefore be concluded that tic severity is not an adequate, unique measurement of treatment refractoriness and should be complemented by the quality of life evaluation. In summary, the authors concluded that they must include lack of tolerability as well as treatment adherence, defined as less than one dose missed on average over a 10-day period, in the refractoriness criteria. A summary of the criteria proposed in this study is found in Table 3, while inclusion criteria for DBS in GTS are demonstrated in Table 4. Importantly, similar criteria were indicated in ESSTS Guidelines (73).
Treatment of Refractory GTS
As stated above, refractoriness in GTS is highly determined by the response to the evidence-based treatments. In the recently published, evidence-based recommendations by the American Academy of Neurology (AAN) (5, 7), the authors revised the available treatments for GTS. A multidisciplinary panel of experts composed of physicians, psychologists, and patient representatives developed recommendations supported by the systematic review. As a result, they formulated 46 recommendations regarding the assessment and management of tics. They underlined that the treatment should be individualized, consulted on by the patient, family, and the provider. Also, the presence of comorbid conditions should be taken into consideration. Therefore, when deciding on treatment of refractory TS, the first decision should be directed toward identification of the main complaint: tics or comorbidities. The authors of the AAN Guidelines comment on currently available treatments for GTS and mention the level of recommendation, also for the refractory cases. They indicate that where the legislation allows, physicians may consider treatment with cannabis-based medication, a treatment option confirmed to be effective at least for some patients (78–82). In cases of refractoriness, a combination of psychotherapy and pharmacotherapy as well as a combination of different compounds with different mechanism of action could be implemented. In some GTS patients, injecting botulinum toxin locally may alleviate the symptoms of motor or/and phonic tics (83). Additionally, the authors discuss the rationale for the use of the DBS in refractory GTS. They indicate that patients with severe GTS, resistant to medical and behavioral therapy, may benefit from the application of DBS. However, this method should be used only in specialized centers with experience in GTS. Moreover, the patients should undergo a multidisciplinary evaluation by a neurologist or psychiatrist, a neurosurgeon, and a psychologist. Additionally, the benefits must outweigh the risks of the procedure. Clinicians should confirm the diagnosis according to the current DSM-5 criteria and exclude secondary tics. Prior to DBS, multiple classes of medication (antipsychotics, dopamine depleters, and α2 agonists) and behavioral therapy must be administered. Finally, DBS should be used only for severe, disabling tics, for example, self-injurious tics. These recommendations are in line with the European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: deep brain stimulation (73) and the recommendations of the Tourette Syndrome Association International Deep Brain Stimulation (DBS) Database and Registry Study Group (74).
Conclusions
As demonstrated by the cacophony of findings presented in this review, the definition of treatment refractory GTS still needs to be definitively established. The primary reasons for the lack of an established definition is the robust clinical variability of GTS as well as the fact that it often co-occurs with a number of other disorders that can contribute to the deterioration in quality of life. Future efforts should focus on the collection of large, international clinical data that could enable analysis with machine learning approaches as this could elucidate the presence of objective clinical clusters (84).
Author Contributions
NS conceived and designed the study, acquired data, and wrote the original draft of the manuscript. NS, AL, JL, MB, and AL-W analyzed and interpreted the data, performed visualization, and reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JL has received grant and research support from National Institutes of Health. He also serves on the scientific advisory boards of the Brain and Behavior Research Foundation, the European Multicentre Tics in Children Studies, the National Organization for Rare Diseases, Fondazione Child, Empathy for Peace, and the Alliance for Decision Education. He has also received royalties from John Wiley and Sons, McGraw-Hill, and Oxford University Press. MB receives additional contracted research support from Biohaven Pharmaceuticals, Janssen Pharmaceuticals, Emalex Biosciences, and Neurocrine Biosciences. He also receives research funding from the NIH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Roessner V, Hoekstra PJ, Rothenberger A. Tourette's disorder and other tic disorders in DSM-5: a comment. Eur Child Adolesc Psychiatry. (2011) 20:71–4. doi: 10.1007/s00787-010-0143-3
2. Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers. (2017) 3:16097. doi: 10.1038/nrdp.2016.97
3. Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. (1989) 28:566–73. doi: 10.1097/00004583-198907000-00015
4. Martino D, Pringsheim TM, Cavanna AE, Colosimo C, Hartmann A, Leckman JF, et al. Systematic review of severity scales and screening instruments for tics: critique and recommendations. Mov Disord. (2017) 32:467–73. doi: 10.1002/mds.26891
5. Pringsheim T, Holler-Managan Y, Okun MS, Jankovic J, Piacentini J, Cavanna AE, et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:907–15. doi: 10.1212/WNL.0000000000007467
6. Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. (2011) 20:173–96. doi: 10.1007/s00787-011-0163-7
7. Pringsheim T, Okun MS, Muller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/WNL.0000000000007466
8. Billnitzer A, Jankovic J. Current management of tics and tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. (2020). doi: 10.1007/s13311-020-00914-6. [Epub ahead of print].
9. Hienert M, Gryglewski G, Stamenkovic M, Kasper S, Lanzenberger R. Striatal dopaminergic alterations in Tourette's syndrome: a meta-analysis based on 16 PET and SPECT neuroimaging studies. Transl Psychiatry. (2018) 8:143. doi: 10.1038/s41398-018-0202-y
10. Maia TV, Conceição VA. Dopaminergic disturbances in Tourette syndrome: an integrative account. Biol Psychiatry. (2018) 84:332–44. doi: 10.1016/j.biopsych.2018.02.1172
11. Kanaan AS, Gerasch S, García-García I, Lampe L, Pampel A, Anwander A, et al. Pathological glutamatergic neurotransmission in gilles de la Tourette syndrome. Brain. (2017) 140:218–34. doi: 10.1093/brain/aww285
12. Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, et al. Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatr Genet. (2011) 21:90–7. doi: 10.1097/YPG.0b013e328341a307
13. Müller-Vahl KR, Szejko N, Wilke F, Jakubovski E, Geworski L, Bengel F, et al. Serotonin transporter binding is increased in Tourette syndrome with obsessive compulsive disorder. Sci Rep. (2019) 9:972. doi: 10.1038/s41598-018-37710-4
14. Mahone EM, Puts NA, Edden RAE, Ryan M, Singer HS. GABA and glutamate in children with tourette syndrome: A (1)H MR spectroscopy study at 7T. Psychiatry Res Neuroimaging. (2018) 273:46–53. doi: 10.1016/j.pscychresns.2017.12.005
15. Puts NA, Harris AD, Crocetti D, Nettles C, Singer HS, Tommerdahl M, et al. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol. (2015) 114:808–17. doi: 10.1152/jn.00060.2015
16. Draper A, Stephenson MC, Jackson GM, Pépés S, Morgan PS, Morris PG, et al. Increased GABA contributes to enhanced control over motor excitability in Tourette syndrome. Curr Biol. (2014) 24:2343–7. doi: 10.1016/j.cub.2014.08.038
17. Müller-Vahl KR, Bindila L, Lutz B, Musshoff F, Skripuletz T, Baumgaertel C, et al. Cerebrospinal fluid endocannabinoid levels in gilles de la Tourette syndrome. Neuropsychopharmacology. (2020) 45:1323–9. doi: 10.1038/s41386-020-0671-6
18. Szejko N, Fichna JP, Safranow K, Dziuba T, Zekanowski C, Janik P. Association of a variant of CNR1 gene encoding cannabinoid receptor 1 with gilles de la Tourette syndrome. Front Genet. (2020) 11:125. doi: 10.3389/fgene.2020.00125
19. Yasmeen S, Melchior L, Bertelsen B, Skov L, Mol Debes N, Tümer Z. Sequence analysis of SLITRK1 for var321 in Danish patients with tourette syndrome and review of the literature. Psychiatr Genet. (2013) 23:130–3. doi: 10.1097/YPG.0b013e328360c880
20. Chou IC, Wan L, Liu SC, Tsai CH, Tsai FJ. Association of the slit and Trk-like 1 gene in Taiwanese patients with Tourette syndrome. Pediatr Neurol. (2007) 37:404–6. doi: 10.1016/j.pediatrneurol.2007.06.017
21. Deng H, Le WD, Xie WJ, Jankovic J. Examination of the SLITRK1 gene in caucasian patients with Tourette syndrome. Acta Neurol Scand. (2006) 114:400–2. doi: 10.1111/j.1600-0404.2006.00706.x
22. Lee CC, Chou IC, Tsai CH, Wang TR, Li TC, Tsai FJ. Dopamine receptor D2 gene polymorphisms are associated in Taiwanese children with Tourette syndrome. Pediatr Neurol. (2005) 33:272–6. doi: 10.1016/j.pediatrneurol.2005.05.005
23. He F, Zheng Y, Huang HH, Cheng YH, Wang CY. Association between tourette syndrome and the dopamine D3 receptor gene rs6280. Chin Med J. (2015) 128:654–8. doi: 10.4103/0366-6999.151665
24. Yuan L, Zheng W, Yang Z, Deng X, Song Z, Deng H. Association of the AADAC gene and Tourette syndrome in a Han Chinese cohort. Neurosci Lett. (2018) 666:24–7. doi: 10.1016/j.neulet.2017.12.034
25. Janik P, Berdyński M, Safranow K, Zekanowski C. Association of ADORA1 rs2228079 and ADORA2A rs5751876 polymorphisms with gilles de la Tourette syndrome in the polish population. PLoS ONE. (2015) 10:e0136754. doi: 10.1371/journal.pone.0136754
26. Janik P, Berdyński M, Safranow K, Zekanowski C. The BTBD9 gene polymorphisms in polish patients with gilles de la Tourette syndrome. Acta Neurobiol Exp. (2014) 74:218–26.
27. Huertas-Fernández I, Gómez-Garre P, Madruga-Garrido M, Bernal-Bernal I, Bonilla-Toribio M, Martín-Rodríguez JF, et al. GDNF gene is associated with Tourette syndrome in a family study. Mov Disord. (2015) 30:1115–20. doi: 10.1002/mds.26279
28. Gomez L, Wigg K, Zhang K, Lopez L, Sandor P, Malone M, et al. Association of the KCNJ5 gene with tourette syndrome and attention-deficit/hyperactivity disorder. Genes Brain Behav. (2014) 13:535–42. doi: 10.1111/gbb.12141
29. Chou IC, Lin HC, Wang CH, Lin WD, Lee CC, Tsai CH, et al. Polymorphisms of interleukin 1 gene IL1RN are associated with Tourette syndrome. Pediatr Neurol. (2010) 42:320–4. doi: 10.1016/j.pediatrneurol.2010.01.006
30. Sun N, Nasello C, Deng L, Wang N, Zhang Y, Xu Z, et al. The PNKD gene is associated with Tourette disorder or tic disorder in a multiplex family. Mol Psychiatry. (2018) 23:1487–95. doi: 10.1038/mp.2017.179
31. Lei J, Deng X, Zhang J, Su L, Xu H, Liang H, et al. Mutation screening of the HDC gene in Chinese Han patients with Tourette syndrome. Am J Med Genet B Neuropsychiatr Genet. (2012) 159b:72–6. doi: 10.1002/ajmg.b.32003
32. Dong H, Liu W, Liu M, Xu L, Li Q, Zhang R, et al. Investigation of a possible role for the histidine decarboxylase gene in Tourette syndrome in the Chinese Han population: a family-based study. PLoS ONE. (2016) 11:e0160265. doi: 10.1371/journal.pone.0160265
33. Yu D, Mathews CA, Scharf JM, Neale BM, Davis LK, Gamazon ER, et al. Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette's syndrome and OCD. Am J Psychiatry. (2015) 172:82–93. doi: 10.1176/appi.ajp.2014.13101306
34. Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, et al. Interrogating the genetic determinants of Tourette's syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry. (2019) 176:217–27. doi: 10.1176/appi.ajp.2018.18070857
35. Baumgaertel C, Skripuletz T, Kronenberg J, Stangel M, Schwenkenbecher P, Sinke C, et al. Immunity in gilles de la Tourette-syndrome: results from a cerebrospinal fluid study. Front Neurol. (2019) 10:732. doi: 10.3389/fneur.2019.00732
36. Martino D, Zis P, Buttiglione M. The role of immune mechanisms in Tourette syndrome. Brain Res. (2015) 1617:126–43. doi: 10.1016/j.brainres.2014.04.027
37. Bos-Veneman NGP, Bijzet J, Limburg PC, Minderaa RB, Kallenberg CG, Hoekstra PJ. Cytokines and soluble adhesion molecules in children and adolescents with a tic disorder. Progr Neuro Psychopharmacol Biol Psychiatry. (2010) 34:1390–5. doi: 10.1016/j.pnpbp.2010.06.028
38. Brander G, Rydell M, Kuja-Halkola R, Fernández de la Cruz L, Lichtenstein P, Serlachius E, et al. Perinatal risk factors in Tourette's and chronic tic disorders: a total population sibling comparison study. Mol Psychiatry. (2018) 23:1189–97. doi: 10.1038/mp.2017.31
39. Abdulkadir M, Tischfield JA, King RA, Fernandez TV, Brown LW, Cheon KA, et al. Pre- and perinatal complications in relation to Tourette syndrome and co-occurring obsessive-compulsive disorder and attention-deficit/hyperactivity disorder. J Psychiatr Res. (2016) 82:126–35. doi: 10.1016/j.jpsychires.2016.07.017
40. McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. (2014) 156:1–7. doi: 10.1016/j.jad.2013.10.043
41. Malhi GS, Parker GB, Crawford J, Wilhelm K, Mitchell PB. Treatment-resistant depression: resistant to definition? Acta Psychiatr Scand. (2005) 112:302–9. doi: 10.1111/j.1600-0447.2005.00602.x
42. Ruhé HG, van Rooijen G, Spijker J, Peeters FP, Schene AH. Staging methods for treatment resistant depression. A systematic review. J Affect Disord. (2012) 137:35–45. doi: 10.1016/j.jad.2011.02.020
43. Roy-Byrne P. Treatment-refractory anxiety; definition, risk factors, and treatment challenges. Dialogues Clin Neurosci. (2015) 17:191–206. doi: 10.31887/DCNS.2015.17.2/proybyrne
44. Bokma WA, Wetzer GAAM, Gehrels JB, Penninx BWJH, Batelaan NM, van Balkom ALJM. Aligning the many definitions of treatment resistance in anxiety disorders: a systematic review. Depression Anxiety. (2019) 36:801–12. doi: 10.1002/da.22895
45. Naguy A, Alamiri B. Treatment-resistant OCD - A psychopharmacological 'touche d'art'. Asian J Psychiatr. (2018) 34:98–9. doi: 10.1016/j.ajp.2018.04.025
46. Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive-compulsive disorder in children. J Am Acad Child Adolesc Psychiatry. (2015) 54:251–62. doi: 10.1016/j.jaac.2015.01.011
47. Sharma E, Math SB. Course and outcome of obsessive-compulsive disorder. Indian J Psychiatry. (2019) 61(Suppl. 1):S43–50. doi: 10.4103/psychiatry.IndianJPsychiatry_521_18
48. Del Casale A, Sorice S, Padovano A, Simmaco M, Ferracuti S, Lamis DA, et al. Psychopharmacological treatment of obsessive-compulsive disorder (OCD). Curr Neuropharmacol. (2019) 17:710–36. doi: 10.2174/1570159X16666180813155017
49. Borza L. Cognitive-behavioral therapy for generalized anxiety. Dialogues Clin Neurosci. (2017) 19:203–8. doi: 10.31887/DCNS.2017.19.2/lborza
50. Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. (2017) 74:9–10. doi: 10.1001/jamapsychiatry.2016.2586
51. Martino D, Ganos C, Pringsheim TM. Tourette syndrome and chronic tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol. (2017) 134:1461–90. doi: 10.1016/bs.irn.2017.05.006
52. Marwitz L, Pringsheim T. Clinical utility of screening for anxiety and depression in children with Tourette syndrome. J Can Acad Child Adolesc Psychiatry. (2018) 27:15–21.
53. Piedad JC, Cavanna AE. Depression in Tourette syndrome: a controlled and comparison study. J Neurol Sci. (2016) 364:128–32. doi: 10.1016/j.jns.2016.03.030
54. Hashemiyoon R, Kuhn J, Visser-Vandewalle V. Putting the pieces together in gilles de la Tourette syndrome: exploring the link between clinical observations and the biological basis of dysfunction. Brain Topogr. (2017) 30:3–29. doi: 10.1007/s10548-016-0525-z
55. Eapen V, Robertson M. Are there distinct subtypes in Tourette syndrome? Pure-Tourette syndrome versus Tourette syndrome-plus, and simple versus complex tics. Neuropsychiatr Dis Treat. (2015) 11:1431–6. doi: 10.2147/NDT.S72284
56. Robertson MM, Cavanna AE. The gilles de la Tourette syndrome: a principal component factor analytic study of a large pedigree. Psychiatr Genet. (2007) 17:143–52. doi: 10.1097/YPG.0b013e328015b937
58. Kano Y, Ohta M, Nagai Y. Differences in clinical characteristics between Tourette syndrome patients with and without 'generalized tics' or coprolalia. Psychiatry Clin Neurosci. (1997) 51:357–61. doi: 10.1111/j.1440-1819.1997.tb02599.x
59. Alsobrook JP 2nd, Pauls DL. A factor analysis of tic symptoms in Gilles de la Tourette's syndrome. Am J Psychiatry. (2002) 159:291–6. doi: 10.1176/appi.ajp.159.2.291
60. Grados MA, Mathews CA. Clinical phenomenology and phenotype variability in Tourette syndrome. J Psychosom Res. (2009) 67:491–6. doi: 10.1016/j.jpsychores.2009.07.011
61. de Haan MJ, Delucchi KL, Mathews CM, Cath DC. Tic symptom dimensions and their heritabilities in Tourette's syndrome. Psychiatr Genet. (2015) 25:112–8. doi: 10.1097/YPG.0000000000000084
62. Sambrani T, Jakubovski E, Müller-Vahl KR. New insights into clinical characteristics of gilles de la Tourette syndrome: findings in 1032 patients from a single German center. Front Neurosci. (2016) 10:415. doi: 10.3389/fnins.2016.00415
63. Pringsheim T. Tic severity and treatment in children: the effect of comorbid attention deficit hyperactivity disorder and obsessive compulsive behaviors. Child Psychiatry Hum Dev. (2017) 48:960–6. doi: 10.1007/s10578-017-0718-z
64. Müller-Vahl KR, Sambrani T, Jakubovski E. Tic disorders revisited: introduction of the term “tic spectrum disorders”. Eur Child Adolesc Psychiatry. (2019) 28:1129–35. doi: 10.1007/s00787-018-01272-7
65. Groth C, Mol Debes N, Rask CU, Lange T, Skov L. Course of Tourette syndrome and comorbidities in a large prospective clinical study. J Am Acad Child Adolesc Psychiatry. (2017) 56:304–12. doi: 10.1016/j.jaac.2017.01.010
66. Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. (2006) 160:65–9. doi: 10.1001/archpedi.160.1.65
67. Abdulkadir M, Mathews CA, Scharf JM, Yu D, Tischfield JA, Heiman GA, et al. Polygenic risk scores derived from a Tourette syndrome genome-wide association study predict presence of tics in the avon longitudinal study of parents and children cohort. Biol Psychiatry. (2019) 85:298–304. doi: 10.1016/j.biopsych.2018.09.011
68. Hirschtritt ME, Darrow SM, Illmann C, Osiecki L, Grados M, Sandor P, et al. Social disinhibition is a heritable subphenotype of tics in Tourette syndrome. Neurology. (2016) 87:497–504. doi: 10.1212/WNL.0000000000002910
69. Darrow SM, Hirschtritt ME, Davis LK, Illmann C, Osiecki L, Grados M, et al. Identification of two heritable cross-disorder endophenotypes for Tourette syndrome. Am J Psychiatry. (2017) 174:387–96. doi: 10.1176/appi.ajp.2016.16020240
70. Kious BM, Jimenez-Shahed J, Shprecher DR. Treatment-refractory Tourette syndrome. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 70:227–36. doi: 10.1016/j.pnpbp.2016.02.003
71. Silva RR, Muñoz DM, Daniel W, Barickman J, Friedhoff AJ. Causes of haloperidol discontinuation in patients with Tourette's disorder: management and alternatives. J Clin Psychiatry. (1996) 57:129–35.
72. Yang C, Hao Z, Zhang LL, Zhu CR, Zhu P, Guo Q. Comparative efficacy and safety of antipsychotic drugs for tic disorders: a systematic review and bayesian network meta-analysis. Pharmacopsychiatry. (2019) 52:7–15. doi: 10.1055/s-0043-124872
73. Muller-Vahl KR, Cath DC, Cavanna AE, Dehning S, Porta M, Robertson MM, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: deep brain stimulation. Eur Child Adolesc Psychiatry. (2011) 20:209–17. doi: 10.1007/s00787-011-0166-4
74. Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord. (2015) 30:448–71. doi: 10.1002/mds.26094
75. Colquhoun M, Stern JS, Collicott N, Williams D, Grabecki K, Simmons H, et al. Severe refractory Tourette syndrome. J Neurol Neurosurg Psychiatry. (2014) 85:e3. doi: 10.1136/jnnp-2014-308883.35
76. Porta M MC, Sassi M, Brambilla A, Defendi A, Servello D, Selvini C, et al. Treatment-refractory Tourette syndrome. Ital J Psychopathol. (2011) 17:225–33. Available online at: https://www.semanticscholar.org/paper/Treatment-refractory-Tourette-syndrome-Porta-Menghetti/988231285aa8bf22c161773891ec84e45a221f38
77. Macerollo A, Martino D, Cavanna AE, Gulisano M, Hartmann A, Hoekstra PJ, et al. Refractoriness to pharmacological treatment for tics: a multicentre European audit. J Neurol Sci. (2016) 366:136–8. doi: 10.1016/j.jns.2016.05.004
78. Muller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, et al. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry. (2003) 64:459–65. doi: 10.4088/JCP.v64n0417
79. Muller-Vahl KR, Schneider U, Koblenz A, Jobges M, Kolbe H, Daldrup T, et al. Treatment of Tourette's syndrome with Delta 9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry. (2002) 35:57–61. doi: 10.1055/s-2002-25028
80. Milosev LM, Psathakis N, Szejko N, Jakubovski E, Muller-Vahl KR. Treatment of gilles de la Tourette syndrome with cannabis-based medicine: results from a retrospective analysis and online survey. Cannabis Cannabinoid Res. (2019) 4:265–74. doi: 10.1089/can.2018.0050
81. Artukoglu BB, Bloch MH. The potential of cannabinoid-based treatments in Tourette syndrome. CNS Drugs. (2019) 33:417–30. doi: 10.1007/s40263-019-00627-1
82. Abi-Jaoude E, Chen L, Cheung P, Bhikram T, Sandor P. Preliminary evidence on cannabis effectiveness and tolerability for adults with Tourette syndrome. J Neuropsychiatry Clin Neurosci. (2017) 29:391–400. doi: 10.1176/appi.neuropsych.16110310
83. Pandey S, Srivanitchapoom P, Kirubakaran R, Berman BD. Botulinum toxin for motor and phonic tics in Tourette's syndrome. Cochrane Database Syst Rev. (2018) 1:Cd012285. doi: 10.1002/14651858.CD012285.pub2
Keywords: Gilles de la Tourette syndrome, treatment-refractoriness, severe tics, psychiatric comorbidities, tics
Citation: Szejko N, Lombroso A, Bloch MH, Landeros-Weisenberger A and Leckman JF (2020) Refractory Gilles de la Tourette Syndrome—Many Pieces That Define the Puzzle. Front. Neurol. 11:589511. doi: 10.3389/fneur.2020.589511
Received: 30 July 2020; Accepted: 20 November 2020;
Published: 18 December 2020.
Edited by:
Daniel Martinez-Ramirez, Tecnológico de Monterrey, MexicoReviewed by:
Prachaya Srivanitchapoom, Mahidol University, ThailandWissam Deeb, UMass Memorial Health Care, United States
Copyright © 2020 Szejko, Lombroso, Bloch, Landeros-Weisenberger and Leckman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalia Szejko, bmF0YWxpYS5zemVqa29AeWFsZS5lZHU=
 Natalia Szejko1,2,3*
Natalia Szejko1,2,3* Adam Lombroso
Adam Lombroso Michael H. Bloch
Michael H. Bloch Angeli Landeros-Weisenberger
Angeli Landeros-Weisenberger James F. Leckman
James F. Leckman