- 1Department of Clinical Neuroscience, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- 2Montreal Neurological Institute, McGill University, Montreal, QC, Canada
- 3Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada
- 4Department of Human Genetics, McGill University, Montreal, QC, Canada
Neuropsychiatric symptoms (NPS) are common in Parkinson's disease (PD) and have demonstrated an association with the p. Val66Met, a polymorphism in the BDNF gene. Mild behavioral impairment (MBI) is a validated syndrome describing emergent and persistent NPS in older adults as a marker of potential cognitive decline and dementia. This study investigated if PD patients with the Met allele were more likely to have MBI and whether they had impairments in specific domains of MBI using the Mild Behavioral Impairment Checklist (MBI-C) as the MBI ascertainment tool. One hundred forty-six PD patients were screened for neuropsychiatric and cognitive impairments with the MBI-C and the Montreal Cognitive Assessment (MoCA). All participants were genotyped for the BDNF p.Val66Met single-nucleotide polymorphism (SNP) using TaqMan Genotyping Assay. Statistical analysis was performed using multiple linear and logistic regression models. Met carriers had a 2 times higher likelihood of being MBI positive (MBI-C total score ≥8) than Val carriers. Met carriers had significantly higher MBI-C total scores and significantly greater impairments in the mood/anxiety and the psychotic domains of MBI-C compared to Val carriers. These findings indicate that the BDNF Met allele is associated with a higher neuropsychiatric burden in PD.
Introduction
Neuropsychiatric symptoms (NPS) are common in Parkinson's disease (PD) patients. These NPS include depression, anxiety, psychosis, etc., which occur more frequently in PD patients than in the general population (1, 2). NPS can be present at early stages of PD and even precede the emergence of cardinal motor symptoms of PD (1). They have a severe social and emotional impact on the quality of life in PD patients and their families/caregivers (3). Mild behavioral impairment (MBI) is a validated syndrome characterized by the emergence of persistent NPS in older adults as an at-risk state for incident cognitive decline, and for some, MBI is the index manifestation of dementia, emerging in advance of cognitive symptoms (4). Early evidence in PD has linked MBI to altered corticostriatal connectivity, middle temporal lobe atrophy, and cognitive impairment which suggest a higher risk of developing dementia (5, 6).
Brain-derived neurotrophic factor (BDNF) is a crucial protein in the central nervous system (CNS) with a substantial role in differentiation, survival, and protection of CNS neurons (7). Studies have investigated a potential role for p.Val66Met (G758A, rs6265), a single-nucleotide polymorphism (SNP) in exon 11 of the BDNF gene, and PD (8–10). The p.Val66Met SNP substitutes a valine (Val) residue at position 66 with a methionine (Met) residue in the pro-domain of the BDNF protein (7).The Met allele has been found to be associated with cognitive impairments in PD patients and late-life psychiatric symptoms in the general population (8, 9, 11). This substitution is not transferred to the final form of BDNF; however, this structural change in the BDNF protein precursor can significantly decrease the secretion of BDNF extracellularly and subsequently reduce its availability to the CNS neurons (7). Recent evidence suggests a role of the Met allele in the induction of long-term depression (LTD) in the brain, likely via altered interaction of BDNF pro-domain with sortilin receptors (12, 13). The altered interaction might explain the connection of this polymorphism with NPS in the general population and in neurodegenerative diseases (14, 15). Recent longitudinal data in Alzheimer disease (AD) patients revealed strong evidence of Met association with depression (15). Moreover, recent meta-analysis reported higher likelihood of mild cognitive impairment in PD patients with the Met allele (9). These evidences suggest a link between the p.Val66Met polymorphism and NPS in PD patients and therefore encourage an investigation of this relationship.
In this study, we tested whether the p.Val66Met SNP in PD patients is associated with MBI burden using the Mild Behavioral Impairment Checklist (MBI-C). Specifically, we hypothesized that PD patients with at least one Met allele (Met carriers) would have greater likelihood of having MBI and higher total MBI-C score than those who are Val homozygotes (Val group). Additionally, we hypothesized that Met carriers would have higher MBI-C domain scores compared to Val group.
Methods
Participants
One hundred forty-six PD patients at Hoehn and Yahr stages II–III were recruited. Patients had a confirmed diagnosis of idiopathic PD by a Movement Disorder Clinic neurologist, meeting the UK Brain Bank criteria for idiopathic PD. All patients were on prescribed dopaminergic medication and were responsive to it. Exclusion criteria were the following: (1) any neurological disorder other than PD; (2) alcohol dependency; (3) history or presence of a severe psychiatric disorder; and (4) cerebrovascular disorders. The severity of motor symptoms was assessed using the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS-III). All participants provided written informed consent according to the declaration of Helsinki, and the study was approved by the Conjoint Health Research Ethics Board (REB14-2463) at the University of Calgary.
Genotyping
A blood sample was collected from each participant, and DNA was extracted using the MagMax DNA Multi-Sample Ultra 2.0 kit and the King Fisher Duo Prime Robot (Thermo Fisher Scientific). DNA samples were screened for the BDNF p.Val66Met SNP (rs6265) using TaqMan SNP Genotyping Assay C-11592758-10 on C-1000 Touch Thermal cycler (Bio-Rad). TaqMan assay reading was done on Applied Biosystems QuantStudio 7 Flex Real-Time PCR system (Fisher Scientific) according to the manufacturer's instructions. The TaqMan assay results were analyzed using the Bio-Rad CFX Maestro software.
Neuropsychiatric and Cognitive Assessment
NPS in all participants was evaluated using the MBI-C (16, 17). The MBI-C contains 34 questions to cover the five domains of MBI including the following: (1) impaired drive/motivation (apathy); (2) emotional dysregulation (mood and anxiety symptoms); (3) impulse dyscontrol (agitation, aggression, abnormal reinforcement, and reward salience); (4) social inappropriateness (impaired social cognition); and (5) abnormal thoughts/perception (psychotic symptoms). This checklist is completed by each patient's caregiver/close family member. Consistent with the MBI criteria, symptoms should have lasted for at least 6 months and present a meaningful change of behavior from longstanding patterns. An MBI-C total score cut point of ≥8 was used to classify a patient as MBI case positive (5, 18, 19). All participants completed the Montreal Cognitive Assessment (MoCA) for a brief cognitive assessment and completed a questionnaire on their demographics and daily activity level.
Statistical Analysis
Statistical analyses of continuous variables were performed using either the student T test or Mann-Whitney (M-W) U test based on the data normality. The Fisher exact and chi-square tests were used to test the categorical variables. Logistic regression was used to test the relationship between the MBI positive condition (the categorical dependent variable) and the two BDNF genotype groups, including any independent variables that were significantly different between the two conditions.
MBI-C total score (the continuous dependent variable) was compared between the two groups using a multiple linear regression model after checking for the multiple linear regression model assumptions. The independent variables that were correlated with MBI-C total score were included in the regression model. Values of p < 0.05 were considered significant for single tests, and significance of 0.01 was used to test MBI-C domains using Bonferroni correction. The same analysis was used to study association of p.Val66Met and MBI-C domain scores. All statistical tests were performed using IBM SPSS Statistics for Mac v. 26 (IBM Corp., Armonk, N.Y., USA). Power analysis was performed using G-Power software 3.1.9.6 (20).
Results
Demographics of Participants
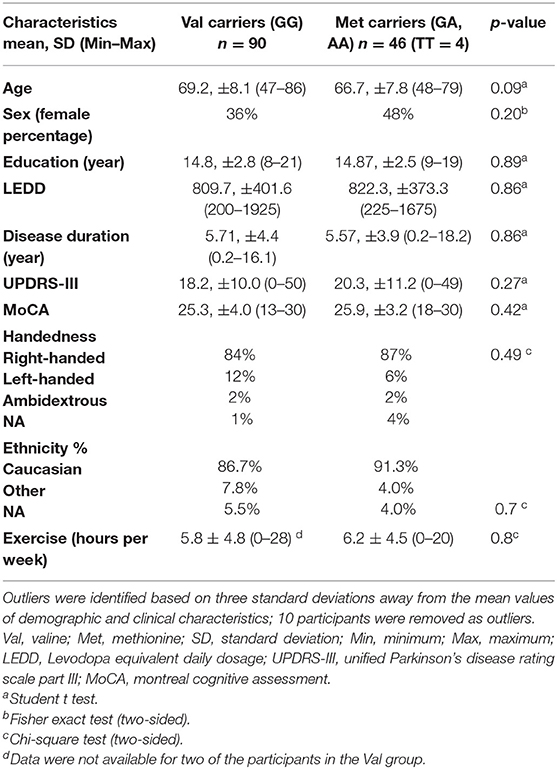
The demographic and clinical characteristics of the participants are summarized in Table 1. Ten patients were identified as outliers based on values that were more than three standard deviation away from the mean of each allelic group for the following variables: age, education, UPDRS, Levodopa equivalent daily dosage (LEDD), disease duration, MoCA, and MBI-C total score.
Among the 136 remaining PD participants, Val homozygous patients (GG) represented the majority of the cohort (n = 90). Because of the low number of homozygous Met/Met patients (n = 4), all Met carriers were pooled as one group. Forty-six patients were heterozygous or homozygous for the Met allele (GA, AA) with a frequency of 0.18, which was in accordance with Hardy-Weinberg equilibrium. The two groups had no significant differences in any of their demographic or clinical characteristics, ethnicity, and weekly exercise level (Table 1).
Association of Val66Met and MBI-C Score
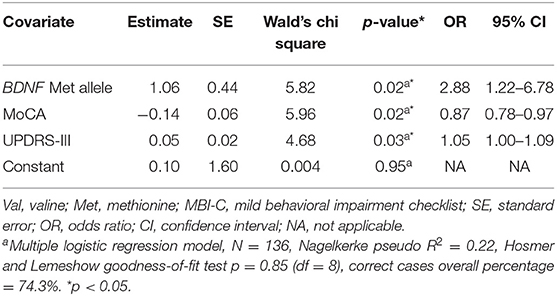
Met carriers were twice as likely to be MBI positive than the Val carriers; 39% of Met carriers were MBI positive, whereas in the Val group only 20% of patients were MBI positive. The Met group had a significantly greater mean value for MBI-C total score than Val carriers (7.39 vs. 4.06, respectively). Two factors were included in the multiple logistic regression as independent variables in addition to the BDNF groups based on significant differences between the two groups in the Mann-Whitney U test; MoCA and UPDRS-III (M-W U = 1230.5, p = 0.005, and M-W U = 2441.5, p = 0.002, respectively). The logistic regression analysis revealed a significant contribution of the Met allele for the likelihood of being MBI positive (OR = 2.88, CI 95% = 1.22–6.78, p = 0.02) (Table 2).
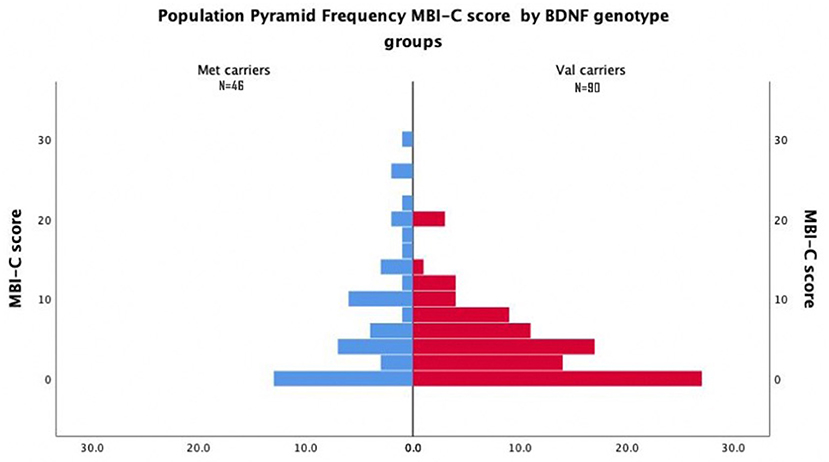
Most Val Carriers had Either Zero or Very Low MBI-C Total Score (Figure 1).
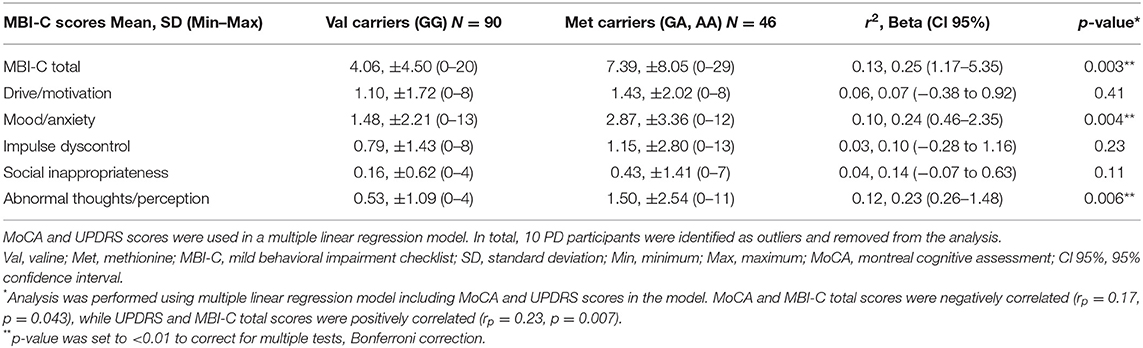
MBI-C total and MoCA scores were negatively correlated [Pearson's r = (134) 0.17, p = 0.04]. Also, UPDRS-III and MBI-C total scores had a positive correlation [Pearson's r = (134) 0.23, p = 0.007]. These factors were included in the multiple linear regression model. The difference between the MBI-C total score between the two allelic groups was statistically significant when controlling for MoCA and UPDRS-III scores in the regression model [r2 = 0.13, Beta = 0.25, F (3, 135) = 6.36, p = 0.013, Cohen's f 2 = 0.15]. A power analysis was conducted, which revealed that our samples size of 136 would yield a power of 0.99 assuming type-I error rate of 0.05.
Association results of BDNF alleles with MBI-C domain scores are shown in Table 3. Patients with the Met allele had significantly higher MBI-C scores for the mood/anxiety (r2 = 0.10, Beta = 0.24, p = 0.004) and the psychosis domains (r2 = 0.12, Beta = 0.23, p = 0.006) when controlling for MoCA and UPDRS-III scores (Table 3).
We performed an extra analysis in order to confirm that MBI classification results were derived from BDNF alleles and not driven by a few participants with marginally higher or lower MBI-C total score than the cutoff value (≥8). All participants with an MBI-C total score of 7 and 8 were excluded from the sample, and analysis was repeated. In total, 10 patients were removed; only one Met-carrier had the score of 7. In the Val group, three patients had the score of 7, and six patients had the score of 8 for MBI-C. Similar to the main analysis, two factors were included in the multiple logistic regression as independent variables in addition to the BDNF groups, based on their significant differences between the two groups in the Mann-Whitney U test; MoCA and UPDRS-III (M-W U = 1024.5, p = 0.02, and M-W U = 1914.5, p = 0.007, respectively). Results revealed a significant contribution of the Met allele for the likelihood of being MBI positive (OR = 4.38, CI 95% = 1.72–11.14, p = 0.002) (Table 4).
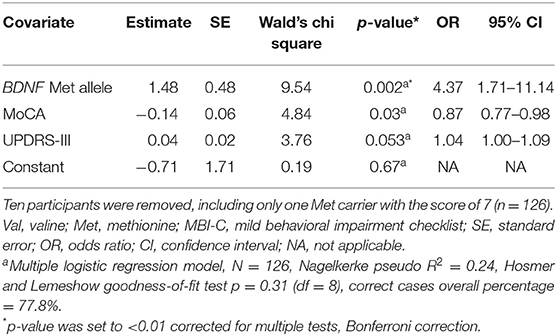
Table 4. Multiple logistic regression analysis of BDNF p.Val66Met and MBI positive likelihood after removing participants with total MBI-C score of 7 and 8.
Each MBI-C domain score was compared between the two groups using Mann-Whitney U test (Table 5). The results were similar to the whole cohort analysis. Met carriers had significantly higher MBI-C total score than the Val group. Also, Met carriers had significantly higher score for the mood/anxiety domain when compared to the Val group and a trend for higher psychosis score (Table 5).

Table 5. MBI-C total and domain scores after removing participants with total MBI-C score of 7 and 8.
Discussion
To our knowledge, the present study is the first to explore the association of the BDNF p.Val66Met SNP and MBI in patients with PD. Patients with at least one Met allele had significantly higher MBI-C total score and significantly higher scores in the emotional dysregulation and the abnormal thoughts/perception domains. Furthermore, PD patients with at least one Met allele had a significantly higher prevalence of MBI than patients in the Val group using MBI-C as the case ascertainment instrument. Our findings implicate the BDNF p.Val66Met SNP in the pathogenesis of MBI in PD patients and suggest this variant as a genetic risk factor for MBI in PD with a medium effect size (Cohen's f 2 = 0.15). These findings are consistent with the evidence in the AD, which imply that the Met allele can be a risk factor for incident cognitive decline and dementia in PD (4, 15, 21).
An increasing body of evidence suggests a link between the development of NPS and cognitive decline in different types of dementia (5, 22–24). Studies demonstrating that BDNF p.Val66Met SNP is found to be associated with both NPS and cognitive impairments in AD are consistent with a biological understanding of NPS (25–29), and previous evidence linking BDNF and NPS (8, 15). The presence of amyloid-β pathology in PD patients together with Lewy body pathology might be a possible explanation of similar NPS profile in AD and PD patients. However, it should be mentioned that different studies have teased apart how the psychiatric profile of PD and AD patients are different (30, 31).
Patients who experience NPS in the early stages of PD show an increased risk of cognitive decline (1, 5), which is consistent with the findings in non-PD dementia (24, 32–36). A recent study reported that PD patients with a variety of NPS, for example, depression, apathy, and hallucinations, displayed impairments in at least one of the main cognitive domains (executive function, language, memory, attention, and visuospatial) and in global cognition (5). These findings hint at the importance of early diagnosis of sustained NPS as markers of cognitive decline in PD patients, in order to identify patients at risk of incident cognitive decline and dementia.
A recent meta-analysis reported an association between the BDNF Met allele and cognitive impairments in PD for 532 patients and 802 controls (p = 0.003). However, the cognitive impairments were found to be more specific to the Caucasian populations (9). Several studies suggested that p.Val66Met SNP might have an association with depression, particularly geriatric depression (14, 37, 38). Nonetheless, this association might differ based on a variety of factors, e.g., the origin of the study population, sex, and the fundamental issue of whether depression is chronic, recurrent, or of later life onset as most depression rating scales do not differentiate (8, 33). The association of BDNF Met allele and geriatric depression was investigated in a meta-analysis including 523 cases and 1,220 controls (age ≥ 60 years) (14). An association between the Met allele and an increased risk for late-life depression was reported (p = 0.004) (8). However, one study reported that the Val allele is associated with anxiety and depression using the Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI) in 104 PD patients. Nevertheless, their population structure was greatly different than the one in our study. Seventeen percent of their participants had early onset PD with a positive family history (39), while in our sample there were only two participants (1.4%) with early onset PD and all of the participants had a confirmed diagnosis of idiopathic PD. Other reasons explaining the different results between the Cagni et al., (39) study and ours are linked to measurement differences of NPS. The BDI and BAI are self-report measures assessing the presence of mood and anxiety symptoms over the last 2 and 4 weeks, respectively. In contrast, the MBI-C measures later life emergent and persistent (for at least 6 months) NPS, identified by a reliable informant. These are quite different approaches to measurement of symptoms, with the MBI-C developed explicitly to capture later life emergent symptoms that either serve as risk factors for cognitive decline and dementia or more likely represent early manifestations of dementia.
Furthermore, BDNF involvement in depression/anxiety disorders has been confirmed through measuring peripheral BDNF levels as well (40, 41). A meta-analysis reported a strong evidence of an association between depression and a decrease in BDNF levels (p < 6.8 × 10−8) (40). These meta-analyses highlight the crucial role of BDNF in depressive disorders and specifically the impact of p.Val66Met SNP on geriatric depression. The Met allele exhibits LTD properties, reduces the neural plasticity, and can also substantially affect the docking of BDNF secretory vesicles into the cellular membrane and decrease its release into the synaptic cleft (7). The BDNF pro-domain with a Met residue is shown to have an independent function by induction of LTD, reducing spine density and neuronal plasticity. These molecular changes are linked to depression and anxiety disorders in both animal models and clinical studies (7, 42, 43). These findings are in agreement with our results that PD patients with at least one Met allele are more susceptible to impairments in the affective/mood dysregulation and abnormal thoughts/perception domains of MBI-C.
We found a strong association between abnormal thoughts/perception in PD patients and Met allele in our cohort. Abnormal thoughts/perception represent psychotic symptoms, specifically hallucinations and delusions, which are associated with impairments in global cognition (1, 44). An abrupt visual memory function is suggested as a potential cause of visual hallucinations. Since BDNF plays a prominent role in the molecular mechanisms of memory in hippocampus, this indicates a possible role for BDNF in the development of such NPS (1, 7). Meta-analytical results of p.Val66Met SNP and psychotic disorders, for example, schizophrenia, are inconclusive at the moment (8, 45). However, the Met allele was found to be linked to higher susceptibility to hippocampal volume loss and deteriorated memory abilities in bipolar patients (46). These findings are in agreement with our results that PD patients with at least one Met allele are more susceptible to symptoms in the affective/mood dysregulation and abnormal thoughts/perception domains.
This study has some limitations that need to be considered. Although the findings of this study showed a fair level of robustness, the sample size is relatively small. The results of this study need to be replicated in a larger sample with an age-matched control group. Nevertheless, the post hoc power analysis indicates sufficient power in our cohort to detect the true effect of BDNF p.Val66Met. A full cognitive assessment of participants would benefit the exploration of p.Val66Met SNP impact on the aging brain, specifically in PD. Antidepressant medications was not considered. It has been shown that antidepressant medications can elevate peripheral BDNF and improve reversible NPS (40).
In conclusion, we observed an association between the BDNF p.Val66Met SNP and susceptibility for the development of late-life behavioral changes in PD patients. PD patients with at least one Met allele had a higher likelihood of MBI compared to non-carriers. Moreover, PD patients with one Met allele had a greater tendency to exhibit mood and anxiety symptoms as well as psychotic symptoms compared to the Val carriers. These findings indicate a potential role for the BDNF p.Val66Met SNP in late-life psychiatric impairment, subsequent cognitive decline, and dementia in PD patients.
Data Availability Statement
The data that support the finding of this article are available from the corresponding author, upon request. Requests to access the datasets should be directed to Oury Monchi, TW9uY2hpb3VyeS5tb25jaGlAdWNhbGdhcnkuY2E=.
Ethics Statement
The studies involving human participants were reviewed and approved by Conjoint Health Research Ethics Board (REB14-2463) at the University of Calgary. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MR: study design, data analysis, interpretation, manuscript writing, and revision. JR, KM, MK, ZJ, IK, TH, JC, EL, DM, JS, ZG-O, and GP: data collection. ZI: study design, analysis, and manuscript revision. OM: study design, data analysis, supervision, and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a project grant from the Canadian Institutes of Health Research (CIHR) (PJT-166123), the Tourmaline Oil Chair in Parkinson's Disease, and the Canada Research Chair in non-motor symptoms of Parkinson's disease to OM. MR received a scholarship from the Parkinson Association of Alberta.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all the PD patients and their families who were willing to participate and provide information for this study.
References
1. Gallagher DA, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis. (2012) 46:581–9. doi: 10.1016/j.nbd.2011.12.041
2. Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord. (2015) 30:919–27. doi: 10.1002/mds.26170
3. Martinez-Martin P, Rodriguez-Blazqueza C, JoãoForjaz M, Frades-Payo B, Agüera-Ortizc L, Weintraub D, et al. Neuropsychiatric symptoms and caregiver's burden in Parkinson's disease. Parkinsonims Relat Disord. (2015) 21:629–34. doi: 10.1016/j.parkreldis.2015.03.024
4. Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. (2016) 12:195–202. doi: 10.1016/j.jalz.2015.05.017
5. Yoon E, Ismail Z, Hanganu A, Kibreab M, Hammer T, Cheetham J, et al. Mild behavioral impairment is linked to worse cognition and brain atrophy in Parkinson's disease. Neurology. (2019) 93:e766–77. doi: 10.1212/WNL.0000000000007968
6. Lang S, Yoon EJ, Kibreab M, Kathol I, Cheetham J, Hammer T, et al. Mild behavioral impairment in Parkinson's disease is associated with altered corticostriatal connectivity. NeuroImage Clin. (2020) 26:102252. doi: 10.1016/j.nicl.2020.102252
7. Hempstead BL. Brain-derived neurotrophic factro: three ligands, many actions. Trans Am Clin Climatol Assoc. (2015) 126:9–19.
8. Tsai SJ. Critical issues in BDNF Val66Met genetic studies of neuropsychiatric disorders. Front Mol Neurosci. (2018) 11:156. doi: 10.3389/fnmol.2018.00156
9. Wang Q, Liu J, Guo Y, Dong G, Zou W, Chen Z. Association between BDNF G196A (Val66Met) polymorphism and cognitive impairment in patients with Parkinson's disease: a meta-analysis. Braz J Med Biol Res. (2019) 52:e8443. doi: 10.1590/1414-431x20198443
10. Mogi M, Togaria A, Kondo T, Mizuno Y, Komure O, Kuno S, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson's disease. Neurosci Lett. (1999) 270:45–8.doi: 10.1016/S0304-3940(99)00463-2
11. Altmann V, Schumacher-Schuh AF, Rieck M, Callegari-Jacques SM, Rieder CR, Hutz MH. Val66Met BDNF polymorphism is associated with Parkinson's disease cognitive impairment. Neurosci Lett. (2016) 615:88–91. doi: 10.1016/j.neulet.2016.01.030
12. Kowianski P, Lietzau G, Czuba E, Waskow M, Steliga A, Morys J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. (2018) 38:579–93. doi: 10.1007/s10571-017-0510-4
13. Hempstead BL. Brain-derived neurotrophic factro: three ligands, many actions. Transactions of the American Clinical and Climatological Association. (2015) 126:9–19.
14. Pei Y, Smith AK, Wang Y, Pan Y, Yang J, Chen Q, et al. The brain-derived neurotrophic-factor (BDNF) val66met polymorphism is associated with geriatric depression: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. (2012) 159B:560–6. doi: 10.1002/ajmg.b.32062
15. Bessi V, Mazzeo S, Bagnoli S, Padiglioni S, Carraro M, Piaceri I, et al. The implication of BDNF Val66Met polymorphism in progression from subjective cognitive decline to mild cognitive impairment and Alzheimer's disease: a 9-year follow-up study. Eur Arch Psychiatry Clin Neurosci. (2019) 270:471–482. doi: 10.1007/s00406-019-01069-y
16. Ismail Z, Aguera-Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, et al. The mild behavioral impairment checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. (2017) 56:929–38. doi: 10.3233/JAD-160979
17. Creese B, Griffiths A, Brooker H, Corbett A, Aarsland D, Ballard C, et al. Profile of mild behavioral impairment and factor structure of the mild behavioral impairment checklist in cognitively normal older adults. Int Psychogeriatr. (2020) 32:705–17. doi: 10.1017/S1041610219001200
18. Mallo SC, Ismail Z, Pereiro AX, Facal D, Lojo-Seoane C, al. e. Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int Psychogeriatr. (2019) 31:231–9. doi: 10.1017/S1041610218000698
19. Mallo SC, Ismail Z, Pereiro AX, Facal D, Lojo-Seoane C, Campos-Magdaleno M, et al. Assessing mild behavioral impairment in people with subjective cognitive complaints (SCCS) with the mild behavioral impairment checklist (MBI-C): a pilot study. J Alzheimer Assoc. (2017) 13:364–5. doi: 10.1016/j.jalz.2017.06.312
20. Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res. (2007) 39:175–91. doi: 10.3758/BF03193146
21. VanDam D, Vermeiren Y, Dekker AD, Naudé PJW, DeDeyn PP. Neuropsychiatric disturbances in alzheimer's disease-what have we learned from neuropathological studies? Curr Alzheimer Res. (2016) 13:1145–64. doi: 10.2174/1567205013666160502123607
22. Chiu PY, Tsai CT, Chen PK, Chen WJ, Lai TJ. Neuropsychiatric symptoms in parkinson's disease dementia are more similar to alzheimer's disease than dementia with lewy bodies: a case-control study. PLoS ONE. (2016) 11:e0153989. doi: 10.1371/journal.pone.0153989
23. López-Pousa S, Vilalta-Franch J, Garre-Olmo J, Pons S, Cucurella MG. Characterisation and prevalence of the psychological and behavioural symptoms in patients with dementia. Europe PMC. (2007) 45:683–8. doi: 10.33588/rn.4511.2007535
24. Taragano FE, Allegri RF, Heisecke SL, Martelli MI, Feldman ML, Sánchez V, et al. Risk of conversion to dementia in a mild behavioral impairment group compared to a psychiatric group and to a mild cognitive impairment group. J Alzheimer's Dis. (2018) 62:227–38. doi: 10.3233/JAD-170632
25. Naude J, Gill S, Hu S, McGirr A, Forkert N, Monchi O, et al. Plasma neurofilament light: a marker of cognitive decline in mild behavioural impairment. J Alzheimes Dis. (2020) 76:1017–27. doi: 10.3233/JAD-200011
26. Andrews SJ, Ismail Z, Anstey KJ, Mortby ME. Association of Alzheimer's genetic loci with mild behavioral impairment. Am J Med Genet B Neuropsychiatr Genet. (2018) 177:727–35. doi: 10.1002/ajmg.b.32684
27. Gill S, Mouches P, Hu S, Rajashekar D, MacMaster FP, Smith EE, et al. Using machine learning to predict dementia from neuropsychiatric symptom and neuroimaging data. J Alzheimes Dis. (2020) 75:277–88. doi: 10.3233/JAD-191169
28. Creese B, Brooker H, Aarsland D, Corbett A, Ballard C, Ismail Z. Genetic risk for Alzheimer disease, cognition and Mild Behavioral Impairment in healthy older adults. medRxiv. [Preprint]. doi: 10.1101/2020.05.13.20100800
29. Lussier FZ, Pascoal TA, Chamoun M, Therriault J, Tissot C, Savard M, et al. Mild behavioral impairment is associated with β-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. (2020) 16:192–9. doi: 10.1002/alz.12007
30. Aarsland D, Cummings JL, Larsen JP. Neuropsychiatric difference between Parkinson's disease with dementia and Alzheimer's disease. Int J Geriatr Psychiatry. (2001) 16:184–91. doi: 10.1002/1099-1166(200102)16:2<184::aid-gps304>3.0.co;2-k
31. Noe E, Marder K, Bell KL, D. M. J, Manly JJ, Yaakov Stern Y. Comparison of dementia with lewy bodies to alzheimer's disease and parkinson's disease with dementia. Mov Disord. (2004) 19:60–7. doi: 10.1002/mds.10633
32. Matsuoka T, Ismail Z, Narumoto J. Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic. journal of alzheimer's disease. (2019) 70:505–13. doi: 10.3233/JAD-190278
33. Ismail Z, Gatchel J, Bateman DR, Barcelos-Ferreira R, Chantillon M, Jaeger J, et al. Affective and emotional dysregulation as pre-dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int Psychogeriatr. (2018) 30:185–96. doi: 10.1017/S1041610217001880
34. Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild behavioral impairment and subjective cognitive decline predict mild cognitive impairment. medRxiv. [Preprint]. (2020). doi: 10.1101/2020.05.24.20112284
35. Creese B, Brooker H, Ismail Z, Wesnes KA, Hampshire A, Khan Z, et al. Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. (2019) 27:823–34. doi: 10.1016/j.jagp.2019.01.215
36. Mortby ME, Black SE, Gauthier S, Miller DS, Porsteinsson A, Smith EE, et al. Dementia clinical trial implications of mild behavioral impairment. Int Psychogeriatr. (2018) 30:171–5. doi: 10.1017/S1041610218000042
37. Taylor WD, Züchner S, McQuoid DR, Steffens DC, Speer MC, Krishnan KRR. Allelic differences in the brain-derived neurotrophic factor Val66Met polymorphism in late-life depression. Am J Geriatr Psychiatry. (2007) 15:850–7. doi: 10.1097/JGP.0b013e318050c9d5
38. Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. (2006) 27):1834–7. doi: 10.1016/j.neurobiolaging.2005.10.013
39. Cagni FC, Campelo C, Coimbra DG, Barbosa MR, Junior LGO, Neto ABS, et al. Association of BDNF Val66MET polymorphism with Parkinson's disease and depression and anxiety symptoms. J Neuropsychiatry Clin Neurosci. (2017) 29:142–7. doi: 10.1176/appi.neuropsych.16040062
40. Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. (2008) 64:527–32. doi: 10.1016/j.biopsych.2008.05.005
41. Suliman S, Hemmings, S. M., Seedat, S. Brain-derived neurotrophic factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. (2013) 7:55. doi: 10.3389/fnint.2013.00055
42. De Vincenti AP, Rios AS, Paratcha G, Ledda F. Mechanisms That modulate and diversify BDNF functions: implications for hippocampal synaptic plasticity. Front Cell Neurosci. (2019) 13:135. doi: 10.3389/fncel.2019.00135
43. Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. (2006) 96:677–85. doi: 10.1152/jn.00336.2006
44. Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson's disease. Mov Disord. (2009) 24:2175–86. doi: 10.1002/mds.22589
45. Goren JL. Brain-derived neurotrophic factor and schizophrenia. Ment Health Clin. (2016) 6:285–8. doi: 10.9740/mhc.2016.11.285
Keywords: BDNF, Parkinson's disease, mild behavioral impairment, neuropsychiatric symptoms, depression
Citation: Ramezani M, Ruskey JA, Martens K, Kibreab M, Javer Z, Kathol I, Hammer T, Cheetham J, Leveille E, Martino D, Sarna JR, Gan-Or Z, Pfeffer G, Ismail Z and Monchi O (2021) Association Between BDNF Val66Met Polymorphism and Mild Behavioral Impairment in Patients With Parkinson's Disease. Front. Neurol. 11:587992. doi: 10.3389/fneur.2020.587992
Received: 27 July 2020; Accepted: 02 December 2020;
Published: 14 January 2021.
Edited by:
Mayela Rodríguez-Violante, Manuel Velasco Suárez Instituto Nacional de Neurología y Neurocirugía, MexicoReviewed by:
Vikram Venkappayya Holla, National Institute of Mental Health and Neurosciences, IndiaJames C. Vickers, University of Tasmania, Australia
Copyright © 2021 Ramezani, Ruskey, Martens, Kibreab, Javer, Kathol, Hammer, Cheetham, Leveille, Martino, Sarna, Gan-Or, Pfeffer, Ismail and Monchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oury Monchi, b3VyeS5tb25jaGlAdWNhbGdhcnkuY2E=
 Mehrafarin Ramezani
Mehrafarin Ramezani Jennifer A. Ruskey2,3
Jennifer A. Ruskey2,3 Kristina Martens
Kristina Martens Etienne Leveille
Etienne Leveille Davide Martino
Davide Martino Ziv Gan-Or
Ziv Gan-Or Gerald Pfeffer
Gerald Pfeffer Oury Monchi
Oury Monchi