
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 October 2020
Sec. Stroke
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.583411
This article is part of the Research Topic Intracranial Bleeding after Reperfusion Therapy in Acute Ischemic Stroke View all 22 articles
Background: Contrast media extravasation can mimic hemorrhage after endovascular thrombectomy (EVT). Dual energy CT (DECT) has the potential to distinguish hemorrhage from iodine contrast.
Methods: We retrospectively examined clinical and radiological data from 106 consecutive acute ischemic stroke patients who received EVT and underwent DECT immediately and 24 h after EVT. Iodine overlay map, virtual non-contrast, and mixed images are reconstructed.
Results: With the use of DECT, the proportion of all patients diagnosed with hemorrhagic transformation on mixed images immediately after EVT was reduced from 74.5% (79 of 106) to 10.4% (11 of 106), with a very poor consistency (κ = 0.076, p = 0.041). Correspondingly, hemorrhagic transformation on mixed images 24 h after EVT was reduced from 41.5% (44 of 106) to 30.2% (32 of 106), with a moderate consistency (κ = 0.757, p < 0.001).
Conclusions: The use of DECT both immediately and 24 h after EVT changes the diagnosis of hemorrhagic transformation in a considerable proportion of acute ischemic stroke patients with EVT. This could affect decision making with respect to antithrombotic strategy.
Intravenous thrombolysis (IVT) is an effective and broadly applicable treatment for acute ischemic stroke (1). One of the most important complications of IVT is intracranial hemorrhagic transformation, which is associated with poor outcome and even death (2). Recently, the benefit of endovascular thrombectomy (EVT) has also been established in acute ischemic stroke patients with large artery occlusion (3). Both IV iodine contrast during advanced imaging and intra-arterial injection of iodine contrast during the EVT procedure can lead to contrast staining due to blood-brain barrier breakdown; as a consequence, hyperdense areas were frequently detected on non-contrast CT immediately after EVT (4). Early differentiation between hemorrhage and contrast staining is important for clinical decision making, such as the use of glycoprotein IIb/IIIa inhibitor (tirofiban) in some patients with high risk of early reocclusion after EVT (5), or initiation of secondary preventive treatment with antiplatelet or anticoagulant agents after 24 h (6).
The use of dual energy CT (DECT) might change the radiologic report regarding post-treatment hemorrhagic transformation in a considerable proportion of patients with EVT compared to conventional non-contrast CT (7). In DECT, iodine overlay map (IOM) and virtual non-contrast (VNC) images are reconstructed from two different X-ray spectra at different kilovoltage (kV) either from one X-ray source using kV switching or from two X-ray sources (8, 9). The differentiation between hemorrhage and contrast medium became feasible since the attenuation characteristics of iodine and blood are different at two energy levels.
However, there are few studies trying to verify the diagnostic confidence in differentiation between hemorrhage and contrast medium extravasation after EVT, and to evaluate the clinical value of DECT at different stages. Therefore, in the current study, we aimed to (1) investigate how DECT immediately after EVT changes the diagnosis of hemorrhagic transformation and compare its radiologic report with follow-up CT of 24 h and (2) investigate how DECT 24 h after EVT changes the diagnosis of hemorrhagic transformation and compare its radiologic report with a follow-up CT of 3 days.
We retrospectively reviewed our prospectively collected database for consecutive patients with acute ischemic stroke received EVT between January 2016 and October 2018. We then enrolled patients who (i) underwent DECT immediately after EVT; (ii) underwent DECT 24 h after EVT; (iii) underwent conventional non-contrast CT 3 days after EVT. We excluded patients who had recent previous ischemic stroke within 3 months to avoid any potential intracranial hemorrhage and contrast staining findings related to subacute blood-brain barrier breakdown.
DECT was performed immediately and 24 h after EVT, and conventional non-contrast CT was performed 3 days after EVT. DECT images were acquired with a dual source 128 slice CT scanner (SOMATOM Force, Siemens Healthcare, Forchheim, Germany). Acquisition and reconstruction of CT parameters were as follows: a dedicated dual-source protocol with simultaneous imaging at 80 kV/392 mAs eff. and 140 kV(Sn)/196 mAs eff., collimation of 0.6 mm and pitch of 0.7 was employed. The raw spiral projection data were rebuilt in three different series, with two sets corresponding to 80 and 140 kV (0.6 mm slice thickness) and a third set corresponding to a mixed map of both energies (80/140 kV), simulating a conventional 120 kV CT. VNC images and IOM were calculated using a dedicated brain hemorrhage algorithm (Syngo; CT Dual-Energy Brain Hemorrhage; Siemens) (Figure 1).
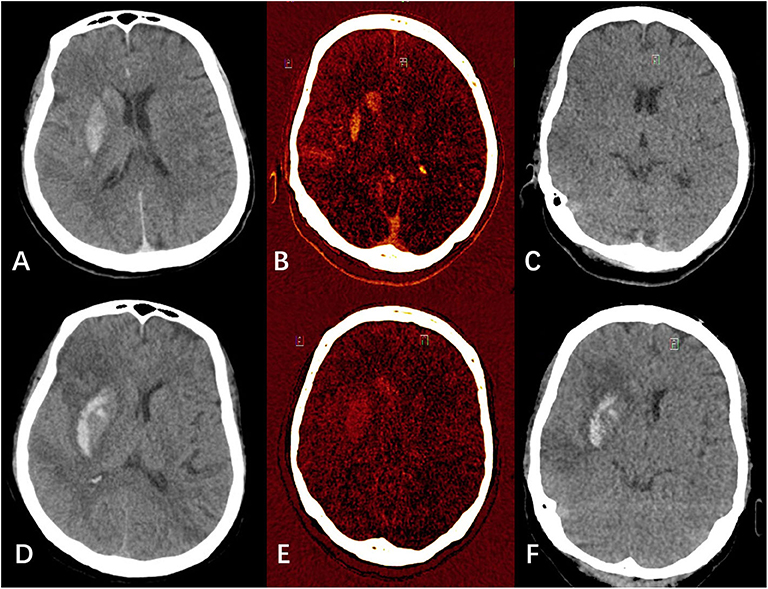
Figure 1. Examples of hemorrhagic transformation and contrast extravasation with iodine overlay map (IOM), virtual non-contrast (VNC), and mixed images. (A–C) were mixed image, IOM, and VNC, respectively from a patient's dual energy CT (DECT) immediately after endovascular thrombectomy (EVT). (A) showed hyperdensities in the right lentiform nucleus and caudate nucleus. In (B,C) combined, the hyperdensities were classified as pure iodine contrast. (D–F) were mixed image, IOM, and VNC, respectively from the same patient's DECT 24 h after EVT. (D) also showed hyperdensities in the right lentiform nucleus and caudate nucleus. In (E,F) combined, the hyperdensities were classified as hemorrhage with iodine contrast.
Hemorrhagic transformation was classified by using the following radiological criteria: hemorrhagic infarction (HI, including HI-1 and HI-2), parenchymal hemorrhage (PH, including PH-1 and PH-2) (10). DECT images were evaluated for the diagnosis and grading of hemorrhagic transformation. Definitions of contrast material extravasation and intracranial hemorrhage on DECT were previously described in detail elsewhere (11). Mixed images of DECT and conventional non-contrast CT images were evaluated for the presence of hyperdense areas. Hyperdensities were defined as areas with objective higher density than the surrounding brain parenchyma. Hyperdensities visible on mixed images of DECT were interpreted as hemorrhage, contrast extravasation, or both. The interpretations of DECT were compared with conventional non-contrast CT 3 days after EVT. Washout or near-complete clearing of the hyperdensities on follow-up CT was classified as contrast material extravasation, while persisting hyperdensities on follow-up CT were classified as hemorrhage.
Two neurologists with 10 years of experience with acute stroke imaging (KL & LJ) separately reviewed first the mixed images alone and, in a second reading, the IOM and VNC images. Disagreement was resolved by consensus, and in cases with remaining disagreement, the final decision was made by an interventional neurologist (CY).
The consensus judgment of DECT and conventional non-contrast CT was used as reference. Categorical variables were presented as number and percentage. Continuous variables were summarized as mean ± SD or median with interquartile range (IQR). Significance of difference between proportions was calculated with the Pearson χ2 or Fisher's exact test. All analyses were performed blind to participant identifying information.
Inter-reader agreement of any hemorrhagic transformation immediately after EVT had a κ value of 0.79 on mixed images, and a κ value of 0.76 on VNC images, respectively. Inter-reader agreement of any hemorrhagic transformation 24 h after EVT had a κ value of 0.81 on mixed images, and a κ value of 0.73 on VNC images, respectively. Inter-reader agreement of any hemorrhagic transformation 3 days after EVT had a κ value of 0.71 on conventional non-contrast CT.
A total of 106 remaining patients were included for the final analysis. Of the patients included, 39 (36.8%) were women, with a median age of 74 years (mean 71.6 ± 10.3 years, range 27–86 years). Mean time from onset to puncture was 291.5 (237.3–367.0) min, and mean time from puncture to reperfusion was 50.5 (40.0–82.3) min. The detailed clinical data and demographics are shown in Table 1.
Based on the IOM and VNC images of DECT immediately after EVT (Table 2), 11 patients (10.4%) were classified as hemorrhagic transformation, and all of them were mixed with iodine contrast. In all, 68 patients (64.2%) were classified as pure iodine contrast, while the remaining 27 patients (25.5%) showed no hyperdensities. Based on the IOM and VNC images of DECT 24 h after EVT (Table 2), 32 patients (30.2%) were classified as hemorrhagic transformation, and 17 of them (53.1%) were mixed with iodine contrast. Twelve patients (11.3%) were classified as pure iodine contrast, while the remaining 62 patients (58.5%) showed no hyperdensities.
The 11 patients diagnosed with hemorrhage immediately after EVT (all mixed with iodine) were still shown as hemorrhage on both mixed images 24 h after EVT and non-contrast CT 3 days after EVT, while 5 of them (45.5%) had clearance of iodine contrast based on the DECT 24 h after EVT (Figure 2). The 27 patients with no hyperdensities immediately after EVT remained clean on both mixed images 24 h after EVT and non-contrast CT 3 days after EVT. In 68 patients classified as pure iodine contrast immediately after EVT, 35 of them (51.5%) showed no hyperdensities on mixed images 24 h after EVT, while 12 of them (17.6%) had clearance of iodine contrast on non-contrast CT 3 days after EVT. And the remaining 21 patients (30.9%) developed hemorrhagic transformation on both VNC images 24 h after EVT and non-contrast CT 3 days after EVT.
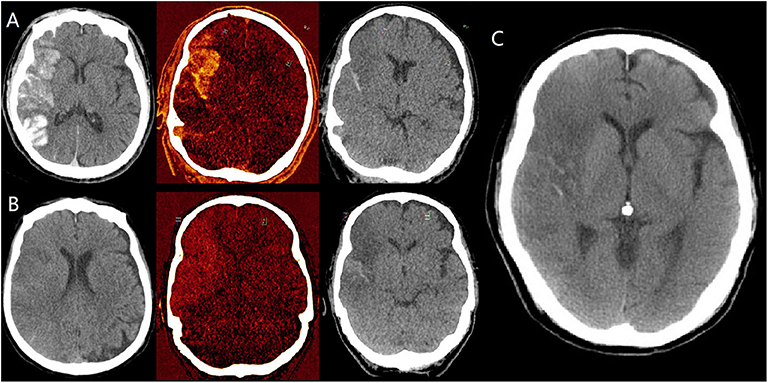
Figure 2. Dynamic changes of hyperdensities in a patient diagnosed with hemorrhagic transformation (mixed with iodine) on dual energy CT (DECT) immediately after EVT. (A) are mixed images, iodine overlay map (IOM), virtual non-contrast (VNC) images immediately after EVT, respectively, indicating the presence of both hemorrhage and iodine contrast. (B) are also mixed images, iodine overlay map (IOM), virtual non-contrast (VNC) images 24 h after EVT, respectively, indicating the persistent hemorrhage and clearance of iodine contrast. (C) is conventional non-contrast CT 3 days after EVT, indicating the partial absorption of hemorrhage.
With the use of DECT, the proportion of all patients diagnosed with hemorrhagic transformation on mixed images immediately after EVT was reduced from 74.5% (79 of 106) to 10.4% (11 of 106), with very poor consistency (κ = 0.076, p = 0.041) (Table 3). Correspondingly, the proportion of all patients diagnosed with hemorrhagic transformation on mixed images 24 h after EVT was reduced from 41.5% (44 of 106) to 30.2% (32 of 106), with moderate consistency (κ = 0.757, p < 0.001) (Table 3).
The classification of hemorrhagic transformation at different stages after thrombectomy was shown in Table 4. New hemorrhage mostly occurred within 24 h after EVT (from 10.4 to 30.2%). Three patients with no hyperdensities on 24 h DECT developed delayed hemorrhagic transformation (1 HI and 2 PH), while one patient with HI on 24 h DECT became PH on 3 day conventional non-contrast CT.
Hyperdense areas were frequently (74.5%) detected on CT immediately after EVT. With the use of DECT, the proportion of patients diagnosed with hemorrhagic transformation immediately after EVT was reduced to 10.4%. Although the phenomenon of contrast medium extravasation became less common at 24 h, the use of DECT still changed the diagnosis of hemorrhagic transformation in a considerable number of patients (11.3%). New hemorrhages mostly occurred within 24 h after EVT (from 10.4 to 30.2%). After excluding some delayed new and progressive hemorrhages, the hemorrhagic transformation classified on 24 h VNC was quite consistent with the 3 day conventional non-contrast CT.
Lummel et al. (12) reported the frequency of hyperdense lesions was 84.2% in patients after EVT, even higher than our current study. Our study showed that 27.2% (12 in 44) of hemorrhage findings in the routine 24 h follow-up group are caused by contrast staining mimicking blood. This proportion increases to 86.1% (68 in 79) in the group scanned immediately after EVT. The former finding is similar to the report from Almqvist et al.'s (7) study of DECT. The latter finding of pure contrast staining proportion is higher than in three previously published studies of a post-interventional DECT strategy within 30, 60, or 120 min (68, 47, and 32%, respectively) (8, 13, 14).
There are several techniques to distinguish contrast staining from hemorrhage. Commonly, the issue can be resolved with a repeated CT examination within 1–3 days (12, 15), which may postpone antithrombotic therapy or anticoagulation. Although iodine can affect several magnetic resonance sequences (16), hemosiderin-sensitive sequences can be used since it is unlikely that iodine could mimic hemorrhage on magnetic resonance imaging (17). By contrast, DECT is a simple and fast solution differentiating hemorrhage and contrast staining, avoiding the delayed time for a repeat examination and the limitations of magnetic resonance, such as contraindications and limited resource issues (7).
Early differentiation between hemorrhage and contrast medium extravasation immediately after EVT is important for clinical decision making, such as whether to start treatment with glycoprotein IIb/IIIa inhibitor (tirofiban) after EVT to prevent early reocclusion due to endothelial damage (5), or might be useful where repeat intervention is necessary. On the other hand, the AHA/ASA guidelines for the management of acute ischemic stroke patients recommended obtaining follow-up imaging 24 h after IVT before starting antiplatelets or anticoagulants (6). However, the high occurrence of hyperdensities on CT after EVT brought concerns to clinicians about the use of antithrombotic agents for secondary preventive treatment, since contrast staining could mimic hemorrhage. Based on the current study, the use of DECT might in our opinion provide this differentiation.
Limitations include a retrospective design in a single stroke center, though we prospectively collected data using a stroke registry, which might present a potential risk of selection bias. Some severe stroke patients might be transferred to an intensive care unit or receive surgical treatment the next day making them unable to undergo follow-up DECT within 24 h. The sample size is moderate; future large multicenter studies and individual patient data meta-analysis are needed to further investigate the importance of DECT in the patients with EVT. On the other hand, the best verification of our confidence in DECT's diagnostic differentiation between hemorrhage and contrast medium extravasation is by performing hemosiderin-sensitive magnetic resonance sequences at the same time, which is clinically difficult. Considering the clearance of iodine contrast, we used a conventional non-contrast CT 3 days after EVT for the verification, although a few patients developed new or progressive hemorrhagic transformation.
Standard non-contrast CT alone should be used with caution for the diagnosis and grading of hemorrhagic transformation after EVT, because contrast staining can mimic hemorrhage. We concluded that DECT with IOM and VNC has potential and is essential for early differentiation of hemorrhage and contrast material extravasation after EVT, offering additional information for clinical decision making regarding antithrombotic and anticoagulant therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by human ethics committee of Hangzhou First Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
KL: drafted/revised the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, and study supervision. LJ: drafted/revised the manuscript, study concept or design, analysis or interpretation of data, and acquisition of data. JR and WX: drafted/revised the manuscript, study concept or design, and analysis or interpretation of data. HH: acquisition of data and analysis or interpretation of data. GN: drafted/revised the manuscript. SY and CY: drafted/revised the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents, tools, patients, study supervision, and obtained funding. All authors contributed to the article and approved the submitted version.
This study was supported by grant from the Zhejiang provincial public welfare research project (LGF18H090017 and LGF20H090008) and the National Natural Science Foundation of China (81701150).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Berkowitz AL, Mittal MK, McLane HC, Shen GC, Muralidharan R, Lyons JL, et al. Worldwide reported use of IV tissue plasminogen activator for acute ischemic stroke. Int J Stroke. (2014) 9:349–55. doi: 10.1111/ijs.12205
2. Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
3. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
4. Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. (2004) 35:876–81. doi: 10.1161/01.STR.0000120726.69501.74
5. Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. (2013) 44:1453–5. doi: 10.1161/STROKEAHA.111.000502
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:46–110. doi: 10.1161/STR.0000000000000163
7. Almqvist H, Holmin S, Mazya MV. Dual energy CT after stroke thrombectomy alters assessment of hemorrhagic complications. Neurology. (2019) 93:1068–75. doi: 10.1212/WNL.0000000000008093
8. Tijssen MP, Hofman PA, Stadler AA, van Zwam W, de Graaf R, van Oostenbrugge RJ, et al. The role of dual energy CT in differentiating between brain haemorrhage and contrast medium after mechanical revascularisation in acute ischaemic stroke. Eur Radiol. (2014) 24:834–40. doi: 10.1007/s00330-013-3073-x
9. Ferda J, Novak M, Mirka H, Baxa J, Ferdova E, Bednarova A, et al. The assessment of intracranial bleeding with virtual unenhanced imaging by means of dual-energy CT angiography. Eur Radiol. (2009) 19:2518–22. doi: 10.1007/s00330-009-1495-2
10. Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. (2001) 32:438–41. doi: 10.1161/01.STR.32.2.438
11. Gupta R, Phan CM, Leidecker C, Brady TJ, Hirsch JA, Nogueira RG, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. (2010) 257:205–11. doi: 10.1148/radiol.10091806
12. Lummel N, Schulte-Altedorneburg G, Bernau C, Pfefferkorn T, Patzig M, Janssen H, et al. Hyperattenuated intracerebral lesions after mechanical recanalization in acute stroke. Am J Neuroradiol. (2014) 35:345–51. doi: 10.3174/ajnr.A3656
13. Morhard D, Ertl L, Gerdsmeier-Petz W, Ertl-Wagner B, Schulte-Altedorneburg G. Dual-energy CT immediately after endovascular stroke intervention: prognostic implications. Cardiovasc Intervent Radiol. (2014) 37:1171–8. doi: 10.1007/s00270-013-0804-y
14. Bonatti M, Lombardo F, Zamboni GA, Vittadello F, Curro Dossi R, Bonetti B, et al. Iodine extravasation quantification on dual-energy CT of the brain performed after mechanical thrombectomy for acute ischemic stroke can predict hemorrhagic complications. Am J Neuroradiol. (2018) 39:441–7. doi: 10.3174/ajnr.A5513
15. Payabvash S, Qureshi MH, Khan SM, Khan M, Majidi S, Pawar S, et al. Differentiating intraparenchymal hemorrhage from contrast extravasation on post-procedural non-contrast CT scan in acute ischemic stroke patients undergoing endovascular treatment. Neuroradiology. (2014) 56:737–44. doi: 10.1007/s00234-014-1381-8
16. Ganguly A, Gold GE, Butts Pauly K, Mayer D, Moseley MM, Pelc NJ, et al. Quantitative evaluation of the relaxivity effects of iodine on GD-DTPA enhanced MR arthrography. J Magn Reson Imaging. (2007) 25:1219–25. doi: 10.1002/jmri.20934
Keywords: dual energy CT (DECT), ischemic stroke, thrombectomy, hemorrhagic transformation (HT), contrast staining
Citation: Liu K, Jiang L, Ruan J, Xia W, Huang H, Niu G, Yan S and Yin C (2020) The Role of Dual Energy CT in Evaluating Hemorrhagic Complications at Different Stages After Thrombectomy. Front. Neurol. 11:583411. doi: 10.3389/fneur.2020.583411
Received: 14 July 2020; Accepted: 03 September 2020;
Published: 07 October 2020.
Edited by:
Bruce Campbell, The University of Melbourne, AustraliaReviewed by:
Julian Maingard, Monash Health, AustraliaCopyright © 2020 Liu, Jiang, Ruan, Xia, Huang, Niu, Yan and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congguo Yin, eWluY2c3MTZAYWxpeXVuLmNvbQ==; Shenqiang Yan, c2hlbnFpYW5neWFuQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.