- 1School of Human Movement and Nutrition Sciences, Faculty of Health and Behavioural Sciences, The University of Queensland, Brisbane, QLD, Australia
- 2Centre for Sensorimotor Performance, The University of Queensland, Brisbane, QLD, Australia
- 3School of Allied Health Sciences, Griffith University, Brisbane, QLD, Australia
- 4Child Health Research Centre, Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia
Background: Cerebral Palsy (CP) is a non-progressive neurological condition that results in motor impairment which increases proximally to distally along the lower extremity (i.e., greatest impairment at the ankle). Consequently, motor impairment and reduced voluntary muscle activation results in reduced neuromuscular control of the lower limb in this population. CP rehabilitation traditionally aims to improve movement proficiency for functional activities, such as walking, by using a range of active movement modalities that require volitional effort; however, the underlying neural mechanisms of improved control and function remain unknown. The primary purpose of this study was to systematically determine the efficacy of lower limb active movement interventions to improve neuromuscular control in individuals with CP.
Methodology: A search for studies involving an active lower limb intervention and neurophysiological outcome measures in individuals with CP was performed in five electronic databases. Studies were assessed for methodological quality using the Downs and Black assessment tool.
Results: Nine of 6,263 articles met the inclusion criteria. Methodological quality of all studies was poor, ranging from 2 to 27 out of a possible score of 32 points on the Downs and Black assessment tool. The study interventions varied extensively in modality and prescription as well as in the outcome measures used.
Conclusions: Whether active movement improves neuromuscular control of the lower limb in CP is unclear due to high variability in intervention protocols and selected outcomes measures. Future active intervention studies must carefully consider the selection of neurophysiological outcome measures.
Introduction
Spastic type Cerebral Palsy (CP) is a motor impairment syndrome resulting from a non-progressive perinatal brain lesion. It is a lifelong condition which reduces an individual's ability to control posture and bodily movements (1). Following a central nervous system lesion, changes in development of the descending pathways causes a reduction in voluntary muscle activation and increased tonic muscle activity which may include spasticity, dystonia, hypertonia, and co-contraction (2–4). Within the lower limb, distal muscles are more impaired (5), which directly impacts lower limb neuromuscular control and the capacity to perform activities of daily living requiring ambulation (6, 7).
Lower limb functional activities of daily living such as ambulation, standing from a chair and climbing stairs, all require control over limb movements to achieve the specific task. To investigate the neuromuscular control required to perform these tasks, and the potential for neuroplasticity following motor learning, both the nervous system and the movement must be considered. Common clinical measures of CP lower limb functional capacity assess gross movement, but do not directly measure neural function during these gross motor tasks. For example, the 6 min walk test measures distance traveled (8), while the selective control assessment of the lower limb rates isolated joint movements (9). Neither assessments involve concurrent measures which probe the nervous system, for example recordings of electrical muscle activity or neural re-organization. To understand underlying neural mechanisms, neurophysiological assessments of the central and peripheral nervous systems are required. Neurophysiological assessment methods including electromyography (EMG) and magnetic resonance imaging (MRI) can determine muscle activation capacity, motor unit firing patterns and firing frequency as well as the amplitude of interference EMG signals and neural tract organization (2, 10, 11). Cross-sectional studies in individuals with CP report reduced capacity to voluntarily activate the plantar flexors and quadriceps by 20–50% (2, 4), reduced electromyography amplitude and motor unit firing rates (12), and altered muscle activation patterns, particularly for distal agonists (13–16).
Active movement training will herein be defined as an intervention requiring volitional muscular effort to achieve a given movement task, exclusive of assisted or passive movements. Intervention studies which aim to improve lower limb joint function, muscular impairment, and functional activity in individuals with CP often use active training modalities including treadmill walking and resistance training which achieve clinically important improvements in strength and walking ability, but typically do not assess neurophysiological outcomes which may indicate neuroplasticity following a perinatal brain lesion (17–19).
Increases in strength achieved with resistance training indicate the trainability of lower limb musculature in CP (20, 21). As knowledge of the neural mechanisms of motor impairment in CP is limited, understanding the neural component of movement control and its adaptability in CP is of great importance for rehabilitation. Preliminary evidence of enhanced neuromuscular control following lower limb active movement training exists in healthy adults and individuals with stroke (22–25), however few studies have used neurophysiological measures to assess changes in neural function and neuromuscular control following interventions in CP. The purpose of this study was to systematically review the current literature to determine the impact of lower limb active movement training in CP on neuromuscular control.
Survey Methodology
Search Strategy
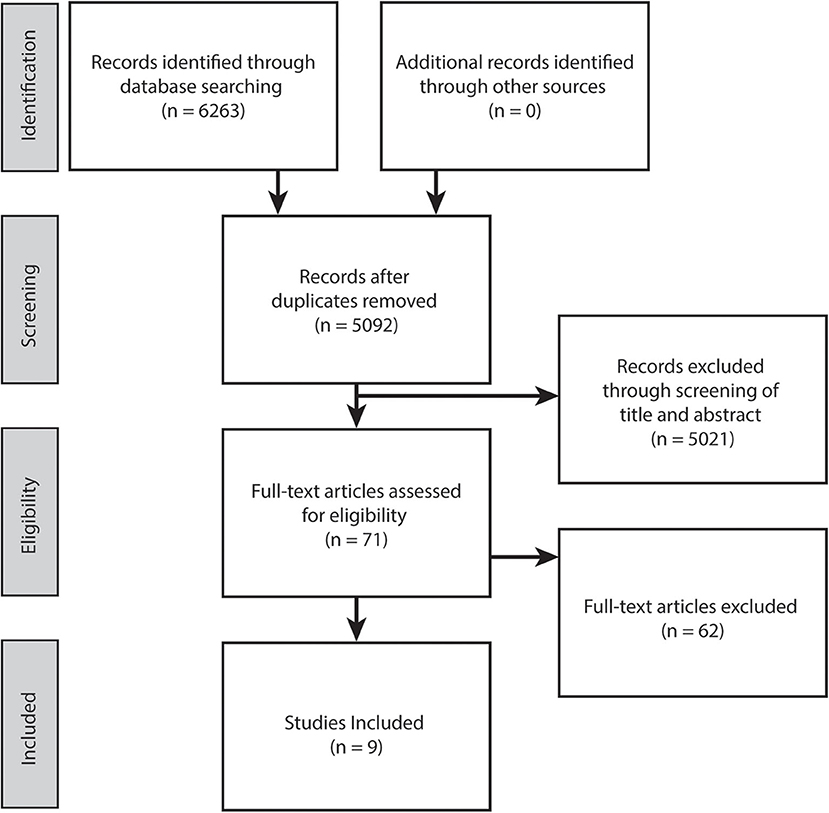
This review was conducted following PRISMA guidelines (Figure 1). An electronic literature search was conducted in September, 2020 within five online databases (Pubmed, CINAHL, Cochrane library, Embase and PEDro). The search strategy comprised of the following keywords: (i) population: (cerebral palsy) AND (lower limb or lower extremity or leg or hip or knee or ankle) AND (ii) intervention: (exercise or training or trial or active movement or rehabilitation or walk* or game* or robot* or therapy or resistance or isometric or isotonic or isokinetic or aerobic or anaerobic) AND (iii) outcome: (neuromuscular or neuromotor or motor control or selective motor control or voluntary activation or volition* or interpolated twitch or electromyography or rate of force development or function or gait or control or coordination or gait analysis or motion analysis). Note the PEDro database keyword search was reduced to “cerebral palsy motor control.” Article full texts that were not available electronically were retrieved through the University of Queensland Library document delivery service.
Inclusion Criteria
Articles were screened via title and abstract by the first author (SO'B) and full texts were retrieved when eligibility could not be established from the abstract. Articles were included if they satisfied the following criteria: (i) ambulatory individuals of any age diagnosed with cerebral palsy, (ii) comprised of an active movement intervention including the lower limb which required volitional muscular effort of the paretic limb/s (any inclusion of passive modalities i.e., botulinum toxin, massage, passive range of motion, stretching, orthosis, surgery, motor driven robotics, and electrical stimulation resulted in exclusion), (iii) reported neurophysiological outcome measures (using electromyography or neuroimaging techniques) and were (iv) peer reviewed primary research, pre/post design with full texts in English. The included articles were selected and agreed upon by SO'B and LB.
Data Extraction, Quality Assessment, and Analysis
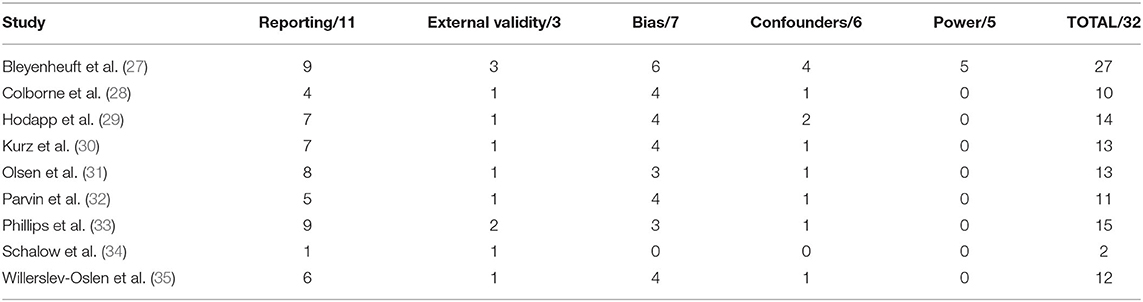
The following data were extracted from each study: number of participants included, participant characteristics (demographic, age, gender, CP type, topographical description, Gross Motor Function Classification Scale (GMFCS) and medication use), intervention protocol (type, length, frequency, session duration, total sessions, initial prescription and progression, equipment used, training location, supervision and adherence), neurophysiological outcome measures (type, muscles tested and the activity used during measurement), functional outcome measures and study results. Studies were assessed for methodological quality by two raters (SO'B and LB) using the Downs and Black (26) quality assessment tool which assesses quality of reporting, internal and external validity, and power across five sub-scales, providing a total score out of 32, and are presented in Table 1. Outcome means and standard deviations were extracted to calculate effect sizes (Meanpost-Meanpre/SDpooled) with 95% confidence intervals. An effect size of ≤0.20 was considered a trivial effect, 0.20 a small effect size, 0.50 a moderate effect size, and ≥0.80 a large effect size (36).
Results
The electronic database search yielded 6,263 potentially relevant studies. Following duplicate removal and initial screening of title and abstract against the inclusion criteria, full texts of the 71 remaining studies were scrutinized, resulting in nine eligible studies included in this review (Figure 1).
Qualitative Assessment
The methodological quality of included studies is presented in Table 1. Overall methodological quality was very poor. The Downs and Black scores ranged from 2 to 27 out of a possible 32 points. All studies except one randomized control trial scored lowest on the external validity and power criteria.
Study Design
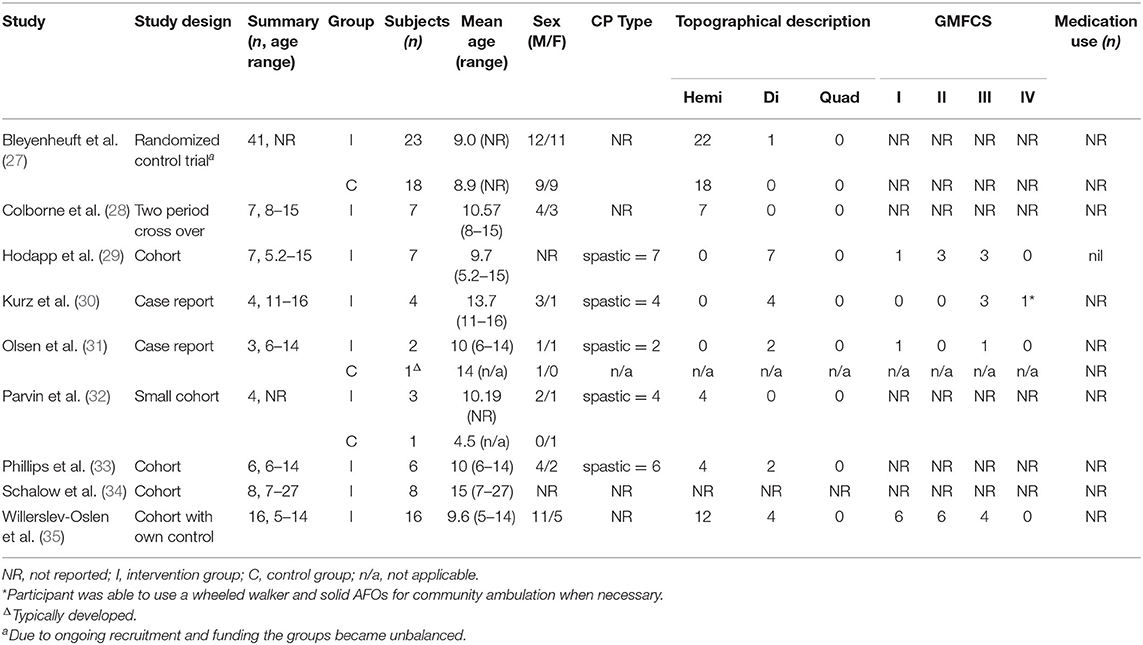
Study design is presented in Table 2. There were three cohort studies (29, 33, 34), one cohort study with own control (35), one case-control study (32), one two period cross-over study (28), and two case reports (30, 31). There was one randomized control trial that measured neuromuscular control following lower limb training, however due to ongoing randomization and limited funding the groups were unbalanced (27).
Participants
The participant's characteristics from each study are presented in Table 2. Total participant numbers ranged from 3 to 41, each with a greater proportion of males. Both adults and children were included, ages spanned 5–27 years. Studies that reported on CP type were all spastic and the topographical classification included hemiplegia (most common) and diplegia. GMFCS levels ranged from I-IV, and all individuals could ambulate independently. Four studies included a control group (27, 31, 32, 35). The first of these control groups did not complete the intervention (27), the second control group consisted of a single typically developed participant (31), the third control group consisted of a single participant who received traditional occupational therapy (32), and the fourth group consisted of the intervention participants who underwent a control period (35). One study reported that no participants were receiving concurrent pharmacological treatment during the period of investigation (29), and eight studies did not report on medication use at the time of the intervention. No studies reported whether participants were receiving concurrent physical therapies during the intervention, and six studies did not report the participant's treatment history (27–29, 32, 32, 34, 35). Three studies reported treatment history including surgery and botulinum toxin injections which had occurred prior to an exclusion period (30, 31, 33).
Interventions
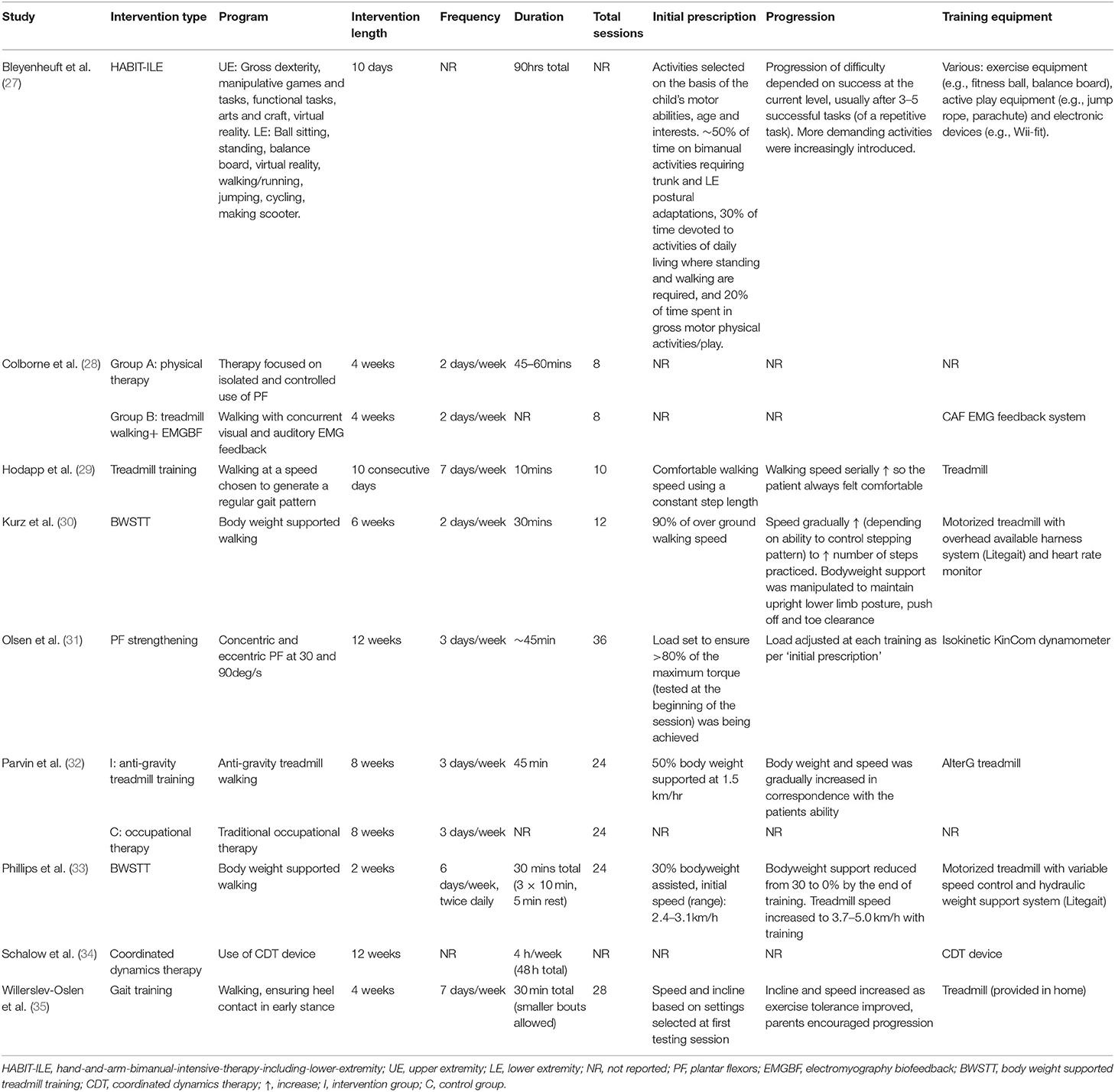
A detailed description of intervention characteristics of the included studies is shown in Table 3. Six studies used treadmill walking (28–30, 32, 33, 35), two with the addition of body weight support through a harness system, one with the assistance of anti-gravity technology, and one with the addition of EMG biofeedback. One study implemented resistance training which only targeted the plantar flexors (31). One study used hand-and-arm-bimanual-intensive-therapy-including-lower-extremity (HABIT-ILE) (27). Coordinated dynamics therapy and physical therapy were each used in one study. Of note, Colborne et al. (28) used two interventions (walking plus biofeedback and physical therapy) in a cross over design. Thus, the current literature comprises a total of 11 different interventions. Intervention length varied from 10 days to 12 weeks and the range of session frequencies employed resulted in the total number of training sessions ranging from eight to 36. Training duration ranged from as little as 10 min up to 1 h. Treadmill speed and incline were commonly progressed throughout the training period, but this was not systematic (Table 3). Progression of the resistance training intervention was also adjusted as required. Neither the physical therapy nor coordinated dynamics therapy interventions reported on initial prescription or the rate of progression. One study reported upon training location, which was at home (35). The training equipment was most often highly specialized (e.g., hydraulic weight support system, coordinated dynamic therapy device, antigravity treadmill, dynamometer, and custom built dorsiflexion machine).
Adherence and Supervision
Adherence was only reported in one study, at 100% compliance with all sessions (30). One study required the therapist to document what was completed in each session in a logbook (28). One study provided parents with a diary to record the duration and specific activity performed during training sessions, factors preventing training, other physical activity performed that day and rate how the child felt during each session (35). Exercise sessions were supervised by trainers (29, 32), physical therapists (28, 30, 31, 33, 34) and parents or family members (34, 35).
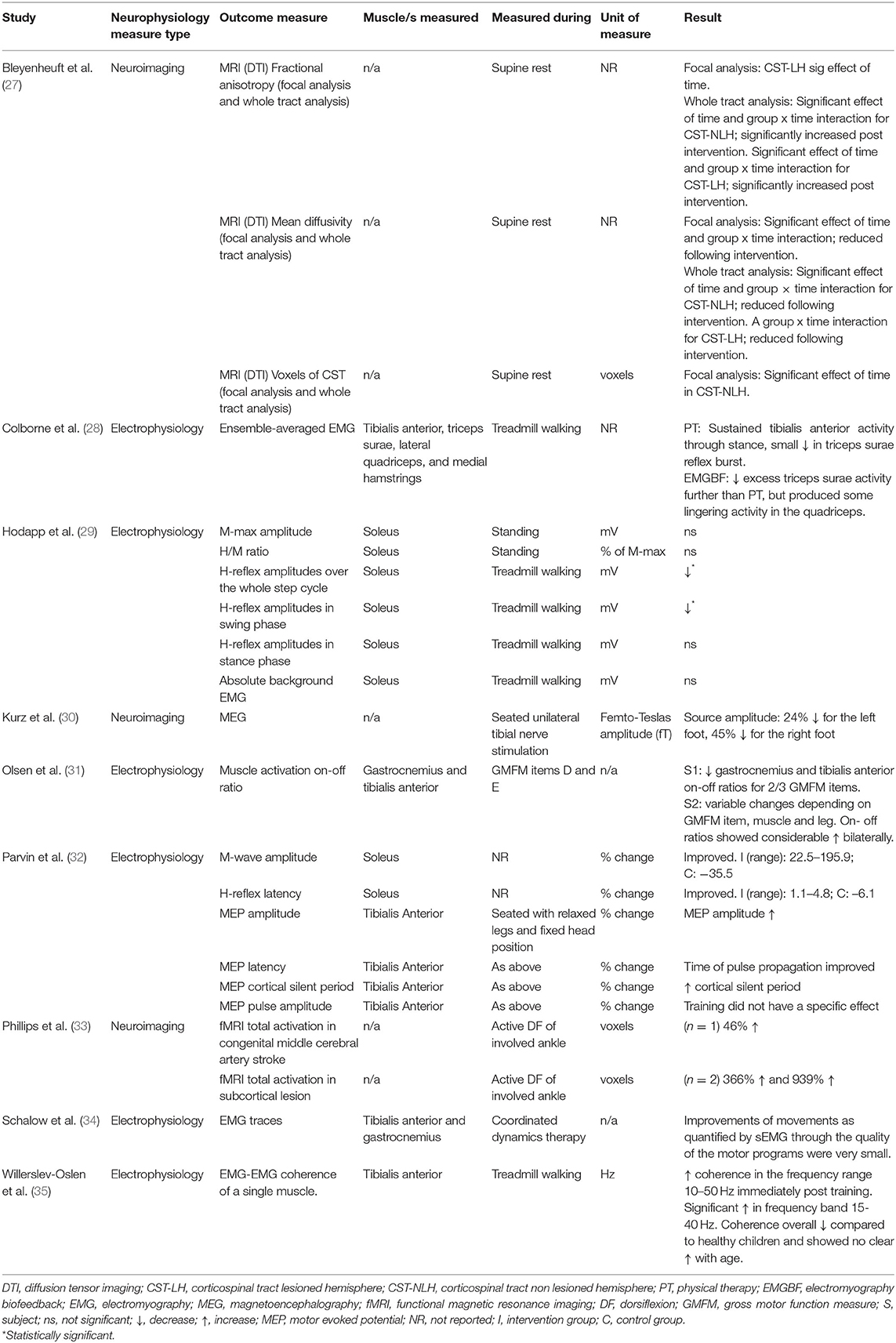
Neurophysiological Outcome Measures
Six studies reported on a total of 16 different electrophysiological outcome measures which are presented in Table 4. Electromyography was recorded from different lower limb muscles (most frequently tibialis anterior) and during different tasks (sitting, standing, walking, dorsiflexion maximum voluntary contraction, Gross Motor Function Measure (GMFM) items, and coordinated dynamic therapy). The results of coordinated dynamic therapy were not presented for all seven subjects, raw EMG of one individual was discussed as an example (34). H-reflexes during the swing phase of gait, H-reflexes over the whole step cycle, and gamma band (35–65 Hz) coherence were the only neurophysiological outcome measures to change statistically significantly following active movement training.
Neuroimaging Outcome Measures
Neuroimaging was used in three studies (27, 30, 33). Five neurophysiological outcome measures were reported (Table 4). Imaging techniques included functional magnetic resonance imaging (fMRI) (33), magnetoencephalography (MEG) (30) and diffusion tensor imaging (DTI), which were measured during different tasks (rest, active dorsiflexion and tibial nerve stimulation). Statistical analysis was not performed in two of these preliminary exploratory studies due to low participant numbers (30, 33). One study performed a two-way repeated measures ANOVA (27).
Functional Outcome Measures
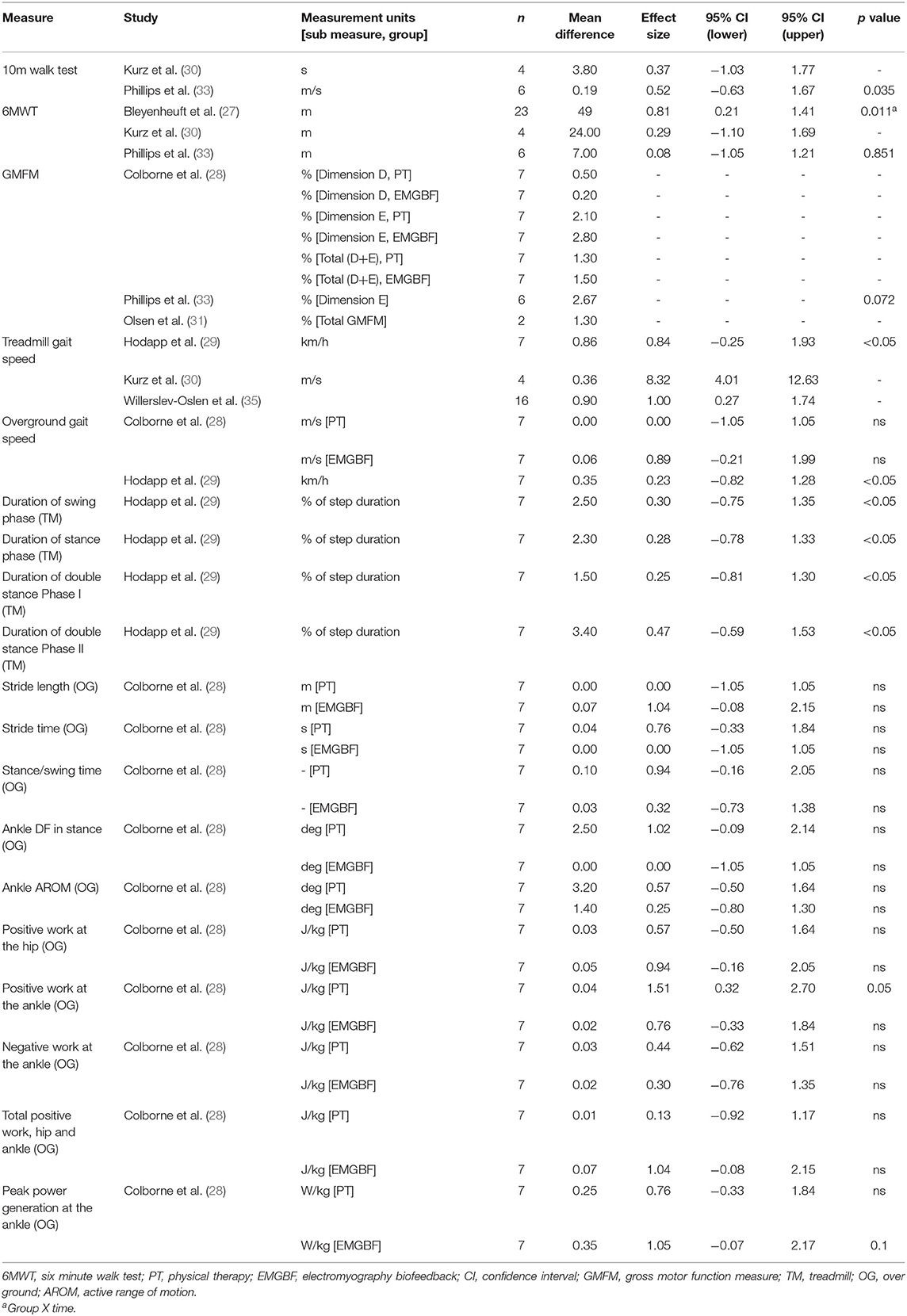
Functional outcome measures and results are presented in Table 6. One study did not measure any functional outcome measures (34). Parvin et al. (32) did not report functional outcome measures due to low participant numbers. No studies achieved the minimum detectable change (61.9, 64.0, and 47.4 m for GMFCS levels I, II, and III, respectively) for the 6 min walk test (37). There was a group x time effect of HABIT-ILE on the 6 min walk test (27). Overground and treadmill gait speed were the only functional outcome measure to improve significantly following active movement training (29, 33). A significant improvement in duration of swing, stance and both double stance phases is likely attributable to the significant increase in treadmill walking speed (29). Overground gait parameters of positive ankle work improved significantly following physical therapy, and peak ankle power significantly improved following treadmill training with EMG biofeedback (28).
Quantitative Assessment
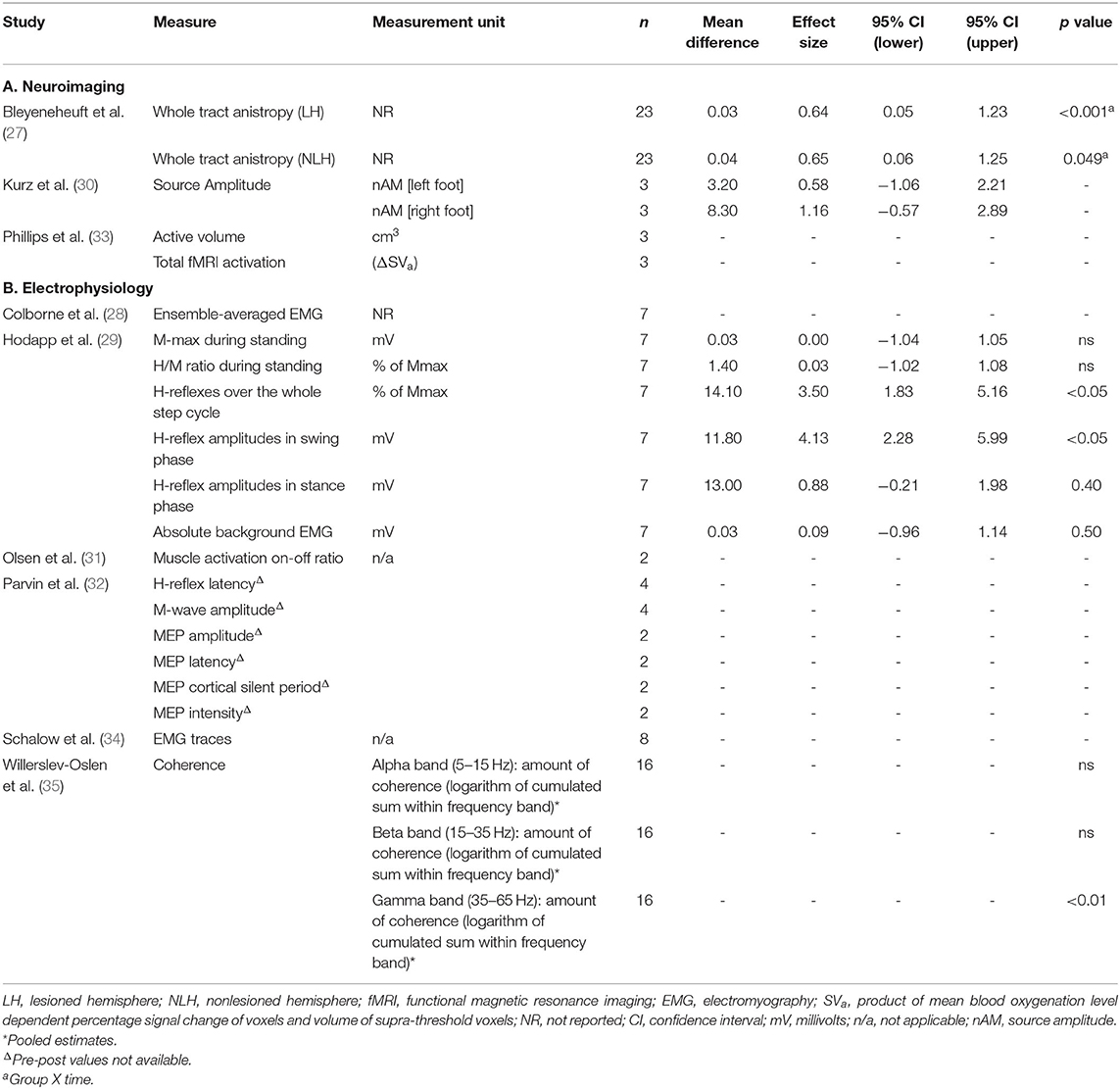
The effect sizes and confidence intervals for neurophysiological and functional outcome measures are summarized in Tables 5, 6. Due to low participant numbers, unavailable or insufficient data, an effect size could not be determined for all outcome measures. Studies with low participant numbers did not complete statistical analysis. The descriptive results for neurophysiological outcomes are presented in Table 4. Effect sizes for neurophysiological measures varied widely. Effect sizes among studies which used the same functional outcome measure ranged from trivial to large.
Discussion
The purpose of this study was to systematically review the current literature to determine the impact of lower limb active movement training on neuromuscular control in CP. Nine studies investigating neurophysiological outcomes of active movement training in individuals with CP were included in this review. Improved cortical representation of the ankle and reduced reflex amplitudes during gait allude to a potential for active movement training interventions to elicit favorable lower limb neuromuscular changes in CP. However, the mechanism/s for improvement are unable to be established due to a limited number of studies and diversity of outcome measures used. No study reported deterioration of outcome measures following interventions.
Treadmill training was the most common intervention. Six of the nine studies incorporated variations of treadmill walking training with a training dose ranging from 1.6 h (across 10 days) to 18 h (across 8 weeks). Following body weight supported treadmill training, favorable central nervous system adaptations for motor skill learning were reported. These include fMRI data suggestive of an increase in the area of the primary motor cortex active during isolated voluntary ankle movement (33), and a more refined representation of the foot in the somatosensory cortices, as identified using magnetoencephalography (MEG) during peripheral nerve stimulation (30). One study quantified changes in corticospinal tract (CST) integrity using diffusion tensor imaging (DTI) following a combined upper and lower limb training protocol (27). There was a significant positive group x time effect of training for the whole tract analyses, and lower limb function quantified using a 6 min walk test. Correlations between focal analyses of CST integrity and hand motor function showed a trend for improvement in hand function, but had no correlation to lower limb performance on the 6 min walk test. Combined upper and lower limb intensive motor training appears to have a favorable impact on CST integrity and hand function. However, it is unclear whether or not lower limb training contributed to the improvements in CST integrity observed in this intervention. Although there was a trend toward a relationship between improved CST changes and hand, but not leg, motor function, this observation does not rule out an effect of lower limb training on CSTs. Any such relationship between CST integrity and motor function may have been biased toward the upper limb due to a larger number of upper limb CST axons in the area of measurement. There was also only one physical lower limb performance assessment measure, which may not have been representative of functions affected strongly by CST integrity. It therefore remains unclear what effect lower limb training has on CST integrity following lower limb motor skill learning.
Favorable peripheral nervous system changes were reported in four gait training studies from varied outcome measures which overall indicate a more functionally useful swing phase and foot placement during gait. Improvements in dorsiflexion prior to foot contact and reflex modulation (reduced soleus H-reflex amplitude) (29), improved tibialis anterior coordination enhancing toe lift (coherence between proximal and distal ends of tibialis anterior of the most affected leg) (35), and triceps surae and tibialis anterior activation patterns (sEMG) more similar to typically developed children were reported (28). The fourth small cohort study (n = 2) reported combined changes in the central and peripheral nervous system, with improved corticospinal tract connectivity [increased motor evoked potential (MEP) amplitude during transcranial magnetic stimulation (TMS)] and reduced inhibition of the tibial nerve (improved H-reflex latency and maximum M-wave amplitude), such that the measures approached values observed in typically developed individuals (32). The impact of training volume and progression on these outcomes is unclear as favorable changes were obtained following a range of 100–840 min of gait training (held across 10–28 sessions), and progression of difficulty was not based on categorical performance criteria being met and poorly described (Table 3). In summary, although there are reports of improved neuromuscular control of the ankle plantar flexors and dorsiflexors during gait following treadmill training, the lack of consistency in training doses and objective outcome measures makes it difficult to make recommendations for interventions and monitoring changes.
Similarly, following coordinated dynamics therapy (crawling, treadmill walking, jumping on a spring-board and use of a coordination dynamic therapy device resembling a combined arm and leg ergometer performed seated or supine), sEMG was reported (through visual inspection) to be changed in a favorable way, but this remained unquantified (34). Olsen et al. (31) demonstrated the feasibility of recording dorsi- and plantar flexion muscle activation patterns during selected GMFM items but was unable to determine whether changes in muscle activation sequencing (sEMG on-off ratio) were favorably improved following resistance training due to outcome variability in the small sample. Resistance training has been shown to increase muscle size and strength in individuals with CP (21, 38). Twelve weeks of lower limb, explosive, progressive, heavy resistance training has recently been shown to increase lower limb muscular strength and rate of force development in tibialis anterior (39). Rate of force development is a measure of torque output during a rapid maximum muscle contraction, but is considered by some as a proxy neurophysiological measure because it is strongly determined by neural drive (40). The increase in rate of force development observed by Kirk et al. (39) was accompanied by improved maximum isometric voluntary dorsiflexion, plantar flexion, knee flexion, and knee extension strength. While it appears that strength training may have provided some improvement in capacity to activate the lower limb musculature in adults with CP, the exact mechanisms (neural vs. muscle adaptation) cannot be determined or quantified by these measures alone.
There have been numerous attempts to understand neuroplasticity and neuromuscular changes following interventions targeting muscular adaptations in humans (41–43), yet there is limited information pertaining to the trainability and measurement of neuromuscular control of the lower limb in CP. Of the nine studies included in this review, no studies performed the same neuromuscular outcome measure under the same conditions. This may have been limited by access to equipment and expertise required to perform such assessments. The location of adaptation within the nervous system following intervention is also unknown. It is particularly difficult to identify site specific changes within the nervous system as the results of non-intrusive neurophysiological measurements are often subject to modulation at multiple neural sites.
Although some nervous system changes were reported following active movement training, only three studies (that incorporated treadmill training and HABIT-ILE) found concomitant functional capacity improvements (27, 29, 33). Functional improvements in self-selected overground walking speed were elicited by a short walking training intervention (100 min across 10 days) (29). A slightly longer training duration of bodyweight supported treadmill walking (720 min across 2 weeks) improved walking speed during the 10 m walk test (33). These relatively short training periods suggest there may be an early adaptation period related to the specificity of the task of walking which improves neuromuscular control. These studies did not report on participant's current physical activity levels, familiarization and confidence with treadmill walking prior to the study.
With few studies measuring both neurophysiology and gross motor function outcomes following active movement training, we are not currently able to predict the impact and relationship of all neuromuscular changes on gross motor function. Further, the sensitivity of functional outcome measures to reflect neural changes also remains unknown. For example, intervention studies have demonstrated improvements in lower limb functional capacity without concurrent neurophysiological measurements (19, 38, 44), which limits our capacity to understand whether central and/or peripheral nervous system changes occur and contribute to these observed functional outcomes.
It is premature to speculate on the recommendations for optimal prescription of active movement training to elicit neuromuscular adaptation, due to the variability of interventions among studies and lack of consistency in choice of neuromuscular outcome measures. Despite treadmill training being a popular training mode, previous systematic reviews in children with CP have not been able to determine optimal protocol parameters (45, 46). Treadmill training is a safe and feasible training intervention, but the magnitude of benefit and improvement in lower limb neuromuscular control for individuals at different GMFCS levels is not clear. Large scale trials are still required before guidelines can be considered.
Individual study results must be interpreted with caution due to low study methodological quality, varied training loads and modalities, lack of control groups and diverse use of neurophysiological and neuroimaging outcome measures. Low participant numbers in small cohort studies do not provide sufficient power or external validity to establish whether changes in neuromuscular control are genuine. The effect of age on motor learning and whether there is an optimal time for neural plasticity is unknown. The large range in age of participants makes it difficult to understand both the age dependent adaptability of the nervous system, and the impact of development. Finally, as all participants in the studies reviewed here were diagnosed with spastic CP, and due to the differential effects of lesion location, the findings of nervous system changes suggested may not be generalisable to other types of CP.
Future Direction
Future research should be conscious of the existing neurophysiological outcome measures used to evaluate training interventions, in order to allow for comparison of training mode and training load on the magnitude of change. Future work should also endeavor to apply greater methodological quality than the existing studies. Careful selection of outcome measures which adequately assess regions of the nervous system targeted by training (cortical, spinal cord, neuromuscular junction) is necessary to draw conclusions regarding the impact of training. No single measure will quantify the adaptability of the nervous system as a whole, but adequate and repeated attempts are required to determine mechanisms underlying motor control changes that may be important for function. Due to the complexity of the motor control system, measures that are sensitive enough to measure change over time are required. There are inherent difficulties with performing appropriate neurophysiological assessments. Equipment, expertise and funds are often not readily accessible to clinicians in clinics where these types of interventions and treatment plans are typically conducted.
Conclusions
The impact of active movement training on lower limb neuromuscular control in individuals with CP cannot yet be established. Due to the small number of investigations and their low scientific quality, it is not possible to determine the mechanisms by which the different active movement interventions elicit change within the nervous system. There is disparity in the choice of outcome measures used between studies, which prevents direct comparisons between interventions and the identification of central vs. peripheral nervous system adaptations. Ultimately, the question of whether modalities such as strength training or gait training can favorably alter neuromuscular control of the lower limb in CP remains unclear and requires further investigation. It also remains unclear how changes in neurophysiological measures relate to changes or improvement in gross motor function in CP.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repositories and references can be found in the article.
Author Contributions
SO'B, LB, GL, and TC: conceptualization and data interpretation. SO'B and LB: search and data extraction. SO'B: writing. LB, GL, and TC: reviewing and editing. All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
SO'B was supported by a National Health and Medical Research Council Postgraduate Scholarship, co-funded by the Cerebral Palsy Alliance Research Foundation [APP1114651].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, et al. Cerebral palsy. Nat Rev Dis Primers. (2016) 2:15082. doi: 10.1038/nrdp.2015.82
2. Hussain AW, Onambele GL, Williams AG, Morse CI. Muscle size, activation, and coactivation in adults with cerebral palsy. Muscle Nerve. (2014) 49:76–83. doi: 10.1002/mus.23866
3. Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW, Task Force on Childhood Motor D. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. (2003) 111:e89–97. doi: 10.1542/peds.111.1.e89
4. Stackhouse SK, Binder-Macleod SA, Lee SCK. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. (2005) 31:594–601. doi: 10.1002/mus.20302
5. Fowler EG, Staudt LA, Greenberg MB. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev Med Child Neurol. (2010) 52:264–9. doi: 10.1111/j.1469-8749.2009.03586.x
6. Barber L, Carty C, Modenese L, Walsh J, Boyd R, Lichtwark G. Medial gastrocnemius and soleus muscle-tendon unit, fascicle, and tendon interaction during walking in children with cerebral palsy. Dev Med Child Neurol. (2017) 59:843–51. doi: 10.1111/dmcn.13427
7. Murphy KP. The adult with cerebral palsy. Orthop Clin North Am. (2010) 41:595–605. doi: 10.1016/j.ocl.2010.06.007
8. Andersson C, Asztalos L, Mattsson E. Six-minute walk test in adults with cerebral palsy. Clin Rehabil. (2006) 20:488–95. doi: 10.1191/0269215506cr964oa
9. Balzer J, Marsico P, Mitteregger E, van der Linden ML, Mercer TH, van Hedel HJ. Construct validity and reliability of the selective control assessment of the lower extremity in children with cerebral palsy. Dev Med Child Neurol. (2016) 58:167–72. doi: 10.1111/dmcn.12805
10. Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. (2003) 45:542–50. doi: 10.1111/j.1469-8749.2003.tb00954.x
11. Rose J, McGill KC. The motor unit in cerebral palsy. Dev Med Child Neurol. (1998) 40:270–7. doi: 10.1111/j.1469-8749.1998.tb15461.x
12. Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. (2005) 47:329–36. doi: 10.1017/S0012162205000629
13. Alves-Pinto A, Blumenstein T, Turova V, Lampe R. Altered lower leg muscle activation patterns in patients with cerebral palsy during cycling on an ergometer. Neuropsychiatr Dis Treat. (2016) 12:1445–56. doi: 10.2147/NDT.S98260
14. Prosser LA, Lee SCK, VanSant AF, Barbe MF, Lauer RT. Trunk and hip muscle activation patterns are different during walking in young children with and without cerebral palsy. Phys Ther. (2010) 90:986–97. doi: 10.2522/ptj.20090161
15. Tedroff K, Knutson LM, Soderberg GL. Synergistic muscle activation during maximum voluntary contractions in children with and without spastic cerebral palsy. Dev Med Child Neurol. (2006) 48:789–96. doi: 10.1017/S0012162206001721
16. Wakeling J, Delaney R, Dudkiewicz I. A method for quantifying dynamic muscle dysfunction in children and young adults with cerebral palsy. Gait Posture. (2007) 25:580–9. doi: 10.1016/j.gaitpost.2006.06.009
17. Andersson C, Grooten W, Hellsten M, Kaping K, Mattsson E. Adults with cerebral palsy: walking ability after progressive strength training. Dev Med Child Neurol. (2003) 45:220–8. doi: 10.1111/j.1469-8749.2003.tb00335.x
18. Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol. (2003) 45:652–7. doi: 10.1111/j.1469-8749.2003.tb00866.x
19. van Vulpen LF, de Groot S, Rameckers E, Becher JG, Dallmeijer AJ. Improved walking capacity and muscle strength after functional power-training in young children with cerebral palsy. Neurorehabilitation Neural Repair. (2017) 31:827–41. doi: 10.1177/1545968317723750
20. Franki I, Desloovere K, De Cat J, Feys H, Molenaers G, Calders P, et al. The evidence-base for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the International Classification of Functioning, Disability and Health as a conceptual framework. J Rehabil Med. (2012) 44:385–95. doi: 10.2340/16501977-0983
21. Gillett JG, Boyd RN, Carty CP, Barber LA. The impact of strength training on skeletal muscle morphology and architecture in children and adolescents with spastic cerebral palsy: a systematic review. Res Dev Disabil. (2016) 56:183–96. doi: 10.1016/j.ridd.2016.06.003
22. Andersen LL, Zeeman P, Jorgensen JR, Bech-Pedersen DT, Sorensen J, Kjaer M, et al. Effects of intensive physical rehabilitation on neuromuscular adaptations in adults with poststroke hemiparesis. J Strength Cond Res. (2011) 25:2808–17. doi: 10.1519/JSC.0b013e31822a62ef
23. Behrens M, Mau-Moeller A, Mueller K, Heise S, Gube M, Beuster N, et al. Plyometric training improves voluntary activation and strength during isometric, concentric and eccentric contractions. J Sci Med Sport. (2016) 19:170–6. doi: 10.1016/j.jsams.2015.01.011
24. Del Balso C, Cafarelli E. Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol. (2007) 103:402–11. doi: 10.1152/japplphysiol.00477.2006
25. Ekblom MM. Improvements in dynamic plantar flexor strength after resistance training are associated with increased voluntary activation and V-to-M ratio. J Appl Physiol. (2010) 109:19–26. doi: 10.1152/japplphysiol.01307.2009
26. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. (1998) 52:377–84. doi: 10.1136/jech.52.6.377
27. Bleyenheuft Y, Dricot L, Ebner-Karestinos D, Paradis J, Saussez G, Renders A, et al. Motor skill training may restore impaired corticospinal tract fibers in children with cerebral palsy. Neurorehabilitation Neural Repair. (2020) 34:533–46. doi: 10.1177/1545968320918841
28. Colborne GR, Wright FV, Naumann S. Feedback of triceps surae EMG in gait of children with cerebral palsy: a controlled study. Arch Phys Med Rehabil. (1994) 75:40–5. doi: 10.1016/0003-9993(94)90335-2
29. Hodapp M, Vry J, Mall V, Faist M. Changes in soleus H-reflex modulation after treadmill training in children with cerebral palsy. Brain. (2009) 132(Pt 1):37–44. doi: 10.1093/brain/awn287
30. Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neurol Phys Ther. (2012) 36:166–72. doi: 10.1097/NPT.0b013e318251776a
31. Olsen JE, Ross SA, Foreman MH, Engsberg JR. Changes in muscle activation following ankle strength training in children with spastic cerebral palsy: an electromyography feasibility case report. Phys Occup Ther Pediatr. (2013) 33:230–42. doi: 10.3109/01942638.2012.723116
32. Parvin S, Taghiloo A, Irani A, Mirbagheri MM. Therapeutic effects of anti-gravity treadmill (AlterG) training on reflex hyper-excitability, corticospinal tract activities, and muscle stiffness in children with cerebral palsy. IEEE Int Conf Rehabil Robot. (2017) 2017:485–90. doi: 10.1109/ICORR.2017.8009295
33. Phillips JP, Sullivan KJ, Burtner PA, Caprihan A, Provost B, Bernitsky-Beddingfield A. Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body-weight-supported treadmill training: a pilot study. Dev Med Child Neurol. (2007) 49:39–44. doi: 10.1017/S0012162207000102.x
34. Schalow G, Jaigma P. Cerebral palsy improvement achieved by coordination dynamics therapy. Electromyogr Clin Neurophysiol. (2005) 45:433–45.
35. Willerslev-Olsen M, Petersen TH, Farmer SF, Nielsen JB. Gait training facilitates central drive to ankle dorsiflexors in children with cerebral palsy. Brain. (2015) 138(Pt 3):589–603. doi: 10.1093/brain/awu399
37. Thompson P, Beath T, Bell J, Jacobson G, Phair T, Salbach NM, et al. Test-retest reliability of the 10-metre fast walk test and 6-minute walk test in ambulatory school-aged children with cerebral palsy. Dev Med Child Neurol. (2008) 50:370–6. doi: 10.1111/j.1469-8749.2008.02048.x
38. Gillett JG, Lichtwark GA, Boyd RN, Barber LA. Functional anaerobic and strength training in young adults with cerebral palsy. Med Sci Sports Exerc. (2018) 50:1549–57. doi: 10.1249/MSS.0000000000001614
39. Kirk H, Geertsen SS, Lorentzen J, Krarup KB, Bandholm T, Nielsen JB. Explosive resistance training increases rate of force development in ankle dorsiflexors and gait function in adults with cerebral palsy. J Strength Cond Res. (2016) 30:2749–60. doi: 10.1519/JSC.0000000000001376
40. Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. (2002) 93:1318–26. doi: 10.1152/japplphysiol.00283.2002
41. Carroll TJ, Selvanayagam VS, Riek S, Semmler JG. Neural adaptations to strength training: moving beyond transcranial magnetic stimulation and reflex studies. Acta Physiol. (2011) 202:119–40. doi: 10.1111/j.1748-1716.2011.02271.x
42. Kidgell DJ, Pearce AJ. What has transcranial magnetic stimulation taught us about neural adaptations to strength training? J Strength Cond Res. (2011) 25:3208–17. doi: 10.1519/JSC.0b013e318212de69
43. Kidgell DJ, Stokes MA, Castricum TJ, Pearce AJ. Neurophysiological responses after short-term strength training of the biceps brachii muscle. J Strength Cond Res. (2010) 24:3123–32. doi: 10.1519/JSC.0b013e3181f56794
44. Verschuren O, Ketelaar M, Gorter JW, Helders PJ, Uiterwaal CS, Takken T. Exercise training program in children and adolescents with cerebral palsy: a randomized controlled trial. Arch Pediatr Adolesc Med. (2007) 161:1075–81. doi: 10.1001/archpedi.161.11.1075
45. Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J Neurol Phys Ther. (2009) 33:27–44. doi: 10.1097/NPT.0b013e31819800e2
Keywords: cerebral palsy, lower limb, intervention, motor control, voluntary activation
Citation: O'Brien SM, Lichtwark GA, Carroll TJ and Barber LA (2020) Impact of Lower Limb Active Movement Training in Individuals With Spastic Type Cerebral Palsy on Neuromuscular Control Outcomes: A Systematic Review. Front. Neurol. 11:581892. doi: 10.3389/fneur.2020.581892
Received: 10 July 2020; Accepted: 22 October 2020;
Published: 26 November 2020.
Edited by:
Emily Keshner, Temple University, United StatesReviewed by:
Maxime T. Robert, Laval University, CanadaAna Carolina De Campos, Federal University of São Carlos, Brazil
Copyright © 2020 O'Brien, Lichtwark, Carroll and Barber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shari M. O'Brien, c2hhcmkub2JyaWVuQHVxY29ubmVjdC5lZHUuYXU=
 Shari M. O'Brien
Shari M. O'Brien Glen A. Lichtwark
Glen A. Lichtwark Timothy J. Carroll
Timothy J. Carroll Lee A. Barber
Lee A. Barber