- 1Cognitive Neurology Institute, Rambam Health Care Campus, Haifa, Israel
- 2Technion – Israel Institute of Technology, Haifa, Israel
Introduction: Variations in lifestyle, socioeconomic status and general health likely account for differences in dementia disparities across racial groups. Our aim was to evaluate the characteristics of Arab (AS) and Jewish (JS) subjects attending a tertiary dementia clinic in Israel.
Methods: Retrospective data regarding subjects attending the Cognitive Neurology Institute at Rambam Health Care Campus between April 1, 2010, and April 31, 2016, for complaints of cognitive decline were collected from the institutional registry. AS and consecutive JS, aged ≥50 years without a previous history of structural brain disease, were included.
Results: The records of 6,175 visits were found; 3,246 subjects were ≥50 years at the initial visit. One hundred and ninety-nine AS and consecutive JS cases were reviewed. Mean age at first visit was 68.4 ± 8.8 for AS and 74.3 for JS (p < 0.0001). Mean education was 7.7 ± 4.8 years for AS and 11.3 years for JS (p < 0.0001). Mean duration of cognitive complaints prior to first visit did not differ between the groups. Initial complaints of both ethnicities were failing memory (97%) and behavioral changes (59%). Functional impairment was reported by 59% of AS and 45% of JS (p = 0.005). MMSE on first evaluation was 19.2 ± 7 for AS and 23.1 ± 5.9 for JS; p = 0.001. Alzheimer's disease was diagnosed in 32% AS and 23% JS, mild cognitive impairment in 12% AS and 21% JS. Normal cognition was diagnosed in 2% AS and 9% JS; p = 0.0001.
Conclusions: Compared to JS, AS attend a tertiary clinic when their cognitive impairment already affects their functional abilities providing a comprehensive benchmark for social health care interventions to reduce disparities.
Introduction
The estimated number of people living with dementia worldwide currently is approaching 50 million. This number is estimated to triple by 2050, as a result of anticipated increase in middle and lower income countries due to aging of their populations (1). Ethnicity is one of the factors influencing dementia prevalence as it impacts health seeking behavior, stigma and perceived futility of the diagnosis (2). Various countries have reported dementia incidence and prevalence to be higher in ethnic minorities (3); alas, they use fewer medical services (4).
Few studies have evaluated the prevalence of dementia in the different ethnic groups in Israel. The largest ethnic groups in Israel are Israeli Jews followed by Israeli Arabs, further divided into Muslims, Christians and Druze, with a smaller number of other ethnicities. The prevalence of Alzheimer's disease (AD) in the 60 years and older Arab population in Israel is estimated as 10–20.5% (5, 6); this is four times higher than the estimated prevalence in the Jewish population (7, 8). Factors, such as high consanguinity rates and genetic pre-disposition (9, 10), high illiteracy rates (5) and lower socioeconomic status (11), have been considered to contribute to this disparity.
The present study sought to determine the usage of tertiary dementia clinic services in Israel among Arab (AS) and Jewish (JS) subjects, explore their characteristics and investigate their diagnosis and compliance with treatment. Exploring the reasons for the differences between these ethnic groups may help to direct service planning, health education and development of interventions.
Materials and Methods
This is a retrospective study regarding subjects attending the Cognitive Neurology Institute at Rambam Health Care Campus (CNIR). It was reviewed and approved by the Institutional Review Boards of Rambam Health Care Campus (# 0680-19-RMB). Written informed consent from the participants was not required to participate in this study in accordance with national legislation and institutional requirements because the study involved medical record review with no subject contact.
The CNIR is a tertiary referral center for diagnosis and treatment of cognitive impairment and dementia, located in Haifa, north Israel. We collected retrospective data on subjects attending the CNIR for complaints regarding acquired cognitive decline between April 2010 and April 2016 from the computerized institutional registry. Ethnicity was determined according to subject's name, records of the ministry of internal affairs, self-report. Inclusion criteria were first clinic visit of subjects 50 years and older with complaints regarding acquired cognitive decline.
Following screening of the CNIR registry, AS meeting inclusion and exclusion criteria were included in the study. First consecutive JS, attending the clinic at nearest date on the calendar, and meeting inclusion/exclusion criteria were added to the study.
Exclusion criteria included history of traumatic brain injury (TBI) or neurosurgery 1 year preceding the emergence of cognitive deterioration, evaluation for ADHD (attention deficit and hyperactivity disorder), evaluation for insurance purposes and cognitive screening prior to neurosurgical procedures. Patient characteristics, medical background, current diagnoses and follow-up visits were recorded.
Ethnicity was retrieved from the demographical data documentation. When unavailable, patient ethnicity was defined by name. Several studies across the world have examined the issue of ethnicity definition by subject's name. A meta-analysis performed by Pablo Mateos of several studies addressing the issue found a sensitivity of 0.67–0.95 and a specificity of 0.8–1 of such reports (12). As we defined only two broad ethnicities, Arab and Jew, and did not subdivide them according to religion or subethnicity, the specificity and sensitivity are probably much higher.
Ethnic demographic data involving northern Israel residents was extracted from the Central Bureau of Statistics registry.
Cognitive complaints were defined as complaints regarding memory, language, orientation, attention or cognitive slowing. Behavioral complaints were defined when significant changes in comportment and behavior, such as aggression, psychosis, disinhibition, apathy and mood disturbances, were reported. Functional impairment was defined as self-report of any difficulties performing ADL (activities of daily living) or IADL (instrumental ADL).
Dementia, AD and mild cognitive impairment (MCI) were diagnosed according to recommendations from the National Institute on Aging-Alzheimer's Association workgroup (NINCDS-ADRDA criteria) (13, 14). VD (vascular dementia) was diagnosed according to AHA/ASA recommendations (15). Subjects who met both criteria for primary degenerative dementia of the Alzheimer type and neuroimaging features of VD were diagnosed as mixed dementia (MD) (16).
A frontotemporal dementia (FTD) diagnosis was based on clinical presentation combined with ancillary tests (17, 18). Dementia associated with extrapyramidal disease (Lewy body dementia and dementia in Parkinson disease) was diagnosed in patients with prominent extrapyramidal manifestations and a corresponding cognitive profile (19, 20).
Pseudo-dementia was diagnosed in subjects who presented with complaints regarding cognitive deterioration that were clinically judged to be secondary to depression but not to a neurodegenerative condition (21).
Comparison of education, MMSE and duration of symptoms between the two ethnic groups was performed by Mann-Whitney non-parametric test. Patients' age, gender, marital status, medical background, diagnosis in the first and the last follow up, as well as number of follow-ups were compared by Chi square test.
Adjustment of MMSE for age and education years (adjusted MMSE: aMMSE) was based on the MMSE norms recommended by MMSE handbook published by Folstein (22).
P-value below 0.005 was considered as statistically significant. Data analysis was performed by SPSS Statistics 25 software.
Results
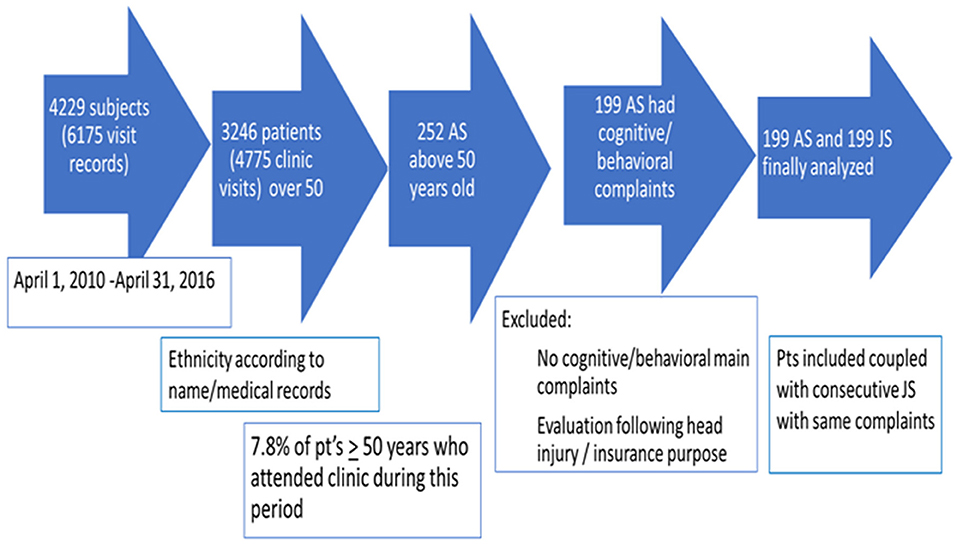
A total of 6,175 visit records of 4,229 subjects attending the CNIR between April 1, 2010, and April 31, 2016, were reviewed; 3,246 subjects were ≥50 years at the initial visit. Two hundred and fifty-two (7.8%) AS ≥50 years were identified. Fifty-three AS were not included for further analysis due to meeting exclusion criteria or missing data. One hundred and ninety-nine AS were included; in 145 subjects, Arab ethnicity was documented in patients' files, ethnicity of the rest was defined by name. AS were coupled with consecutive JS who met inclusion and exclusion criteria (Figure 1).
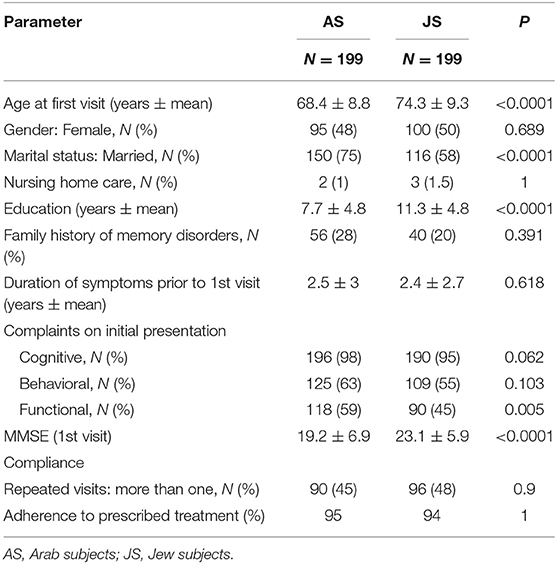
Mean age at first visit was 68.4 ± 8.8 for AS and 74.3 ± 9.3 years for JS (p < 0.0001). Mean education was 7.7 ± 4.8 years for AS and 11.3 ± 4.8 years for JS (p < 0.0001). Seventy-five percent AS and 58% JS were married; 1% AS and 1.5% JS were referred from nursing homes.
Mean duration of cognitive complaints prior to the first visit did not differ between the groups (AS 2.5 ± 3 years, JS 2.4 ± 2.7 years). Initial complaints of both ethnicities included failing memory (97%) and behavioral changes (59%). Functional impairment was reported by 59% of AS and 45% of JS (p = 0.005).
MMSE on first evaluation was 19.2 ± 6.9 for AS and 23.1 ± 5.9 for JS; p = 0.001 (Table 1). MMSE differences remained significant after correction for age and education years (25.4 ± 22.5 AS, 34.5 ± 22.7 JS, p < 0.0001) (22) (adjusted MMSE: aMMSE).
A low aMMSE (<15) was more prevalent in AS (41.2% AS, 25.8% JS, p = 0.002), female gender (41.5% female, 25.8% male, p = 0.002) and patients with behavioral and functional complaints (43% behavioral, 46% functional complaints; 20% and 19% without behavioral and functional complaints, respectively; p < 0.0001). The presence of cognitive complaints was not associated with lower scores on the aMMSE (33% with cognitive complaints, 22% without this complaint had aMMSE below 15, p = 0.477).
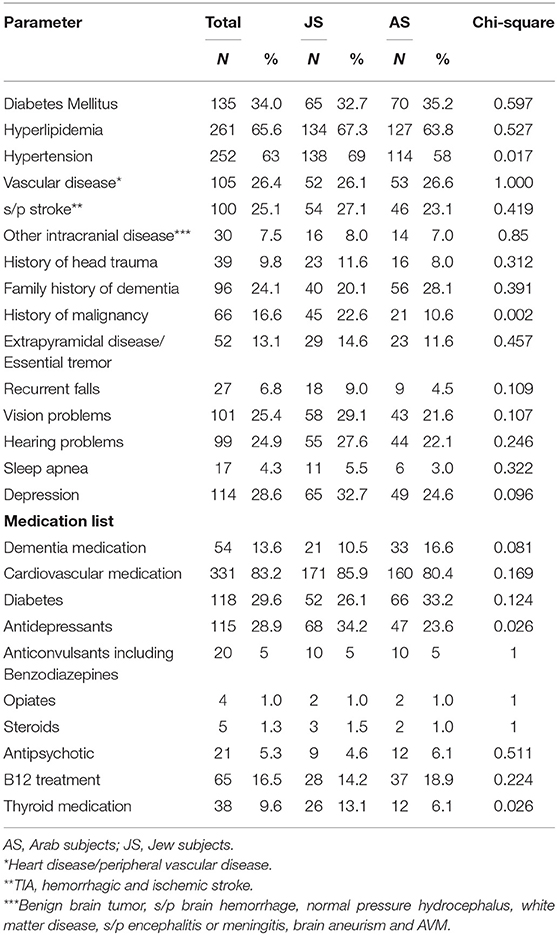
Past medical diagnoses and prescribed medications as written in the referral are described in Table 2. Vascular risk factors, family history of dementia, history of previous head injury and a background extrapyramidal disease did not differ between the groups. Hypertension diagnosis was more prevalent in JS (58% AS, 69% JS; p = 0.017). Background depression (based on medical records) was more prevalent in JS, although not significantly (25% AS, 33% JS; p = 0.096). History of malignancy was more prevalent in the Jewish population (11% AS, 23% JS; p = 0.002). JS used more antidepressants (24% AS, 34% JS; p = 0.026) and thyroid (6% AS, 13% JS; p = 0.026) medications.
Sixty-three AS (32%) and 46 JS (23%) were diagnosed with AD, 29 AS (15%) and 15 JS (8%) were diagnosed with VD. Twenty-three AS (12%) and 41 JS (21%) were diagnosed with MCI. Thirty-seven (19%) AS and 40 (20%) JS were diagnosed with MD. Normal cognition was diagnosed in three AS (2%) and 18 JS (9%). Frontotemporal dementia (FTD) was diagnosed in 0.5% AS and 3% JS. Pseudo-dementia was more prevalent in AS (6.5% AS; 0.5% JS). Dementia associated with extrapyramidal disease was diagnosed in 5 AS and 4 JS. The diagnosis could not be established at the initial visit in 11%. These subjects were referred for further evaluations.
Following initial recommendations, 90 (45%) AS and 96 (48%) JS returned to at least one follow-up visit; 25% AS and 25% JS returned to recurrent (≥2) follow-up visits; mean number of follow up visits was 1.15 ± 1.8 for AS and 1.2 ± 2 for JS, p = 0.9. Two months to 10 years elapsed between the first to last visit; mean follow up was 2.58 ± 1.7 years for AS and 2.88 ± 2.38 years for JS; p = 1. Ninety-five percent of subjects followed the prescribed treatment.
During the last follow-up visit, normal cognition was recorded in four (4%) JS and no AS. Fifty-three AS (59%) and 39 JS (41%) were diagnosed with AD, three AS (3%) and 15 (16%) JS were diagnosed with MCI, 3% AS and 3% JS were found to have VD. Similar rates of MD were found in both groups; 19% AS and 23% JS (Table 3). Diagnosis was still deferred in 3% of subjects. Compared to the first visit, patients had similar rates of MD and two times higher rates of AD in both ethnicities. Normal cognition, MCI and VD were less prevalent on the last visits.
Discussion
Despite the fact that the Arab population constitutes 37% of northern Israel residents 55 years and older and 17% of the Haifa district residents (23), AS constituted only 7.8% of the subjects that were evaluated in the CNIR.
In Israel, evaluations for cognitive deterioration within tertiary dementia clinics are only partially reimbursed by the national health care providers. According to the Israeli bureau of statistics, Arab salaried employees earn about 65% less than their Jewish counterparts (11). Accordingly, Arab households spend 42% less money on supplementary healthcare insurance than do Jewish ones (24). Indeed, prior studies support a correlation between lower income and self-reported health in the Israeli Arab population (25). Therefore, the poorer financial status of AS may partially account for their underrepresentation in the clinic.
An additional cause for the under-representation of AS may be ethnicity dependent beliefs regarding dementia. AD may be associated with futility and stigma regarding “mental diseases and dementia” which may impede help seeking (26). Previous research exploring stigma among caregivers of persons with AD in Israel report pronounced stigma in Arab compared to Jewish population and suggest that a lower level of education may account for this difference (27). Also, prior works suggest that ethnic minorities often perceive cognitive decline as part of normal aging, delaying seeking medical help (28).
A low referral rate of ethnic minorities to cognitive clinics was shown in previous works (29); this may be another reason for AS underrepresentation and stand as an important intervention point.
To adjust for the imbalance in the number of subjects attending our clinic, we evaluated AS and immediately consecutive JS dyads, as stated. AS differed significantly from JS with regard to demographic and clinical characteristics.
AS were younger at their first visit compared to JS (68.4 ± 8.8 vs. 74.3 ± 9.3; p < 0.0001), nevertheless, they were referred for evaluation when they already reported functionally impairment and had a lower MMSE (Table 1). Similar findings were reported in Asian and black minorities residing in London, UK (30) and in the Latino minority in Philadelphia (31).
AS had a higher rate of AD (32 vs. 23% in JS) and of VaD (15 vs. 8% in JS). JS were more frequently diagnosed with normal cognition (2% in AS; 9% in JS) and with MCI (12% in AS; 21% in JS). This data correlates with previous studies that show higher rates of AD and VaD in the Arab population aged ≥60 (5, 6, 32).
A systematic review and meta- analysis found that, worldwide, people from minority ethnic groups with dementia access healthcare services in later stages of their illness (33). Our study supports this finding. Although our study did not show any differences in the duration of the reported symptoms between the onset of cognitive complaints and the first CNIR visit (2.5 ± 3 in AS, 2.4 ± 2.7 in JS), this value may be under-reported. This is supported by low rates of MCI, lower MMSE and higher rates of functional impairment documented on the first visit in AS in our study.
Other reasons for similar duration of symptoms despite poorer cognitive performance may be lower basic MMSE and a higher pace of cognitive decline in AS populations.
Higher AD prevalence and younger age at presentation of AS may have several reasons.
Alzheimer's disease is frequently heritable (34) and high rates of consanguineous marriages are reported between Israel Arabs (9, 35).
Compared to JS, AS had fewer schooling years (Table 1). Lower formal education in the Arab population in Israel was reported previously and is thought to reflect socio-demographic and ethno-religious elements (11, 36). Lower mean years of formal education tend to characterize members of minority groups compared to the majority group and were associated with AD in previous studies (37) and may partially explain higher AD rates in Arab population (8).
Vascular risk factors, such as diabetes and hypertension, are commonly associated with an increased risk for AD and dementia (38, 39). In the present study, the prevalence of diabetes was similar between AS and JS (Table 2), but, according to previous work, diabetes prevalence is higher in the Arab population (40). Similarly, hypertension was less prevalent in AS (Table 2), although poor blood pressure control was previously reported in the Arab population in Israel (41).
Depression is also regarded as a risk factor for AD (42, 43). Kaplan et al. (44) found depression rates 2.5 times higher in the Arab population compared to the Jewish population in Israel. In the present study, clinical, co-morbid diagnosis of depression was similar in both populations, while antidepressant use was higher in JS. As we collected community diagnoses, a possible reason for the discrepancy between our data and previous data is under-diagnosis and/or under-treatment of depression in ethnic minorities (45–47).
Interestingly, pseudo-dementia was diagnosed far more commonly in AS (13 vs. 1). Eleven of those AS patients had a previous depression diagnosis and nine were treated medically for depression. Somatization disorders in general are more frequently found in the Arab society (48, 49) and due to cultural and religious elements depression particularly tends to present with somatic complaints in Arab society (50, 51).
The prevalence of dementia in our study was similar to prior works. First visit diagnosis was AD in 27% of subjects, VaD in 11% and MD in 19%. Previous pathological and clinical studies in elderly onset dementia report AD in 20–40% of patients (52–54), VaD in 7.5–24% (53, 54), and MD in 13–40% (16, 54). During follow-up visits AD diagnosis was more frequent than in the first visit (50%), probably as MCI patients converted to AD and patients with deferred diagnosis were diagnosed with AD.
Our study showed a higher rate of past malignancy diagnosis in JS (21 AS, 45 JS). A higher rate of malignancy in the Jewish compared to the Arab population is consistent with the Israel cancer register (55) and may be due to differences in diet, genetic factors, the lower mean age of the Arab population and the more prevalent urban way of life of the Jewish population (56).
A low aMMSE (<15) was more prevalent in patients with behavioral and functional complaints. The presence of cognitive complaints was not associated with lower scores on the aMMSE. Prior studies did not show any difference in the number of cognitive complaints between MCI and dementia patients (57). Memory complaints were more common in mild-moderate dementia than in severe dementia (58). The presence of cognitive complaints usually does not correlate with dementia severity as those complaints usually evolve in the early stages of MCI and dementia and are the basic criteria for MCI and dementia diagnosis. As dementia progresses it usually affects more behavioral aspects (59) and functional abilities, correlating in our study with low aMMSE.
Although Arab patients are underrepresented in our study and may arrive at the tertiary clinic with more advanced disease, they have the same rate of compliance with treatment and continue follow up as the Jewish population. This good compliance with treatment and follow-up may reinforce the importance of creating public policies to bring Arab patients to the clinics earlier.
To the best of our knowledge, our study is the first that directly compares prevalence and characteristics of dementia in the two main ethnic groups in Israel (8). Its limitation is that it is retrospective and descriptive in nature, raising the need for future prospective comparative research.
In summary, our study reveals that AS arrive for evaluation younger but already report functional impairment and have lower MMSE, suggesting that AS under-representation in the clinic most probably reflects lower usage of this medical service. Reasons can be poorer awareness of dementia and lower referral rate, lower socioeconomic status and educational level and poorer control of risk factors in this population.
The importance of this study is in planning preventive measures and future interventions in order to reduce the disparity between ethnicities. Proactive educational interventions in the Arab community along with raising awareness about the importance of memory clinics among family physicians, district neurologists and the population itself, should be considered.
Data Availability Statement
The datasets presented in this article are not readily available because the data has been sufficiently de-identified and is available upon request. Requests to access the datasets should be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Boards of Rambam Health Care Campus (# 0680-19-RMB). Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PS and JA conceived the research idea. PS, RB, NY, and JA performed evaluations of the subjects in the Rambam cognitive clinic. PS collected retrospect information about the clinic visits, prior diagnosis, treatment and follow ups and wrote the manuscript in consultation with JA and TF. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M. World Alzheimer's report 2015: the global impact of dementia. Alzheimer's Disease International (2015).
2. Phillipson L, Magee C, Jones S, Reis S, Skladzien E. Dementia attitudes and help-seeking intentions: an investigation of responses to two scenarios of an experience of the early signs of dementia. Aging Ment Heal. (2015) 19:968–77. doi: 10.1080/13607863.2014.995588
3. Smith K, Flicker L, Lautenschlager N, Almeida OP, Atkinson D, Dwyer A. High prevalence of dementia and cognitive impairment in Indigenous Australians. Neurology. (2008) 71:1470–3. doi: 10.1212/01.wnl.0000320508.11013.4f
4. Bowes A, Wilkinson H. “We didn't know it would get that bad”: South Asian experiences of dementia and the service response. Health Soc. Care Commun. (2003) 11:387–97. doi: 10.1046/j.1365-2524.2003.00440.x
5. Bowirrat A, Friedland RP, Farrer L, Baldwin C, Korczyn A. Genetic and environmental risk factors for alzheimer's disease in israeli arabs. J Mol Neurosci. (2002) 19:239–45. doi: 10.1007/s12031-002-0040-4
6. Afgin AE, Massarwa M, Schechtman E, Israeli-Korn SD, Strugatsky R, Abuful A, et al. High prevalence of mild cognitive impairment and alzheimer's disease in arabic villages in Northern Israel: impact of gender and education. J Alzheimers Dis. (2012) 29:431–9. doi: 10.3233/JAD-2011-111667
7. Korczyn AD, Kahana E, Galper Y. Epidemiology of dementia in Ashkelon, Israel. Neuroepidemiology. (1991) 10:424–8.
8. Werner P, Friedland RP, Inzelberg R. Alzheimer's disease and the elderly in Israel: are we paying enough attention to the topic in the arab population? Am J Alzheimers Dis Other Demen. (2015) 30:448–53. doi: 10.1177/1533317515577130
9. Vardi-Saliternik R, Friedlander Y, Cohen T. Consaguinity in a population sample of Israel Muslim Arabs, Christian Arabs and Druze. Ann Hum Biol. (2002) 29:422–31. doi: 10.1080/03014460110100928
10. Farrer LA, Bowirrat A, Friedland RP, Waraska K, Korczyn AD BC. Identification of multiple loci for Alzheimer's disease in a consanguineous Israeli-Arab community. Hum Mol Genet. (2003) 12:415–22. doi: 10.1093/hmg/ddg037
11. Gharrah Ramsees. Arab Society in Israel (7) Population, Society, Economy. Jerusalem: Van Leer Institute Press (2015).
12. Mateos P. A review of name-based ethnicity classification methods and their potential in population studies. Popul Space Place. (2007) 13:243–63. doi: 10.1002/psp.457
13. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
14. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
15. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:2672–713. doi: 10.1161/STR.0b013e3182299496
16. Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. Prog Geriatr. (2002) 50:1431–8. doi: 10.1046/j.1532-5415.2002.50367.x
17. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. (2011) 76:1006–14. doi: 10.1212/WNL.0b013e31821103e6
18. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
19. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. (2007) 22:1689–707. doi: 10.1002/mds.21507
20. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. (2017) 89:88–100. doi: 10.1212/WNL.0000000000004058
21. Kang H, Zhao F, You L, Giorgetta C, D V, Sarkhel S, et al. Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. (2014) 17:147–54. doi: 10.4103/0972-2327.132613
23. CBC Central Bureau of Statistics. Population, by Population Group, Religion, Age and Sex, District and Sub-district. Jerusalem (2017) 2.19.
24. Hayan I, Kornilenko I, Hertel D, Georgi M, Rotem N. Religion and Self-Definition of Level of Religiosity. In: Society in Israel. 10th ed. Central Bureau of Statistics (Israel). (2016). p. 228–79.
25. Baron-Epel O, Weinstein R, Haviv-Mesika A, Garty-Sandalon N, Green MS. Individual-level analysis of social capital and health: a comparison of Arab and Jewish Israelis. Soc Sci Med. (2008) 66:900–10. doi: 10.1016/j.socscimed.2007.10.025
26. Xiao LD, Habel L, De Bellis A. Perceived challenges in dementia care by Vietnamese family caregivers and care workers in South Australia. J Cross Cult Gerontol. (2015) 30:333–52. doi: 10.1007/s10823-015-9264-y
27. Werner P, AboJabel H. Who internalizes courtesy stigma and how? A study among Israeli Arab family caregivers of persons with dementia. Aging Ment Heal. (2019) 0:1–8. doi: 10.1080/13607863.2019.1584790
28. Gray HL, Jimenez DE, Cucciare MA, Tong HQ, Gallagher-Thompson D. Ethnic differences in beliefs regarding alzheimer disease among dementia family caregivers. Am J Geriatr Psychiatry. (2009) 17:925–33. doi: 10.1097/JGP.0b013e3181ad4f3c
29. Subramaniam H, Mukaetova-Ladinska EB, Wilson A, Bankart J. Representation of Black, Asian and minority ethnic patients in secondary care mental health services: analysis of 7-year access to memory services in Leicester and Leicestershire. BJPsych Bull. (2020) 44:145–152. doi: 10.1192/bjb.2020.3
30. Mukadam N, Lewis G, Mueller C, Werbeloff N, Stewart R, Livingston G. Ethnic differences in cognition and age in people diagnosed with dementia: a study of electronic health records in two large mental healthcare providers. Int J Geriatr Psychiatry. (2019) 34:504–10. doi: 10.1002/gps.5046
31. Livney MG, Clark CM, Karlawish JH, Cartmell S, Negrón M, Nuñez J. Ethnoracial Differences in the Clinical Characteristics of Alzheimer Disease at Initial Presentation at an Urban Alzheimer's Disease Center. Am J Geriatr Psychiatry. (2011) 19:430–9. doi: 10.1097/JGP.0b013e3181f7d881
32. Bowirrat A, Treves TA, Friedland RP, Korczyn AD. Prevalence of Alzheimer's type dementia in an elderly Arab population. Eur J Neurol. (2001) 8:119–23. doi: 10.1046/j.1468-1331.2001.00183.x
33. Cooper C, Tandy A, Balamurali T, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. (2010) 18:193–203. doi: 10.1097/JGP.0b013e3181bf9caf
34. Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. (2006) 63:168–74. doi: 10.1001/archpsyc.63.2.168
35. Sherva R, Baldwin C, Inzelberg R, Vardarajan B, Cupples LA, Lunetta K, et al. Identification of novel candidate genes for Alzheimer's disease by autosygocity mapping using genome wide SNP data from an Israeli-Arab community. J Alzheimers Dis. (2011) 23:349–59. doi: 10.3233/JAD-2010-100714
36. Okun BS, Friedlander D. Educational stratification among Arabs and Jews in Israel: historical disadvantage, discrimination, and opportunity. Popul Stud. (2005) 59:163–80. doi: 10.1080/00324720500099405
37. Chin A, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer's. Alzheimers Dis Assoc Disord. (2011) 25:187–95. doi: 10.1097/WAD.0b013e318211c6c9
38. Carnevale D, Perrotta M, Lembo G, Trimarco B. Pathophysiological links among hypertension and Alzheimer's disease. High Blood Press Cardiovasc Prev. (2016) 23:3–7. doi: 10.1007/s40292-015-0108-1
39. Shinohara M, Sato N. Bidirectional interactions between diabetes and Alzheimer's disease. Neurochem Int. (2017) 108:296–302. doi: 10.1016/j.neuint.2017.04.020
40. Jaffe A, Giveon S, Wulffhart L, Oberman B, Baidousi M, Ziv A, et al. Adult Arabs have higher risk for diabetes mellitus than Jews in Israel. PLoS ONE. (2017) 12:e0176661. doi: 10.1371/journal.pone.0176661
41. Abu-Saad K, Chetrit A, Eilat-Adar S, Alpert G, Atamna A, Gillon-Keren M, et al. Blood pressure level and hypertension awareness and control differ by marital status, sex, and ethnicity: a population-based study. Am J Hypertens. (2014) 27:1511–20. doi: 10.1093/ajh/hpu081
42. Wiels W, Baeken C, Engelborghs S. Depressive symptoms in the elderly-an early symptom of dementia? A systematic review. Front Pharmacol. (2020) 11:34. doi: 10.3389/fphar.2020.00034
43. Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer's disease: the function of AQP4 and the glymphatic system. Psychopharmacol. (2017) 234:365–79. doi: 10.1007/s00213-016-4473-9
44. Kaplan G, Glasser S, Murad H, Atamna A, Alpert G, Goldbourt U, et al. Depression among Arabs and Jews in Israel: a population-based study. Soc Psychiatry Psychiatr Epidemiol. (2010) 45:931–9. doi: 10.1007/s00127-009-0142-1
45. Shao Z, Richie W, Bailey R. Racial and ethnic disparity in major depressive disorder. J Racial Ethn Heal Disparities. (2016) 3:692–705. doi: 10.1007/s40615-015-0188-6
46. Pickett Y, Weissman J, Bruce M. Racial differences in antidepressant use among older home health care patients. Psychiatr Serv. (2012) 63:827–9. doi: 10.1176/appi.ps.201100233
47. Bailey R, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. (2019) 15:603–9. doi: 10.2147/NDT.S128584
48. El-Rufaie OE, Al-Sabosy MM, Bener A, Abuzeid MS. Somatized mental disorder among primary care arab patients. J Psychosom Res. (1999) 46:549–55. doi: 10.1016/S0022-3999(98)00101-9
49. Abu-Kaf S, Shahar G. Depression and somatic symptoms among two ethnic groups in Israel: testing three theoretical models. Isr J Psychiatry. (2017) 54:32–40.
50. Deisenhammer E, Coban-Başaran M, Mantar A, et al. Ethnic and migrational impact on the clinical manifestation of depression. Soc Psychiatry Psychiatr Epidemiol. (2012) 47:1121–9. doi: 10.1007/s00127-011-0417-1
51. Hamdi E, Amin Y, Abou-Saleh MT. Problems in validating endogenous depression in the Arab culture by contemporary diagnostic criteria. J Affect Disord. (1997) 44:131–43. doi: 10.1016/S0165-0327(97)00037-2
52. Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer's disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. (2018) 15:1–7. doi: 10.1016/j.jalz.2018.07.216
53. Stevens T, Livingston G, Kitchen G, Manela M, Walker Z, Katona C. Islington study of dementia subtypes in the community. Br J Psychiatry. (2002) 180:270–6. doi: 10.1192/bjp.180.3.270
54. Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly–An update. J Alzheimers Dis. (2006) 9:61–70. doi: 10.3233/JAD-2006-9S308
56. Miha B. Tendency in incidence of malignant diseases in Israel. Review. (2002) 6. Available online at: https://www.tnuva.co.il/Child_Nutrition/%D7%9E%D7%9B%D7%95%D7%9F-%D7%AA%D7%A0%D7%95%D7%91%D7%94-%D7%9C%D7%9E%D7%97%D7%A7%D7%A8/review/%D7%92%D7%99%D7%9C%D7%99%D7%95%D7%9F-6-%D7%AA%D7%96%D7%95%D7%A0%D7%94-%D7%95%D7%A1%D7%A8%D7%98%D7%9F
57. Salem LC, Vogel A, Ebstrup J, Linneberg A, Waldemar G. Subjective cognitive complaints included in diagnostic evaluation of dementia helps accurate diagnosis in a mixed memory clinic cohort. Int J Geriatric Psychiatry. (2015) 30:1177–85. doi: 10.1002/gps.4272
58. Grut M, Jorm AF, Fratiglioni L, Forsell Y, Viitanen M, Winblad B. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J Am Geriatr Soc. (1993) 41:1295–300. doi: 10.1111/j.1532-5415.1993.tb06478.x
Keywords: dementia, cognitive, ethnicity, disparities, cohort, epidemiology, Arab, Jew
Citation: Specktor P, Ben Hayun R, Yarovinsky N, Fisher T and Aharon Peretz J (2021) Ethnic Differences in Attending a Tertiary Dementia Clinic in Israel. Front. Neurol. 11:578068. doi: 10.3389/fneur.2020.578068
Received: 30 June 2020; Accepted: 10 December 2020;
Published: 13 January 2021.
Edited by:
Suvarna Alladi, Nizam's Institute of Medical Sciences, IndiaReviewed by:
Elisa De Paula Franca Resende, Federal University of Minas Gerais, BrazilJennifer A. Deal, Johns Hopkins University, United States
Copyright © 2021 Specktor, Ben Hayun, Yarovinsky, Fisher and Aharon Peretz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Polina Specktor, cG9seV9zcGVja3RvciYjeDAwMDQwO3lhaG9vLmNvbQ==
 Polina Specktor
Polina Specktor Rachel Ben Hayun
Rachel Ben Hayun Natalia Yarovinsky
Natalia Yarovinsky Tali Fisher
Tali Fisher Judith Aharon Peretz
Judith Aharon Peretz