- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
Objective: To determine the relationship between stress hyperglycemia and prognosis of acute ischemic stroke people with and without diabetes.
Methods: A subgroup of 8,622 acute ischemic stroke people with baseline levels of fasting blood glucose and HbA1c from the China National Stroke Registry II were analyzed. Stress hyperglycemia was measured by glucose/glycated hemoglobin (HbA1c) ratio, calculated by fasting blood glucose divided by HbA1c. Diabetes was diagnosed according to medical history or a HbA1c level of ≥6.5%. The outcomes included severe neurological deficit defined as modified Rankin Scale score of 3 to 5 and all-cause death at 1 year. The associations between glucose/HbA1c ratio and neurological deficit and all-cause death were analyzed via logistic regression model and Cox proportional-hazards model, respectively. Subgroup analyses of participants with or without diabetes were performed separately.
Results: Totally 1,189 (13.7%) participants had severe neurological deficit, and 678 (7.9%) died within 1 year. Compared with the lowest quartile, the highest quartiles of glucose/HbA1c ratio were associated with elevated risk of worse neurological deficit (20.1% vs. 13.0%; adjusted OR, 1.83; 95%CI, 1.31–2.54, p = 0.001), and mortality (12.1% vs. 6.6%; adjusted HR, 2.04; 95% CI, 1.47–2.83, p < 0.0001) after adjusted for potential covariates. The association of glucose/HbA1c ratio with neurological deficit remained in the participants with and without diabetes, while it was only significant in the participants without diabetes as for the outcome of mortality.
Conclusions: Stress hyperglycemia, measured by glucose/HbA1c ratio, was associated with increased risk of severe neurological deficit and mortality within 1 year in the acute ischemic stroke people.
Introduction
Stress hyperglycemia is the relative transient increase in glucose when an acute stress such as stroke occurred (1). It is present in approximately half of people with acute ischemic stroke (2, 3). Some (3) but not all (4) prior studies indicated acute stress hyperglycemia predicted increased risk of mortality and poor functional outcome after ischemic stroke. Most previous studies defined stress hyperglycemia according to absolute glucose level, including the fasting and random glucose levels, without considering the background glucose concentration, although they were analyzed by status of diabetes (3, 4). Glycated hemoglobin (HbA1c) is a well-validated marker for glucose control over 2–3 months, reflecting the background glucose level before the event (5). Recent studies demonstrated that relative hyperglycemia, defined as glucose/HbA1c ratio or admission glucose divided by estimated average glucose derived from HbA1c, might be a more reliable method to measure the degree of stress hyperglycemia and a better predictor for outcomes of acute illness than absolute hyperglycemia (6–8). Our recent study showed that relative hyperglycemia of glucose/HbA1c ratio was related to mortality in the ischemic stroke people without diabetes (9). However, its association with neurological functional disability is still undefined. Furthermore, people with diabetes could also develop stress hyperglycemia, which has been overlooked in many previous studies (1). In this study, we therefore aimed to investigate the relationship between stress hyperglycemia, measured by glucose/HbA1c ratio, and outcomes of acute ischemic stroke people with and without diabetes in a large stroke registry study in China.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Site Selection
The cohort from China National Stroke Registry II (CNSR II) was used in this study. As described previously (10), the CNSR II was the nationwide initiative to establish a reliable national stroke database for assessing the delivery of stroke care in clinical practice and to identify areas that needed further improvement compared with CNSR I in 2007 (11). The criteria for site selection in CNSR II were similar as CNSR I (11), briefly including: (1) having at least one stroke neurologist; (2) at least two hospitals included from each of the 31 provinces and municipalities in mainland China; (3) participating voluntarily; and (4) ability to perform research (11). A total of 219 hospitals participated in CNSR II. The study was approved by the central Institutional Review Board at Beijing Tiantan Hospital. All participants or their legal proxies provided signed informed consent before enrollment.
Study Participants
Participants were consecutively recruited from centers from June 2012 to January 2013, if meeting the following criteria: (1) age>18 years; (2) diagnosis within 7 days of the index event, including ischemic stroke, TIA, spontaneous intracerebral hemorrhage, or subarachnoid hemorrhage; (3) direct hospital admission from the emergency department or physician's clinic; and (4) informed consent provided by the participant or legally authorized representative. Specifically, ischemic stroke was diagnosed according to the World Health Organization criteria combined with brain computed tomography (CT) or magnetic resonance imaging (MRI) confirmation (12). Of 25,018 participants with acute cerebrovascular events in CNSR II, 19,604 were diagnosed with acute ischemic stroke. Among these participants, 8,622 with baseline levels of fasting blood glucose and HbA1c were included (Figure 1 and Supplementary Table 1). Participants with diabetes were diagnosed according to a prior history of diabetes or a HbA1c level of ≥6.5% (13).
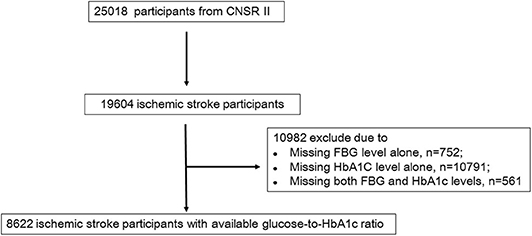
Figure 1. Flow chart showing the participant selection; CNSR, China National Stroke Registry; FBG, Fasting blood glucose.
Data Collection
Trained research coordinators at each hospital collected clinical data including demographics, detailed medical history, prestroke mRS, vascular risk factors, stroke severity, laboratory tests, treatments, complication, cerebrovascular events during hospitalization, and discharge status. Demographic variables included age and sex. The vascular risk factors included cigarette smoking, and medical history of hypertension, diabetes, hypercholesterolemia, ischemic stroke, TIA, myocardial infarction, heart failure and atrial fibrillation. Stroke severity was evaluated using National Institutes of Health Stroke Scale (NIHSS) score by physicians (14). Levels of fasting blood glucose, HbA1c, low-density lipoprotein cholesterol (LDL_C), triglyceride and high-sensitive C-reactive protein (hsCRP) on admission were extracted from medical records. All participants received routine stroke care according to their conditions.
Follow-Up and Outcomes
The detailed follow-up procedure has been previously described (15). In brief, participants were followed up by telephone interview at 3, 6, and 12 months after stroke onset by trained research personals blinded to participant information, following standard scripts to collect information on stroke-related disability and all-cause death. Totally 960 (11.1%) patients were lost at 1 year. The outcomes included severe neurological deficit defined as modified Rankin Scale (mRS) score of 3–5 and all-cause death at 1 year.
Statistical Analysis
Categorical variables were presented as percentages and continuous variables as medians with interquartile ranges. Baseline characteristics were analyzed by x2 statistics for the categorical variables and Kruskal-Wallis test for the continuous variables.
The glucose/HbA1c ratio was calculated by fasting blood glucose (mmol/L) divided by HbA1c (%) (6). The associations between glucose/HbA1c ratio and neurological deficit were analyzed by using logistic regression model, and the Cox proportional-hazards model was used for analyzing the association with mortality. Kaplan–Meier survival curve was applied to depict the occurrence of mortality and analyzed using the log-rank univariate test. Inflammatory response maybe a causative factor leading to high glucose level associated with poor outcomes in the patients with acute ischemic stroke, apart from stress. We therefore included demographic factors, prior published traditional or clinical risk factors and levels of hsCRP in the multivariate model. Hazard ratios (HRs) or Odd ratios (ORs) and their 95% confidence intervals (CIs) were calculated. A 2-sided P < 0.05 was considered to indicate statistical significance. SAS software, version 9.4 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
Result
Participants Characteristics
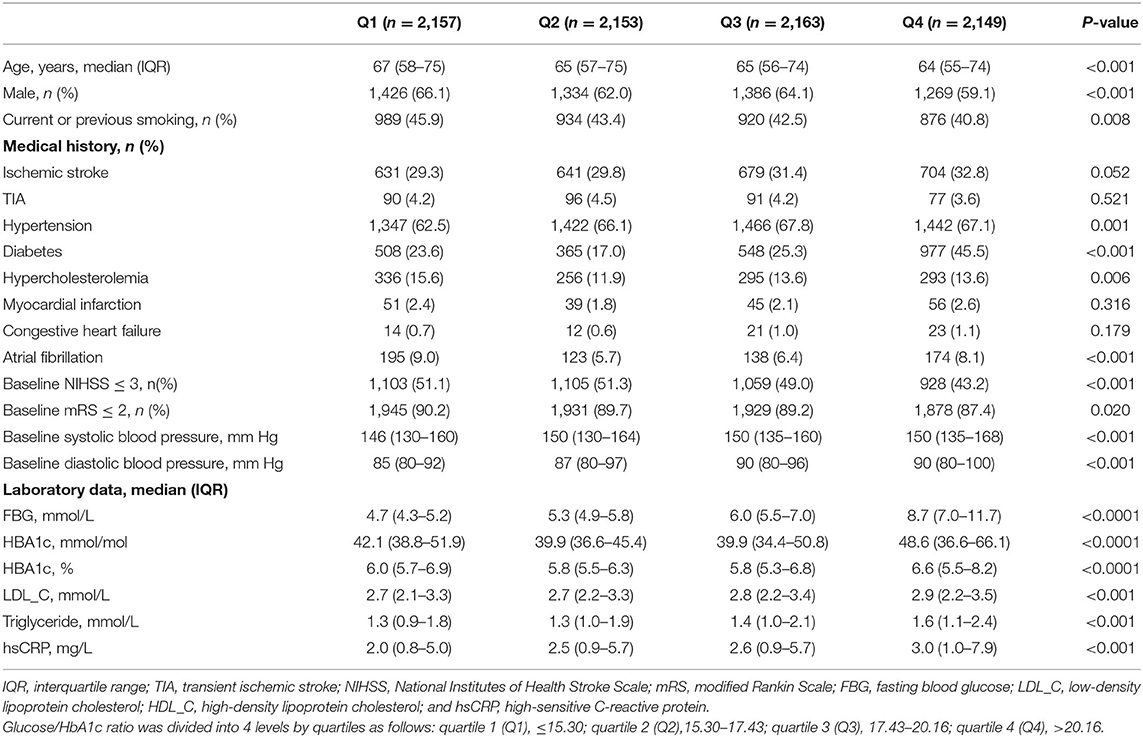
Among 8,622 participants included in the final study, 2,398 (27.8%) had prior diabetes, 2984 (34.6%) had a HbA1c level of ≥6.5% and 3488 (40.5%) had either a history of diabetes or a HbA1c level of ≥6.5%. The medians of fasting blood glucose and HbA1c for all participants, participants with diabetes and participants without diabetes were 5.7 mmol/L (interquartile, 5.0–7.4 mmol/L) and 42.1 mmol/mol (6.0%) [interquartile, 36.6–53.0 mmol/mol (5.5–7.0%)], 7.8 mmol/L (interquartile, 6.1–10.3 mmol/L) and 58.5 mmol/mol (7.5%) [interquartile, 49.7–74.9 mmol/mol (6.7–9.0%)], and 5.2 mmol/L (interquartile, 4.7–5.8 mmol/L) and 37.7 mmol/mol (5.6%) [interquartile, 34.4–41.0 mmol/mol (5.3–5.9%)], respectively. The baseline characteristics of participants by quartiles of glucose/HbA1c ratio were shown in Table 1. Participants with higher glucose/HbA1c ratio were significantly younger, female, non-smokers, and had histories of hypertension and diabetes but not hypercholesterolemia and atrial fibrillation, higher baseline NIHSS and mRS scores, higher systolic/diastolic blood pressure, and higher levels of fasting blood glucose, HbA1c, LDL_C, triglyceride, and hsCRP (Table 1).
Associations of Glucose/HbA1c Ratio With Outcomes
A total of 1189 (13.7%) participants had severe neurological deficit (mRS, 3–5), and 678 (7.9%) deaths occurred within 1 year. Compared with the lowest quartile, the third (19.0% vs. 13.0%; adjusted OR, 1.68; 95% CI, 1.24–2.26; p = 0.001) and highest (20.1% vs. 13.0%; adjusted OR, 1.83; 95% CI, 1.31–2.54, p = 0.001) quartiles of glucose/HbA1c ratio were independently associated with elevated risk of worse neurological deficit (Table 2).
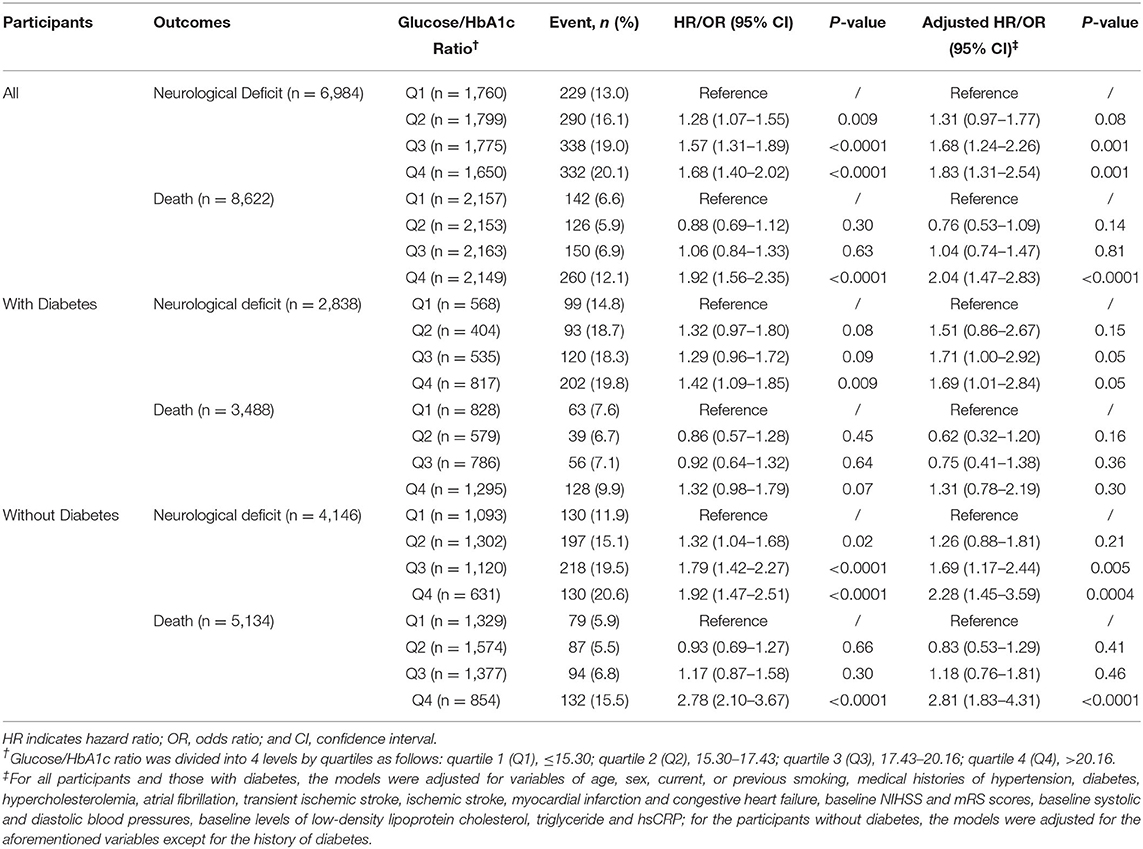
Table 2. Associations of Glucose/HbA1c Ratio with Neurological Deficit and All-cause Death at 1 year.
As for the outcome of mortality, participants in the highest quartiles of glucose/HbA1c ratio had around 2-fold of risk of mortality at 1 year than those in the lowest quartiles after fully adjusted for potential covariates (12.1% vs. 6.6%; adjusted HR, 2.04; 95% CI, 1.47–2.83, p < 0.0001) (Table 2 and Figure 2).
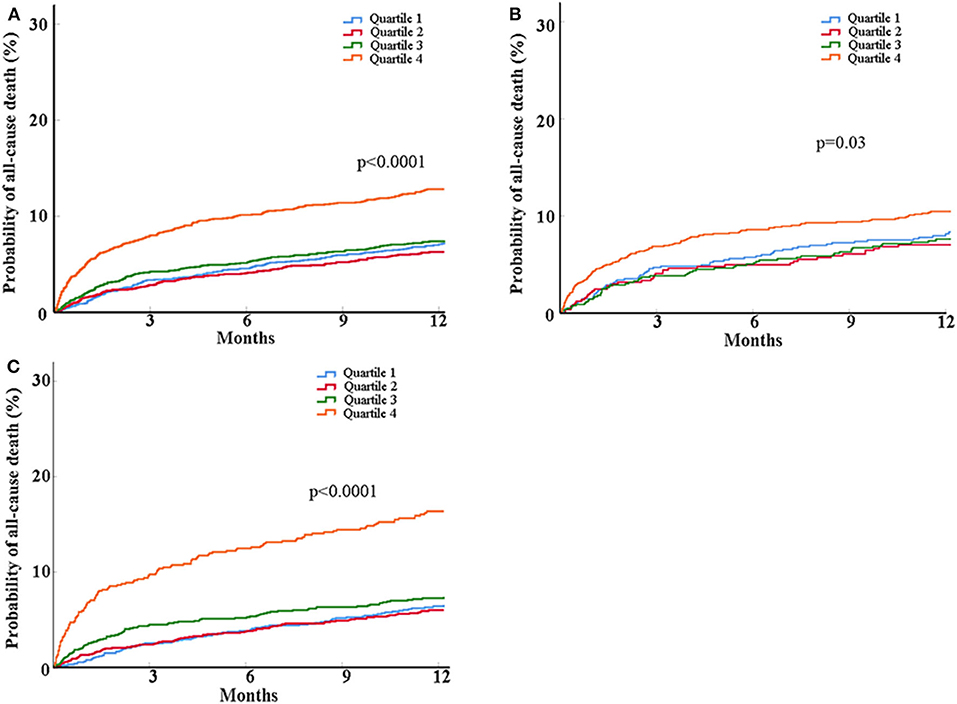
Figure 2. Kaplan-Meier curves for probability of all-cause death within 1 year in all patients (A) and the patients with (B) and without diabetes (C). Patients were classified into four groups based on glucose/HbA1c ratio as follows: quartile 1 (Q1), ≤15.30; quartile 2 (Q2), 15.30–17.43; quartile 3 (Q3), 17.43–20.16; quartile 4 (Q4), >20.16. Log-rank tests were performed and the p-values were presented.
Associations of Glucose/HbA1c Ratio With Outcomes in People With and Without Diabetes
When stratifying participants into those with and without diabetes, in the people with diabetes, the highest quartiles of glucose/HbA1c ratio was related to increased risk of poor neurological function recovery (adjusted OR, 1.69; 95% CI, 1.01–2.84, p = 0.047) but not mortality, compared with the lowest quartiles (Table 2 and Figure 2). In the people without diabetes, the associations of glucose/HbA1c ratio with neurological deficit (third vs. first quartiles of glucose/ HbA1c ratio: adjusted OR, 1.69; 95% CI, 1.17–2.44; p = 0.005; forth vs. first quartiles of glucose/HbA1c ratio: adjusted OR, 2.28; 95% CI, 1.45–3.59; p = 0.0004) and mortality (highest vs. lowest quartiles of glucose/HbA1c ratio: adjusted HR, 2.81; 95% CI, 1.83–4.31; p < 0.0001) were both significant in the crude and multivariate models (Table 2 and Figure 2).
Discussion
The major finding of this study is that relative stress hyperglycemia measured by Glucose/HbA1c ratio independently predicts severe neurological deficit within 1 year in the acute ischemic stroke people, irrespective of diabetes status. And its association with mortality at 1 year is more apparent in those people without diagnosed or underlying diabetes.
There was controversy regarding the association between stress hyperglycemia and prognosis of ischemic stroke. An early meta-analysis found that acute stress hyperglycemia was associated with an increased risk of in-hospital mortality and poor function recovery in ischemic stroke people (3). Other studies subsequently showed that hyperglycemia predicted worse functional outcome at 6-month (16) and 1 year (17) in the people with acute ischemic stroke. And Roquer et al. also reported that hyperglycemia was related with 3-month mortality in acute ischemic stroke people without diabetes or with diabetes and previous good glycemic control, but not in those with diabetes and previous poor glycemic control (18). However, a recent prospective study did not demonstrate significant relationship between stress hyperglycemia and risk of in-hospital mortality and poor functional outcome after stroke (4). Difference of study population and definition of stress hyperglycemia might partly account for these discrepancies. Besides that, the discordance of included pertinent confounding factors, such as stroke severity, might also affect the predictive value of stress hyperglycemia (4, 19, 20). On the other hand, stress hyperglycemia was usually diagnosed according to absolute hyperglycemia, excluding prior diabetes or preexisting diabetes with deterioration of premorbid glycemic control, which in fact might also have stress hyperglycemia (1, 3). Furthermore, this definition failed to differentiate stress hyperglycemia from newly diagnosed or underlying albeit undiagnosed diabetes and did not consider the background glucose concentration. Unlike absolutely hyperglycemia, relative measures of hyperglycemia, such as glucose/ HbA1c ratio, reflected the relative acute rapid increase of blood glucose level compared with premorbid glucose status. Previous study showed that such relative measures controlling for background glycemia better predicted prognosis of critical illness than absolute hyperglycemia (6–8). We previously found that glucose/HbA1c ratio was related to all-cause death in people with acute ischemic stroke (9). In the current study, we further added evidence that glucose/HbA1c ratio could predict neurological deficit, in addition to mortality. In light of disability from stroke bringing huge personal and societal burden, early recognition and management of high-risk patients is of great importance. Blood markers are promising to aid in the risk stratification and individual treatment of ischemic stroke (21, 22). A line of evidence have shown that elevated blood glucose levels are associated with unfavorable outcome in the patients with ischemic stroke (3, 23–26). However, since hyperglycemia at the acute phage of ischemic stroke could be due to poor control of glucose in the diabetic patients, most prior studies excluded the diabetic patients (25, 26). People with diabetes could have stress hyperglycemia as well. Our study at least suggested a practical and economical measurement of stress hyperglycemia to predict the prognosis of stroke in the people with or without diabetes. Furthermore, taking background glucose into account has been emphasized in the current study base on the observation that for the patients with elevated fasting blood glucose, the prognosis might not be as poor as expected if accompany with high HbA1c. Our findings might shed some light on the individual glucose management in the acute ischemic stroke.
On the other hand, it has been observed that there is discordance in the associations of absolutely hyperglycemia with functional outcome and mortality between people with and without diabetes (3, 25, 26). Similarly, the relationship of higher glucose/HbA1c ratio with mortality was only apparent in the people without diabetes in our study. However, we did not find any difference in the predictive value of stress hyperglycemia in neurological function deficit between people with and without diabetes. The clear reasons for the discrepancy were still unknown. One possible explanation could be the difference in the definition of stress hyperglycemia as elaborated above. Secondarily, unlike majority of the previous studies, we diagnosed diabetes according to HbA1c levels in addition to prior history, being capable of differentiating people without diabetes from those with underlying but undiagnosed diabetes (25, 26). Thirdly, poor functional outcome was defined as a mRS score of >3 which included mortality in some study (25), which was different from ours as well.
Strengths of our study included a large sample size of ischemic stroke people with outcomes of interest such as functional disability and mortality, distinguishing people with underlying diabetes by using the measurement of HbA1c, as well as the inclusion of stroke severity when analyzing the association between stress hyperglycemia and outcomes. Nonetheless, some limitations should be noticed while interpreting our results. First, the major one was its retrospective design. Further randomized controlled trials are needed to confirm our findings. Second, participants with missing baseline fasting blood glucose or HbA1c levels were not included, so a selection bias might exist. Third, the infarct size was not available. Though it has been suggested that hyperglycemia contributed to the worsening of the initial infarct, irrespective of volume, and severity (25), debate continues as to if it is a contributing factor to the more severe stroke or merely a stress response to a larger infarct. Forth, the lack of exact cause of death impeded the understanding of the underlying causes for elevated glucose/HbA1c ratio. Fifth, as our cohort only comprised Chinese adult people with ischemic stroke, these results may not be generalizable to other races and ethnicities.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Central Institutional Review Board at Beijing Tiantan Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL and YoW conceptualized this work. YiW, XZ, ZL, HL, LL, and YoW supervised the study. JL, YP, and HL performed the statistical analysis. JL and KQ prepared the manuscript. YiW, XZ, ZL, and LL revised the manuscript. All authors approved the protocol.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81671128, 81870905, and 81825007), the Young Scientist Program of Beijing Tiantan Hospital (No. YSP201702), Beijing Municipal Administration of Hospitals' Youth Programme (QML20190502), the Ministry of Science and Technology of the People's Republic of China (2016YFC0901000, 2016YFC0901001, 2016YFC0901002, 2017YFC1310901, 2018YFC1311700, and 2018YFC1311706), and Beijing Municipal Commission of Health and Family Planning (No. 2016-1-2041, SML20150502).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all participating hospitals, physicians and nurses, and the China National Stroke Registry II Steering Committee members.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.576895/full#supplementary-material
References
1. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5
2. Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet. (1999) 353:376–7. doi: 10.1016/S0140-6736(05)74948-5
3. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
4. Tziomalos K, Dimitriou P, Bouziana SD, Spanou M, Kostaki S, Angelopoulou SM, et al. Stress hyperglycemia and acute ischemic stroke in-hospital outcome. Metabolism. (2017) 67:99–105. doi: 10.1016/j.metabol.2016.11.011
5. Di Angelantonio E, Gao P, Khan H, Butterworth AS, Wormser D, Kaptoge S, et al. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. (2014) 311:1225–33. doi: 10.1001/jama.2014.1873
6. Su YW, Hsu CY, Guo YW, Chen HS. Usefulness of the plasma glucose concentration-to-HbA1c ratio in predicting clinical outcomes during acute illness with extreme hyperglycaemia. Diabetes Metab. (2017) 43:40–7. doi: 10.1016/j.diabet.2016.07.036
7. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
8. Chen X, Liu Z, Miao J, Zheng W, Yang Q, Ye X, et al. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:1668–73. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.022
9. Zhu B, Pan Y, Jing J, Meng X, Zhao X, Liu L, et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front Neurol. (2019) 10:1003. doi: 10.3389/fneur.2019.01003
10. Yang X, Li Z, Zhao X, Wang C, Liu L, Wang C, et al. Use of warfarin at discharge among acute ischemic stroke patients with nonvalvular atrial fibrillation in China. Stroke. (2016) 47:464–70. doi: 10.1161/STROKEAHA.115.011833
11. Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. (2011) 6:355–61. doi: 10.1111/j.1747-4949.2011.00584.x
12. Stroke−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
13. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl. 1):S13–27. doi: 10.2337/dc18-S002
14. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
15. Gu HQ, Li ZX, Zhao XQ, Liu LP, Li H, Wang CJ, et al. Insurance status and 1-year outcomes of stroke and transient ischaemic attack: a registry-based cohort study in China. BMJ Open. (2018) 8:e021334. doi: 10.1136/bmjopen-2017-021334
16. Luitse MJA, van Seeters T, Horsch AD, Kool HA, Velthuis BK, Kappelle LJ, et al. Admission hyperglycaemia and cerebral perfusion deficits in acute ischaemic stroke. Cerebrovasc Dis. (2013) 35:163–7. doi: 10.1159/000346588
17. Ntaios G, Egli M, Faouzi M, Michel P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke. (2010) 41:2366–70. doi: 10.1161/STROKEAHA.110.592170
18. Roquer J, Giralt-Steinhauer E, Cerdà G, Rodríguez-Campello A, Cuadrado-Godia E, Jiménez-Conde J, et al. Glycated hemoglobin value combined with initial glucose levels for evaluating mortality risk in patients with ischemic stroke. Cerebrovasc Dis. (2015) 40:244–50. doi: 10.1159/000440735
19. Christensen H, Boysen G. Blood glucose increases early after stroke onset: a study on serial measurements of blood glucose in acute stroke. Eur J Neurol. (2002) 9:297–301. doi: 10.1046/j.1468-1331.2002.00409.x
20. Candelise L, Landi G, Orazio EN, Boccardi E. Prognostic significance of hyperglycemia in acute stroke. Arch Neurol. (1985) 42:661–3. doi: 10.1001/archneur.1985.04060070051014
21. Tu WJ, Dong X, Zhao SJ, Yang DG, Chen H. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol. (2013) 25:771–8. doi: 10.1111/jne.12052
22. Li J, Wang Y. Blood biomarkers in minor stroke and transient ischemic attack. Neurosci Bull. (2016). 32:463–8. doi: 10.1007/s12264-016-0038-5
23. Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. (1997) 314:1303–6. doi: 10.1136/bmj.314.7090.1303
24. Wada S, Yoshimura S, Inoue M, Matsuki T, Arihiro S, Koga M, et al. Outcome prediction in acute stroke patients by continuous glucose monitoring. J Am Heart Assoc. (2018) 7:e008744. doi: 10.1161/JAHA.118.008744
25. Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, et al. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. (2009) 10:181–6. doi: 10.1007/s12028-008-9080-0
Keywords: stroke, glucose, glycated hemoglobin A, prognosis, China
Citation: Li J, Quan K, Wang Y, Zhao X, Li Z, Pan Y, Li H, Liu L and Wang Y (2020) Effect of Stress Hyperglycemia on Neurological Deficit and Mortality in the Acute Ischemic Stroke People With and Without Diabetes. Front. Neurol. 11:576895. doi: 10.3389/fneur.2020.576895
Received: 27 June 2020; Accepted: 20 August 2020;
Published: 24 September 2020.
Edited by:
Ashfaq Shuaib, University of Alberta, CanadaReviewed by:
Wen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaChun-Feng Liu, Second Affiliated Hospital of Soochow University, China
Copyright © 2020 Li, Quan, Wang, Zhao, Li, Pan, Li, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjun Wang, eW9uZ2p1bndhbmdAbmNyY25kLm9yZy5jbg==
†These authors have contributed equally to this work
 Jiejie Li1,2,3,4†
Jiejie Li1,2,3,4† Kehua Quan
Kehua Quan Yilong Wang
Yilong Wang Xingquan Zhao
Xingquan Zhao Zixiao Li
Zixiao Li Yuesong Pan
Yuesong Pan Yongjun Wang
Yongjun Wang