
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 25 September 2020
Sec. Movement Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.576569
This article is part of the Research Topic Managing Parkinson's Disease with a Multidisciplinary Perspective View all 13 articles
Background: Parkinson's disease (PD) is now known to be a multisystemic heterogeneous neurodegenerative disease, including a wide spectrum of both motor and non-motor symptoms. PD patients' management must encompass a multidisciplinary approach to effectively address its complex nature. There are still challenges in terms of treating axial (gait, balance, posture, speech, and swallowing) and cognitive symptoms that typically arise with disease progression becoming poorly responsive to dopaminergic or surgical treatments.
Objective: The objectives of the study are to further establish the presentation of axial and cognitive symptoms in early PD [Hoehn and Yahr (H&Y) scale ≤ 2] and to discuss the evidence for non-pharmacological approaches in early PD.
Results: Mild and subtle changes in the investigated domains can be present even in early PD. Over the last 15 years, a few randomized clinical trials have been focused on these areas. Due to the low number of studies and the heterogeneity of the results, no definitive recommendations are possible. However, positive results have been obtained, with effective treatments being high-intensity treadmill and cueing for gait disturbances, high-intensity voice treatment, video-assisted swallowing therapy for dysphagia, and warm-up exercises and Wii FitTM training for cognition.
Conclusions: Considering the association of motor, speech, and cognitive function, future trials should focus on multidisciplinary approaches to combined non-pharmacological management. We highlight the need for a more unified approach in managing these “orphan” symptoms, from the very beginning of the disease. The concept “the sooner the better” should be applied to multidisciplinary non-pharmacological management in PD.
Parkinson's disease (PD) is a neurodegenerative disease characterized by a spectrum of both motor and non-motor symptoms. Effective management of patients with PD (PwPD) must encompass a multidisciplinary individualized approach (1–3). Medical management of PD has improved parkinsonian symptoms and quality of life (QoL) either through pharmacological or neurosurgical interventions (4). Nevertheless, there are still challenges in terms of treating the axial (gait, posture, balance, speech, and swallowing) and cognitive symptoms that typically arise with disease progression becoming poorly responsive to both dopaminergic and surgical treatments (5, 6). Particular attention should be paid to axial and cognitive disabilities, which are predictions of dependency development and mortality (7, 8). Identifying the extent to which these symptoms are present in early PD and treating these symptoms early could help reduce their burden in later disease stages. Indeed, the ideal time frame to apply a certain treatment is actually a topic of discussion, especially for trials on disease-modifying treatment (9). What seems to be clear is that if we apply a neuroprotective treatment in early/intermediate PD, it is too late, as the neurodegenerative process is too advanced (9). The same concept could be applied for non-pharmacological treatment for those troublesome symptoms that characterize the advanced PD stage; we need to elaborate and identify strategies that could prevent disabilities and act before their manifested appearance (10).
In this mini review, the presentation of axial and cognitive symptoms in early PD [i.e., Hoehn and Yahr scale (H&Y) ≤ 2] is reviewed, and the evidence for non-pharmacological treatments for these symptoms in early PD is discussed. Randomized clinical trials (RCTs) on non-pharmacological treatments and written in English from 2005 to 2020 were identified in MEDLINE. Non-invasive brain stimulation was not included in our search. The following search terms (and derivatives) that were used included Parkinson, early, treatment, falls, balance, posture, gait, speech, communication, voice, dysphagia, swallowing, cognition, dementia, and executive function. Relevant treatment studies were selected according to the flow diagram shown in Figure 1. Overall, 11 RCTs (7 studies for gait, one of which had balance as secondary outcome and 0 for posture, 1 for speech, 2 for swallowing, and 1 for cognition) were selected and reviewed (Table 1).
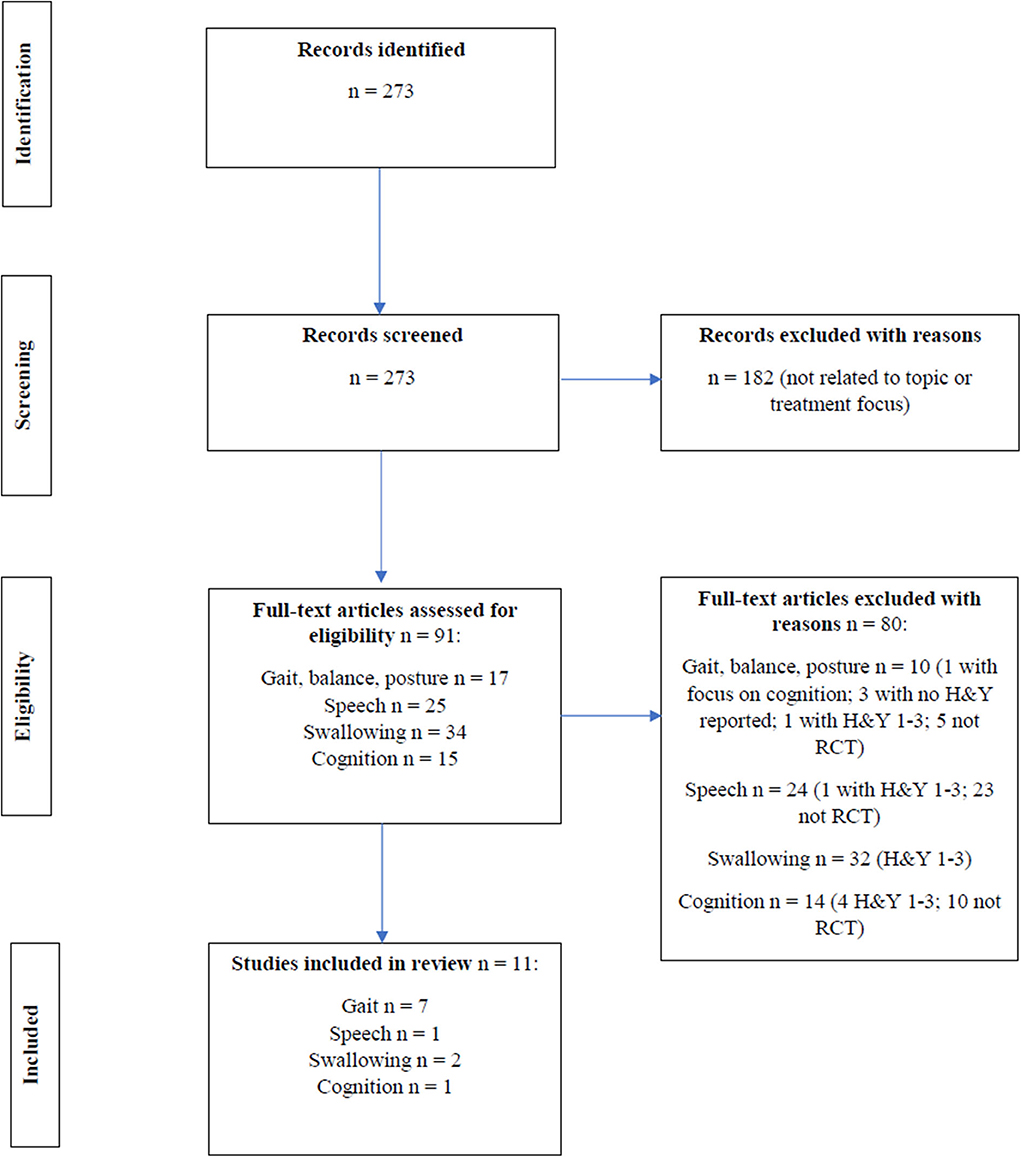
Figure 1. Flow diagram for RCT intervention studies across gait, balance, posture, speech, swallowing and cognition.
Gait, balance, posture, speech, and swallowing problems represent the typical axial clinical features of PwPD. Axial impairment is very common among PwPD, inexorably worsening with disease progression, with a severe impact on autonomy and QoL (22, 23). Axial signs are often resistant to pharmacological and surgical treatments. Therefore, non-pharmacological approaches may offer a valuable add-on treatment rescue.
Gait is controlled by cortical and subcortical neuronal networks, which are both altered in PwPD due to basal ganglia loop disruption and the frontal lobe dysfunction, the latter usually appearing with disease progression (24, 25). However, gait disorders are not only present in advanced PD. It has been shown that in the first 5 years from diagnosis, several gait parameters differ between PwPD and controls, such as lower step width, stride duration, and swing velocity that contribute to reduced gait velocity and a stooped posture (26, 27). Freezing of gait (FoG) can be present in 15–25% of early PD (28, 29). It is associated with the akineto-rigid PD subtype, older age, higher daily levodopa (L-dopa) dose, postural instability, and declining executive function (30), as well as with a higher risk of falls and fear of falling (31). It is often resistant to pharmacological treatment and rarely even worsened by L-dopa (32).
Overall, seven RCTs have specifically looked at gait interventions for early PD, such as treadmill programs, global postural reeducation, high-intensity exercise, or multidisciplinary intensive rehabilitation (11–17). The main findings ranged from no to mild-moderate improvements when compared to traditional care/physiotherapy (Table 1). Studies involving participants at different stages of the disease have also shown similar findings for gait intervention. For example, a Cochrane meta-analysis of 39 RCTs (H&Y 1–4) reported that physical activity and exercise improved gait speed, FoG, and functional mobility over a short term (<3 months) with no clear differences among physiotherapy techniques (33). Further, a recent phase II trial (H&Y 1–3) reported that a high-intensity treadmill program is safe and feasible (34). Finally, cueing has also been shown to be a useful rescue strategy for patients with drug-resistant FoG (35). Recently, performance-based cueing devices tailored to specific patients' gait performance have been developed, thanks to the use of wearable technology (36). The only small cross-over trial study on patients with disabling FoG based in the laboratory setting suggested positive results (37).
Static balance depends on the interactions between the characteristics of the person, the task, and the environment. Balance disturbances are related to different subsystem dysfunctions, including muscle weakness, proprioceptive sensory loss, increase in sensory thresholds, alteration of motor coordination, and cognitive decline. Interestingly, subtle balance impairments have been observed in patients with idiopathic REM behavior disorder, when compared to healthy controls (38) as well as altered postural sway during cognitive multitasking in early PD (39). For clinical assessment, the most reliable predictive factor of balance impairment is a 12-month history of falls, followed by FoG episodes in the previous month, and comfortable gait speed of <1.1 m/s (40).
Overall, there are no RCTs available on rehabilitation effect specifically for early PD patients. This is partly due to the fact that postural instability is usually clinically relevant in H&Y ≥ 3 patients. Only one single-blind RCT evaluated the effect of Nintendo Wii™-based motor cognitive training vs. balance exercise therapy without feedback or cognitive stimulation on activities of daily living (UPDRS-II). This study also explored the balance Berg scale as a secondary outcome among H&Y 1–2 patients finding an improvement in both groups (14) (Table 1). The most consistent treatment results are seen with motor training and traditional Chinese medical exercise (i.e., Tai Chi and Qigong) as shown by several meta-analyses of randomized RCTs and participants with H&Y 1–4 (41, 42). Of note, reduction in falls has been reported only by Tai Chi trials. It has been recently suggested that aquatic exercise may have equal or greater benefit on balance and fear of falling than land-based exercise (43).
PD postural abnormalities include: (a) camptocormia, defined as forward trunk bending ≥30° at the lumbar fulcrum (lower) or ≥45° at the thoracic fulcrum (upper); (b) Pisa syndrome, defined as ≥10° of lateral trunk bending that resolve almost completely when lying supine; and (c) anterocollis, ≥45° of forward neck bending. While in advanced stage postural abnormalities may be present in 30% of PwPD, few data is available to characterize postural abnormalities in early PD, which seems to occur in ~5% of H&Y 1–2 patients, and it appears usually isolated (44). Indeed, early and severe postural abnormalities, especially anterocollis, is a red flag for multiple system atrophy diagnosis (45). Multifactorial pathophysiology underlines postural abnormalities, which can be related to a higher disability due to increased risk of falls, back pain, and reduced mobility (46). Postural abnormalities and associated pain treatment remain an unmet clinical need. Camptocormia might improve with dopaminergic treatment, if associated with off periods, or with DBS if it is not yet fixed, while a few positive results have been obtained with botulinum toxin injections for fixed posture.
There are no RCTs available on rehabilitation effect specifically for early PD patients. However, high-intensity programs including stretching, strengthening, gait and balance training, or patient-tailored proprioceptive and tactile stimulation have shown promise in a small group of patients (H&Y 2–4) with moderate postural abnormalities. The positive effect was reported up to 6 months post-intervention (47, 48). However, postural deformities are potentially reversible with early recognition and management. Therefore, patients with mild postural abnormalities might be the best target for rehabilitation.
Studies have confirmed that speech difficulties are present in early PD, most commonly mild and including features from one or more of the speech subsystems of respiration (e.g., reduced phonation time), phonation (e.g., breathy or rough vocal quality, increased jitter and shimmer), articulation (e.g., imprecise articulation and slow alternating movements), and prosody (e.g., monopitch and monoloudness) (49–56). Variation was noted across studies in relation to the speech subsystem that was most impaired including articulation, phonation, or prosody (52, 55).
Several associations were also found between speech difficulties and/or motor or cognitive abnormalities in early PD. Speech difficulties have been associated with limb bradykinesia and rigidity, more for participants with akineto-rigid motor phenotype (70%) compared to tremor-dominant phenotype (19%) (49). Moreover, monoloudness was found to be correlated to bradykinesia (50), possibly linked to bradykinesia and rigidity at the laryngeal level (53). Furthermore, speech difficulties were found to be a predictor of cognitive decline in PD (53).
Only one RCT is available on the rehabilitation effect specifically for early PD patients. Levy and colleagues (18) found that only the high-intensity voice treatment, Lee Silverman Voice Treatment (LSVT) LOUD®, resulted in significant improvements in intelligibility for patients with mean H&Y 2.1. This was compared to patients receiving a high-intensity articulation treatment or no treatment. The findings are also consistent with the earlier reports from the RCT by Ramig et al. (57) where participants with H&Y 1–3 showed significant improvements in loudness level and functional communication only in the LSVT LOUD® group. Improvements were noted at 1 and 7 months post-treatment.
The most common cause of death in PD patients is aspiration pneumonia, resulting from preexisting dysphagia (58, 59), and therefore, its management should be prioritized. Dysphagia is not just a symptom of late stage PD. In a recent study, Pflug et al. (60) used fiberoptic endoscopic evaluation of swallowing (FEES) on 119 consecutive PwPD and found that 20% of the patients with a disease duration of <2 years had aspiration and that 12% (7 out of 57 patients) with H&Y 2 suffered from severe aspiration. Thus, there is a need to manage dysphagia and avoid complications as early as possible.
A number of existing clinical tools have been shown to be unreliable in detecting PD-related dysphagia. For example, swallowing questionnaires were found to detect swallowing problems in 12–27% of the PwPD, with <10% of the PwPD reporting spontaneously about dysphagia (61–63). Clinical bedside predictors of aspiration used for stroke, like the “normal” water swallow test (64), have been shown to be unreliable in PD (65). The Dutch guidelines estimating the maximum swallowing volume or the maximum swallowing speed (66) were not a suitable screening instrument to predict aspiration in PD patients (60). Increased drooling (sialorrhea) was also deemed a sign of penetration or aspiration (67) until Nienstedt et al. (62, 68) found that drooling cannot be considered an early sign of dysphagia. Therefore, instrumental methods [FEES and Videofluoroscopic Swallow Study (VFSS)] are the most valid and reliable methods of detecting risk of aspiration and penetration in PwPD (67, 68), particularly if they present with the following four symptoms: delayed mastication, reduced lingual motility prior to transfer, aspiration, and total swallow time (69). This information is crucial when evaluating studies on swallowing therapy and the validity of outcome measures.
Three RCTs were identified (70), comparing specific rehabilitative techniques to conventional dysphagia therapy, defined as dietary and postural changes. Logemann et al. (71) studied the validity of compensatory strategies and found that the thickness of the bolus is more effective than postural adjustments (“chin down” maneuver) in preventing the incidence of aspiration in the largest sample to date of PwPD (N = 711).
Two RCTs have looked specifically at swallowing intervention in early PD. The use of electrical stimulation therapy (SES) by the Baijens et al. (20, 72) was based on the hypothesis that sensory electrical stimulation (delivered through the VitaStim therapy device for 80 Hz, 700 ms, 0–25 mA) on the submental muscles would have a positive effect on FEES and VFS dysphagia ratings. They compared patients (H&Y ≤1–4) undergoing conventional dysphagia therapy with those undergoing sensory- or motor-level stimulation in the submental muscles, 30 min daily for 15 days. There was no significant difference between the groups. This could be due to the lack of physiological rationale over the choice of electrical stimulation in the submental region and the lack of specificity of the muscles stimulated.
Manor et al. (19) compared specific swallowing exercise therapy to conventional therapy and published the results of video-assisted swallowing therapy (VAST) in a group of 21 patients. This therapy was based on the hypothesis that six sessions of visual information and biofeedback from the swallowing process can be more effective than traditional therapy alone. There was a significant improvement in swallowing function from both interventions, with just the pharyngeal residue parameter being significantly better in the experimental group.
The third RCT examined another form of exercise, the strengthening of the expiratory muscles in order to increase the hyolaryngeal movement that protects the airway from liquid or food penetration or aspiration. The expiratory muscle strength training (EMST) device—a calibrated, spring-loaded valve to mechanically overload the expiratory and submental muscles—can improve submental muscle contraction that helps to elevate the hyolaryngeal complex during swallowing and to strengthen the protective cough. In a study of PD participants with mean H&Y of 2.5, Troche et al. (73) compared the EMST with a sham device. The primary outcome measure, the penetration–aspiration score, significantly improved in the EMST group.
Mild cognitive impairment with specific involvement of memory, visuospatial, and executive function has been described in early PwPD (74, 75). The executive dysfunction is characterized by deficits in internal control of attention, set shifting, planning, inhibitory control, dual task performance, and on a range of decision making and social cognition tasks (76). Moreover, mild cognitive impairment represents a significant risk factor for early dementia (77). Frontal dysfunction has also been noted using the executive and social cognition battery (78).
Kluger et al. found that fatigue, assessed by the Fatigue Severity Scale, was correlated to reduced visuospatial abilities (79). Cognitive decline in early PD has been associated with worse motor and non-motor symptoms, suggesting that this reflects a faster progressive phenotype (80). Postural control strategies are strictly correlated to cognitive performance, and Fernandes et al. (81) found that cognitive tasks might improve the postural control strategies during gait initiation.
One RCT has focused on improvements in cognition in early PD based on physical activity. Warm-up exercises and Nintendo Wii Fit™ motor and cognitive skills improved performance in both types of skills in PwPD. However, the ability to learn, retain, and transfer performance improvements after extensive training on Wii Fit games depended largely on the demands, particularly cognitive, of the specific games involved. Thus, those in which patients exhibited no learning deficit have the greatest indication for therapeutic use (21).
Two additional studies with participants at H&Y 1–3 stages have also suggested benefits of physical activity on cognition. Tanaka et al. (82) observed significant improvements in components of executive function on completion of an exercise program. Of note, abstract thinking and the ability to make appropriate decisions under certain circumstances were the primary subsets targeted and improved with the above exercise program. Moreover, the physical activity program resulted in positive trends in verbal fluency and organization of words as well as fewer errors in spatial working memory.
Further, cognitive function showed an improvement after a training period when they were monitored through the Parkinson's Disease Questionnaire (PDQ-39) by Dibble and colleagues (83). Indeed, when PDQ-39 results were compared between PwPD who exercised and those who did not, an improvement in cognitive function was found in the first group.
The findings from this mini review have highlighted that subtle changes in the fundamental areas of axial symptoms and cognition can be present even in early PD (Table 2). Further database searches should be conducted to substantiate the findings from this mini review, which does not aim to present a systematic review of the topic.
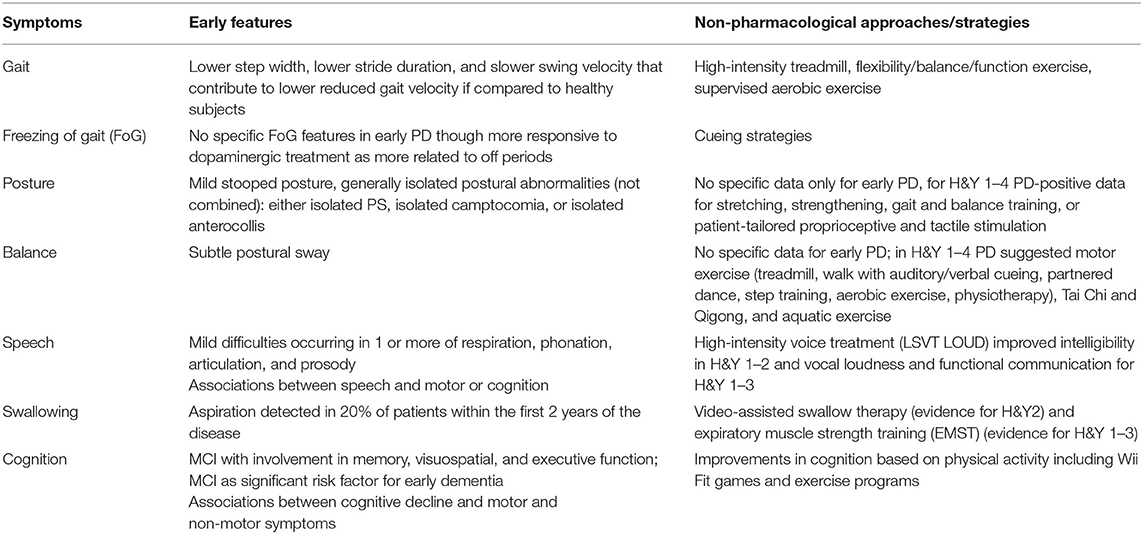
Table 2. Gait, balance, posture, speech, swallowing, and cognitive symptoms in early PD and current non-pharmacological treatment strategies.
In relation to non-pharmacological interventions over the last 15 years, only a limited number of RCTs have been conducted across areas of axial and cognition with the specific focus on early PD as defined by H&Y ≤ 2. However, in spite of this, positive results have been obtained in the studies, with improvements shown across a range of parameters, allowing to suggest several non-pharmacological approaches for gait, speech, swallowing, and cognitive symptoms in early PD (Table 2). Overall high-intensity treadmill and cueing strategies have obtained some positive results on gait and FoG, Tai Chi and Qigong, and aquatic exercise for balance, LSVT LOUD for speech, VAST and EMST for swallowing and warm-up exercises, and Nintendo Wii Fit™ skills for cognitive symptoms (Table 2). As these symptoms are particularly troublesome and resistant to dopaminergic and surgical treatment in more advanced stages of PD, attention should be given to the development of multidisciplinary non-pharmacological interventions as early as possible before these symptoms become troublesome, as already suggested for palliative care intervention (84). An early treatment approach could help to delay or reduce the high burden and/or medical complications that are often associated with the appearance of these disability milestones in later PD, in spite of the absence of any strong evidences of a clear disease-modifying effect. Although beyond the scope of this mini review, further consideration should also be given to the timing of treatment in early PD and whether the greatest benefit would be for pre-symptomatic or symptomatic patients with PD. In addition, we should clarify if those symptoms need to be targeted directly or rather their risk factors represent the most effective approach. Indeed, we have observed that axial and cognitive symptoms may be subtle in early stage of the disease and eventually difficult to treat or to define a “minimally clinically relevant improvement” for patients. At the same time, recognized risk factors for their occurrence are an older age at disease onset, the need for high L-dopa dose, and the presence of other axial symptoms, such as freezing of gait, falls, or patients who have a postural instability and gait disorder phenotype (30, 85, 86). However, once the abovementioned risk factors are present, it may be too late to achieve optimal gains due to the increased symptoms across axial and cognition domains. This could suggest the need to define if we should apply from the very beginning of the disease specific non-pharmacological strategies to a subgroup of patients with a postural instability and gait disorder phenotype or if we should focus on the pre-symptomatic population, who only present a clinical defined biomarker of possible parkinsonism and dementia development, such as patients with a REM behavior disorder (87).
Early and individualized rehabilitation across the presented axial and cognitive domains would help individuals with PD to maintain a positive impact on QoL and maintain employment and family/social life, while dysphagia and fall management could help to further reduce or delay hospitalization and consequent complications in later stages. A recent longitudinal observational study on the physical activity and early PD has already shown that higher self-reported physical activity is associated with slower disease progression (88). This lends further support to intensive rehabilitation and physical activity in early PD. Despite the few RCTs on early PD, the available evidences suggest that “the sooner the better” is a concept that could be suitable for non-pharmacological multidisciplinary interventions for these four “orphan” symptoms, from the very beginning of the disease.
Further large-scale RCTs are needed to specifically look at interventions for early PD across the areas axial and cognitive domains in a longitudinal manner. To further increase clinical relevance, studies should evaluate treatments that are based on the neurophysiology of PD such as increasing amplitude of movement, sensory–motor calibration, and visual cueing and feedback, using functional outcomes (19, 57, 73). Further, as associations were noted between motor, speech, and cognitive functions, future research should focus on multidisciplinary approaches to combined non-pharmacological management. Advances in this area will continue to empower patients early on in the disease process. The final aim should be to pull together the trends toward a more unified approach in managing these “orphan symptoms” in early PD using a multidisciplinary and individualized care approach that could reduce the disease burden in later PD.
ET and GS were responsible for drafting and revising the manuscript, study concept and design, acquisition, analysis and interpretation of data, and study execution. AM and MF were responsible for drafting/revising the manuscript, study concept and design, interpretation of data, and study execution. All authors contributed to the article and approved the submitted version.
This work was supported by the National Brain Appeal, Small Acorns Fund, National Hospital for Neurology and Neurosurgery, UCLH NHS Trust (nationalbrainappeal.org), the University College London Hospitals Charity, and the Australian Catholic University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ET would like to acknowledge the assistance from the National Brain Appeal, Small Acorns Fund, UCLH NHS Trust.
1. Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord. (2018) 33:1248–66. doi: 10.1002/mds.27372
2. Qamar MA, Harington G, Trump S, Johnson J, Roberts F, Frost E. Multidisciplinary care in Parkinson's disease. Int Rev Neurobiol. (2017) 132:511–23. doi: 10.1016/bs.irn.2017.02.001
3. Bloem BR, Henderson EJ, Dorsey ER, Okun MS, Okubadejo N, Chan P, et al. Integrated and patient-centred management of Parkinson's disease: a network model for reshaping chronic neurological care. Lancet Neurol. (2020) 19:623–34. doi: 10.1016/S1474-4422(20)30064-8
4. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. (2020) 323:548–60. doi: 10.1001/jama.2019.22360
5. Constantinescu R, Eriksson B, Jansson Y, Johnels B, Holmberg B, Gudmundsdottir T, et al. Key clinical milestones 15 years and onwards after DBS-STN surgery-a retrospective analysis of patients that underwent surgery between 1993 and 2001. Clin Neurol Neurosurg. (2017) 154:43–48. doi: 10.1016/j.clineuro.2017.01.010
6. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. (2008) 23:837–44. doi: 10.1002/mds.21956
7. Macleod AD, Counsell CE. Predictors of functional dependency in Parkinson's disease. Mov Disord. (2016) 31:1482–8. doi: 10.1002/mds.26751
8. Lo RY, Tanner CM, Albers KB, Leimpeter AD, Fross RD, Bernstein AL, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. (2009) 66:1353–8. doi: 10.1001/archneurol.2009.221
9. Lang AE, Espay AJ. Disease modification in Parkinson's disease: current approaches, challenges, future considerations. Mov Disord. (2018) 33:660–77. doi: 10.1002/mds.27360
10. Antonini A, Bravi D, Sandre M, Bubacco L. Immunization therapies for Parkinson's disease: state of the art and considerations for future clinical trials. Expert Opin Invest Drugs. (2020) 12:1–11. doi: 10.1080/13543784.2020.1771693
11. Canning CG, Allen NE, Dean CM, Goh L, Fung VS. Home-based treadmill training for individuals with Parkinson's disease: a randomized controlled pilot trial. Clin Rehabil. (2012) 26:817–26. doi: 10.1177/0269215511432652
12. Nadeau A, Pourcher E, Corbeil P. Effects of 24 wk of treadmill training on gait performance in Parkinson's disease. Med Sci Sports Exerc. (2014) 46:645–55. doi: 10.1249/MSS.0000000000000144
13. Agosti V, Vitale C, Avella D, Rucco R, Santangelo G, Sorrentino P, et al. Effects of global postural reeducation on gait kinematics in parkinsonian patients: a pilot randomized three-dimensional motion analysis study. Neurol Sci. (2016) 37:515–22. doi: 10.1007/s10072-015-2433-5
14. Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira Tde P, Zomignani AP, et al. Effect of Nintendo Wii-based motor and cognitive training on activities of daily living in patients with Parkinson's disease: a randomised clinical trial. Physiotherapy. (2012) 98:196–204. doi: 10.1016/j.physio.2012.06.004
15. Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. (2008) 89:1221–9. doi: 10.1016/j.apmr.2008.01.013
16. Park A, Zid D, Russell J, Malone A, Rendon A, Wehr A, et al. Effects of a formal exercise program on Parkinson's disease: a pilot study using a delayed start design. Parkinsonism Relat Disord. (2014) 20:106–11. doi: 10.1016/j.parkreldis.2013.10.003
17. Frazzitta G, Maestri R, Bertotti G, Riboldazzi G, Boveri N, Perini M, et al. Intensive rehabilitation treatment in early Parkinson's disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. (2015) 29:123–31. doi: 10.1177/1545968314542981
18. Levy ES, Moya-Gale G, Chang YHM, Freeman K, Forrest K, Brin MF, et al. The effects of intensive speech treatment on intelligibility in Parkinson's disease: a randomised controlled trial. Clin Med. (2020) 24:100429. doi: 10.1016/j.eclinm.2020.100429
19. Manor Y, Mootanah R, Freud D, Giladi N, Cohen JT. Video-assisted swallowing therapy for patients with Parkinson's disease. Parkinsonism Relat Disord. (2013) 19:207–11. doi: 10.1016/j.parkreldis.2012.10.004
20. Baijens LW, Speyer R, Passos VL, Pilz W, van der Kruis J, Haarmans S, et al. Surface electrical stimulation in dysphagic Parkinson patients: a randomized clinical trial. Laryngoscope. (2013) 123:E38–44. doi: 10.1002/lary.24119
21. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, Guedes da Silva K, Oliveira Tde P, Peterson Zomignani A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson's disease–effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. (2012) 98:217–23. doi: 10.1016/j.physio.2012.06.001
22. Debu B, De Oliveira Godeiro C, Lino JC, Moro E. Managing gait, balance, and posture in Parkinson's disease. Curr Neurol Neurosci Rep. (2018) 18:23. doi: 10.1007/s11910-018-0828-4
23. Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. (2014) 71:884–90. doi: 10.1001/jamaneurol.2014.753
24. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. (2013) 28:1483–91. doi: 10.1002/mds.25669
25. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. (2017) 10:1–17. doi: 10.14802/jmd.16062
26. Galna B, Lord S, Burn DJ, Rochester L. Progression of gait dysfunction in incident Parkinson's disease: impact of medication and phenotype. Mov Disord. (2015) 30:359–67. doi: 10.1002/mds.26110
27. Panyakaew P, Bhidayasiri R. The spectrum of preclinical gait disorders in early Parkinson's disease: subclinical gait abnormalities and compensatory mechanisms revealed with dual tasking. J Neural Transmission. (2013) 120:1665–72. doi: 10.1007/s00702-013-1051-8
28. Zhang H, Yin X, Ouyang Z, Chen J, Zhou S, Zhang C, et al. A prospective study of freezing of gait with early Parkinson disease in Chinese patients. Medicine. (2016) 95:e4056. doi: 10.1097/MD.0000000000004056
29. Hall JM, Shine JM, O'Callaghan C, Walton CC, Gilat M, Naismith SL, et al. Freezing of gait and its associations in the early and advanced clinical motor stages of Parkinson's disease: a cross-sectional study. J Parkinson's Dis. (2015) 5:881–91. doi: 10.3233/JPD-150581
30. Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. A 12-year population-based study of freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. (2015) 21:254–8. doi: 10.1016/j.parkreldis.2014.12.020
31. Walton CC, Shine JM, Hall JM, O'Callaghan C, Mowszowski L, Gilat M, et al. The major impact of freezing of gait on quality of life in Parkinson's disease. J Neurol. (2015) 262:108–15. doi: 10.1007/s00415-014-7524-3
32. Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR. Freezing of gait: a practical approach to management. Lancet Neurol. (2015) 14:768–78. doi: 10.1016/S1474-4422(15)00041-1
33. Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Database Syst Rev. (2013) Cd002817. doi: 10.1002/14651858.CD002817.pub4
34. Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. (2012) 92:1395–410. doi: 10.2522/ptj.20110472
35. Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. (2007) 78:134–40. doi: 10.1136/jnnp.200X.097923
36. Ginis P, Nieuwboer A, Dorfman M, Ferrari A, Gazit E, Canning CG, et al. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson's disease: a pilot randomized controlled trial. Parkinsonism Relat Disord. (2016) 22:28–34. doi: 10.1016/j.parkreldis.2015.11.004
37. Barthel C, Nonnekes J, van Helvert M, Haan R, Janssen A, Delval A, et al. The laser shoes: a new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology. (2018) 90:e164-71. doi: 10.1212/WNL.0000000000004795
38. Martens KAE, Hall JM, Gilat M, Georgiades MJ, Walton CC, Lewis SJG. Anxiety is associated with freezing of gait and attentional set-shifting in Parkinson's disease: a new perspective for early intervention. Gait Post. (2016) 49:431–6. doi: 10.1016/j.gaitpost.2016.07.182
39. Chen T, Fan Y, Zhuang X, Feng D, Chen Y, Chan P, et al. Postural sway in patients with early Parkinson's disease performing cognitive tasks while standing. Neurol Res. (2018) 40:491–8. doi: 10.1080/01616412.2018.1451017
40. Duncan RP, Cavanaugh JT, Earhart GM, Ellis TD, Ford MP, Foreman KB, et al. External validation of a simple clinical tool used to predict falls in people with Parkinson disease. Parkinsonism Relat Disord. (2015) 21:960–3. doi: 10.1016/j.parkreldis.2015.05.008
41. Allen NE, Sherrington C, Suriyarachchi GD, Paul SS, Song J, Canning CG. Exercise and motor training in people with Parkinson's disease: a systematic review of participant characteristics, intervention delivery, retention rates, adherence, and adverse events in clinical trials. Parkinson's Dis. (2012) 2012:854328. doi: 10.1155/2012/854328
42. Yang Y, Qiu WQ, Hao YL, Lv ZY, Jiao SJ, Teng JF. The efficacy of traditional Chinese medical exercise for Parkinson's disease: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0122469. doi: 10.1371/journal.pone.0122469
43. Cugusi L, Manca A, Bergamin M, Di Blasio A, Monticone M, Deriu F, et al. Aquatic exercise improves motor impairments in people with Parkinson's disease, with similar or greater benefits than land-based exercise: a systematic review. J Physiother. (2019) 65:65–74. doi: 10.1016/j.jphys.2019.02.003
44. Tinazzi M, Geroin C, Gandolfi M, Smania N, Tamburin S, Morgante F, et al. Pisa syndrome in Parkinson's disease: an integrated approach from pathophysiology to management. Mov Disord. (2016) 31:1785–95. doi: 10.1002/mds.26829
45. Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. (2015) 372:1375–6. doi: 10.1056/NEJMc1501657
46. Doherty KM, van de Warrenburg BP, Peralta MC, Silveira-Moriyama L, Azulay JP, Gershanik OS, et al. Postural deformities in Parkinson's disease. Lancet Neurol. (2011) 10:538–49. doi: 10.1016/S1474-4422(11)70067-9
47. Capecci M, Serpicelli C, Fiorentini L, Censi G, Ferretti M, Orni C, et al. Postural rehabilitation and kinesio taping for axial postural disorders in Parkinson's disease. Arch Phys Med Rehabil. (2014) 95:1067–75. doi: 10.1016/j.apmr.2014.01.020
48. Bartolo M, Serrao M, Tassorelli C, Don R, Ranavolo A, Draicchio F, et al. Four-week trunk-specific rehabilitation treatment improves lateral trunk flexion in Parkinson's disease. Mov Disord. (2010) 25:325–31. doi: 10.1002/mds.23007
49. Rusz J, Cmejla R, Ruzickova H, Klempir J, Majerova V, Picmausova J, et al. Evaluation of speech impairment in early stages of Parkinson's disease: a prospective study with the role of pharmacotherapy. J Neural Transmission. (2013) 120:319–29. doi: 10.1007/s00702-012-0853-4
50. Rusz J, Tykalova T, Klempir J, Cmejla R, Ruzicka E. Effects of dopaminergic replacement therapy on motor speech disorders in Parkinson's disease: longitudinal follow-up study on previously untreated patients. J Neural Transmission. (2016) 123:379–87. doi: 10.1007/s00702-016-1515-8
51. Rusz J, Cmejla R, Ruzickova H, Ruzicka E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson's disease. J Acoustical Soc Am. (2011) 129:350–67. doi: 10.1121/1.3514381
52. Defazio G, Guerrieri M, Liuzzi D, Gigante AF, di Nicola V. Assessment of voice and speech symptoms in early Parkinson's disease by the Robertson dysarthria profile. Neurol Sci. (2016) 37:443–9. doi: 10.1007/s10072-015-2422-8
53. Polychronis S, Niccolini F, Pagano G, Yousaf T, Politis M. Speech difficulties in early de novo patients with Parkinson's disease. Parkinsonism Relat Disord. (2019) 64:256–61. doi: 10.1016/j.parkreldis.2019.04.026
54. Tykalova T, Rusz J, Klempir J, Cmejla R, Ruzicka E. Distinct patterns of imprecise consonant articulation among Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Brain Lang. (2017) 165:1–9. doi: 10.1016/j.bandl.2016.11.005
55. Huh YE, Park J, Suh MK, Lee SE, Kim J, Jeong Y, et al. Differences in early speech patterns between Parkinson variant of multiple system atrophy and Parkinson's disease. Brain Lang. (2015) 147:14–20. doi: 10.1016/j.bandl.2015.04.007
56. Midi I, Dogan M, Koseoglu M, Can G, Sehitoglu MA, Gunal DI. Voice abnormalities and their relation with motor dysfunction in Parkinson's disease. Acta Neurol Scand. (2008) 117:26–34. doi: 10.1111/j.1600-0404.2007.00965.x
57. Ramig L, Halpern A, Spielman J, Fox C, Freeman K. Speech treatment in Parkinson's disease: randomized controlled trial (RCT). Mov Disord. (2018) 33:1777–91. doi: 10.1002/mds.27460
58. Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. (2015) 14:625–39. doi: 10.1016/S1474-4422(15)00007-1
59. Fabbri M, Coelho M, Abreu D, Guedes LC, Rosa MM, Godinho C, et al. Dysphagia predicts poor outcome in late-stage Parkinson's disease. Parkinsonism Relat Disord. (2019) 64:73–81. doi: 10.1016/j.parkreldis.2019.02.043
60. Pflug C, Niessen A, Buhmann C, Bihler M. Swallowing speed is no adequate predictor of aspiration in Parkinson's disease. Neurogastroenterol Motil. (2019) 31:e13713. doi: 10.1111/nmo.13713
61. Buhmann C, Flugel T, Bihler M, Gerloff C, Niessen A, Hidding U, et al. Is the Munich dysphagia test-Parkinson's disease (MDT-PD) a valid screening tool for patients at risk for aspiration? Parkinsonism Relat Disord. (2019) 61:138–43. doi: 10.1016/j.parkreldis.2018.10.031
62. Nienstedt JC, Bihler M, Niessen A, Plaetke R, Potter-Nerger M, Gerloff C, et al. Predictive clinical factors for penetration and aspiration in Parkinson's disease. Neurogastroenterol Motil. (2019) 31:e13524. doi: 10.1111/nmo.13524
63. Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson's disease. Neurology. (1989) 39:1309–14. doi: 10.1212/WNL.39.10.1309
64. DePippo KL, Holas MA, Reding MJ. Validation of the 3-oz water swallow test for aspiration following stroke. Arch Neurol. (1992) 49:1259–61. doi: 10.1001/archneur.1992.00530360057018
65. Lam K, Lam FK, Lau KK, Chan YK, Kan EY, Woo J, et al. Simple clinical tests may predict severe oropharyngeal dysphagia in Parkinson's disease. Mov Disord. (2007) 22:640–4. doi: 10.1002/mds.21362
66. Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord. (2012) 18:311–5. doi: 10.1016/j.parkreldis.2011.11.006
67. Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos-Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:992–8. doi: 10.1016/j.parkreldis.2014.06.008
68. Nienstedt JC, Buhmann C, Bihler M, Niessen A, Plaetke R, Gerloff C, et al. Drooling is no early sign of dysphagia in Parkinson's disease. Neurogastroenterol Motil. (2018) 30:e13259. doi: 10.1111/nmo.13259
69. Tomita S, Oeda T, Umemura A, Kohsaka M, Park K, Yamamoto K, et al. Video-fluoroscopic swallowing study scale for predicting aspiration pneumonia in Parkinson's disease. PLoS ONE. (2018) 13:e0197608. doi: 10.1371/journal.pone.0197608
70. Park MS, Choi JY, Song YJ, Choi H, Park EJ, Ji ES. Systematic review of behavioral therapy to improve swallowing functions of patients with Parkinson's disease. Gastroenterol Nurs. (2019) 42:65–78. doi: 10.1097/SGA.0000000000000358
71. Logemann JA, Gensler G, Robbins J, Lindblad AS, Brandt D, Hind JA, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson's disease. J Speech Lang Hear Res. (2008) 51:173–83. doi: 10.1044/1092-4388(2008/013)
72. Baijens LW, Speyer R, Passos VL, Pilz W, Roodenburg N, Clave P. The effect of surface electrical stimulation on swallowing in dysphagic parkinson patients. Dysphagia. (2012) 27:528–37. doi: 10.1007/s00455-011-9387-4
73. Troche MS, Okun MS, Rosenbek JC, Musson N, Fernandez HH, Rodriguez R, et al. Aspiration and swallowing in parkinson disease and rehabilitation with EMST: a randomized trial. Neurology. (2010) 75:1912–9. doi: 10.1212/WNL.0b013e3181fef115
74. Seubert-Ravelo AN, Yanez-Tellez MG, Salgado-Ceballos H, Escartin-Perez RE, Neri-Nani GA, Velazquez-Osuna S. Mild cognitive impairment in patients with early-onset Parkinson's disease. Dement Geriatr Cogn Disord. (2016) 42:17–30. doi: 10.1159/000447533
75. Tang Y, Ge J, Liu F, Wu P, Guo S, Liu Z, et al. Cerebral metabolic differences associated with cognitive impairment in Parkinson's disease. PLoS ONE. (2016) 11:e0152716. doi: 10.1371/journal.pone.0152716
76. Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. (2013) 7:193–224. doi: 10.1111/jnp.12028
77. Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. (2013) 70:580–6. doi: 10.1001/jamaneurol.2013.2110
78. Esteves S, Gleichgerrcht E, Torralva T, Chade A, Gomez Arevalo G, Gershanik O, et al. Performance of patients with early Parkinson disease on an executive and social cognition battery. Cogn Behav Neurol. (2018) 31:142–50. doi: 10.1097/WNN.0000000000000159
79. Kluger BM, Garimella S, Garvan C. Minimal clinically important difference of the modified fatigue impact scale in Parkinson's disease. Parkinsonism Relat Disord. (2017) 43:101–4. doi: 10.1016/j.parkreldis.2017.07.016
80. Hu MT, Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Rolinski M, Murray C, et al. Predictors of cognitive impairment in an early stage Parkinson's disease cohort. Mov Disord. (2014) 29:351–9. doi: 10.1002/mds.25748
81. Fernandes A, Sousa ASP, Rocha N, Tavares J. The influence of a cognitive task on the postural phase of gait initiation in Parkinson's disease: an electromyographic-based analysis. Motor Control. (2017) 21:249–64. doi: 10.1123/mc.2015-0032
82. Tanaka K, Quadros AC Jr, Santos RF, Stella F, Gobbi LT, Gobbi S, et al. Benefits of physical exercise on executive functions in older people with Parkinson's disease. Brain Cogn. (2009) 69:435–41. doi: 10.1016/j.bandc.2008.09.008
83. Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson's disease: a preliminary study. Parkinsonism Relat Disord. (2009) 15:752–7. doi: 10.1016/j.parkreldis.2009.04.009
84. Bouca-Machado R, Lennaerts-Kats H, Bloem B, Ferreira JJ. Why palliative care applies to Parkinson's disease. Mov Disord. (2018) 33:750–53. doi: 10.1002/mds.27309
85. Hiorth YH, Larsen JP, Lode K, Pedersen KF. Natural history of falls in a population-based cohort of patients with Parkinson's disease: an 8-year prospective study. Parkinsonism Relat Disord. (2014) 20:1059–64. doi: 10.1016/j.parkreldis.2014.06.023
86. Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. (2012) 83:601–6. doi: 10.1136/jnnp-2011-301874
87. Postuma RB, Iranzo A, Hu M, Hogl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. (2019) 142:744–59. doi: 10.1093/brain/awz030
Keywords: early Parkinson's disease, speech, swallowing, axial symptoms, cognition, multidisciplinary care
Citation: Sharpe G, Macerollo A, Fabbri M and Tripoliti E (2020) Non-pharmacological Treatment Challenges in Early Parkinson's Disease for Axial and Cognitive Symptoms: A Mini Review. Front. Neurol. 11:576569. doi: 10.3389/fneur.2020.576569
Received: 26 June 2020; Accepted: 17 August 2020;
Published: 25 September 2020.
Edited by:
Daniel Martinez-Ramirez, Tecnológico de Monterrey, MexicoReviewed by:
Abhishek Lenka, MedStar Georgetown University Hospital, United StatesCopyright © 2020 Sharpe, Macerollo, Fabbri and Tripoliti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margherita Fabbri, bWFyZ2hlcml0YWZhYmJyaW1kQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.