- 1Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, China
- 2Department of Radiology and Nuclear Medicine, Erasmus Medical Center, Rotterdam, Netherlands
- 3Department of Epidemiology, Erasmus MC University Medical Center, Rotterdam, Netherlands
- 4Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong, China
- 5Department of Imaging and Interventional Radiology, Chinese University of Hong Kong, Hong Kong, China
- 6Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hung Hom, Hong Kong
Background: Intracranial arterial calcification (IAC) is highly prevalent in ischemic stroke patients. However, data on the association of IAC with stroke recurrence and mortality remains limited. We examined the effect of IAC on the long-term recurrence of stroke and the risk of post-stroke mortality.
Methods: Using a prospective stroke registry, we recruited 694 patients (mean age 71.6 ± 12.4; male sex 50.3%) since December 2004. IAC was visualized using the computed tomography exam that was made at hospital admission and was quantified with the Agatston method. All patients were regularly followed up till July 2016. The impacts of IAC on stroke recurrence and mortality were assessed using Cox-regression models with adjustments for age, sex, and relevant cardiovascular risk factors.
Results: During a median follow-up period of 8.8 years, 156 patients (22.5%) suffered a recurrent stroke and 84 died (12.1%). We found that a higher IAC Agatston score related to a higher risk of stroke recurrence (HR per 1-SD increase in IAC: 1.30; 95% CI, 1.08–1.56, p = 0.005) and a higher risk of post-stroke mortality (HR per 1-SD increase, 1.44; 95% CI, 1.06–1.96, p = 0.019). After investigating etiology-specific risks of stroke-recurrence, we found that a higher IAC Agatston score specifically associated with small-vessel occlusive stroke.
Conclusions: IAC is a strong risk factor for recurrent stroke and post-stroke mortality. Among stroke subtypes, IAC relates to higher risk of stroke recurrence among patients with small-vessel disease, which indicates chronic calcification detected in large cerebral arteries may have potential effects on the cerebrovascular beds extending to small vessels.
Worldwide, stroke is one of the leading causes of death (1), and permanent disability (2). Ischemic stroke has a complex etiology with many contributing causes such as large-artery atherosclerosis or small-vessel occlusion or cardiac emboli. Effective prevention could significantly reduce the rates of stroke recurrence and mortality (3), which may benefit from the validation of an useful imaging biomarker.
Intracranial arterial calcification (IAC) is frequently observed on non-contrast computed tomography (CT), which is a routine imaging investigation for every patient with suspicious stroke. Our previous clinical studies found a nearly 70% prevalence of cerebral arteries calcification in Chinese population (4) and a prevalence of over 80% among stroke patients (5). Inconsistent with its high prevalence, the effects of IAC on ischemic stroke has not received much attention. A recent population-based study in White individuals demonstrated the role of IAC in increasing the risk of a first stroke (6). Assessing IAC by using quantitative Agatston score, our recent study found that IAC was closely associated with cerebral artery stenosis and intracranial micro-embolism (7–9). However, data on its effects on stroke recurrence and mortality after stroke remain scarce.
Considering that IAC is a chronic disease within cerebrovascular system, this prospective study based on a local stroke registry aimed to investigate the effects of IAC on stroke recurrence and vascular mortality caused by ischemic stroke.
Methods
Setting
All consecutive patients that were admitted to the Prince of Wales Hospital between 1 December 2004 and 31 March 2005 with ischemic stroke or transient ischemic attack (TIA) were included in the current study. This study was part of the Prince of Wales Hospital stroke registry which was described in detail elsewhere (10). In brief, the Prince of Wales Hospital was the principal teaching hospital of the Chinese University of Hong Kong and was a tertiary referral center for the New Territories of Hong Kong. The acute stroke unit (ASU), to which all patients with stroke or TIA in this study were admitted, provided all the stroke beds available in the region (population size: 650,000). This study provided a recruitment method that was almost identical to that of a population study.
For the current study, we excluded patients whose incident stroke was due to venous infarcts, intracranial hemorrhage, subdural hematoma or subarachnoid hemorrhage. Patients with other potential causes of IAC, such as hyperparathyroidism or end-stage renal failure were excluded. Patients who refused secondary prevention with antiplatelet or anticoagulant therapies were excluded. Thirty patients were excluded due to obvious artifacts in the CT images that were not measurable for IAC. Four patients were excluded due to emigration outside the geographical area and could not be reached. This left 694 patients in the current study.
This study was approved by the institutional review board (the Clinical Research Ethics Committee of the Chinese University of Hong Kong).
Assessment of Intracranial Arterial Calcification
At admission, all patients underwent a non-contrast CT examination of the skull, which was used to assess IAC. All examinations were performed on a 16-slice multi-detector row CT system (Light speed 16 plus, General Electric, Milwaukee, WI, USA) with the following scan parameters: 140 kVp, 170 mAs, 2 s per rotation. Axial images were reconstructed at 0.625 mm intervals.
We evaluated IAC Agatston score using a semi-automatic procedure which we previously described (7, 8). In brief, CT source images for each patient were reconstructed to three dimensional (3D) images by MATLAB (R2015a, the MathWorks Corporation, MA, USA), and subsequently transferred to 3D software (Analyze, version 12.0, AnalyzeDirect Inc., KS, USA) for IAC segmentation with “seeding method” and manual editing. Then IAC volume was automatically generated by ITK-SNAP (version 3.4.0, open source software, www.itk-snap.org) (11). Next, 3D CT images with IAC segments were reconstructed at 3 mm intervals by MATLAB. Finally, IAC Agatston score was automatically generated using a custom-made program with the Agatston algorithm (12). According to this algorithm, on each 3 mm-thick CT slice, a weighted value was assigned to the highest artery calcium density within the slice. Weighted value of 1 for 130–199 Hounsfield units (HU), 2 for 200–299 HU, 3 for 300–399 HU, and 4 for 400 HU or greater. This weighted value was then multiplied by the area of calcification in the same slice, and total Agatston score is a summed result of all CT slices.
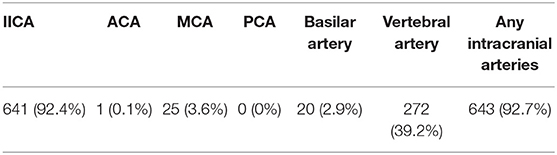
We defined IAC as hyperdense foci with attenuation number ≥130 HU within any of the following cerebral arteries: right and left intracranial internal carotid artery (IICA), right and left anterior cerebral arteries (ACA), right and left middle cerebral arteries (MCA), right and left posterior cerebral arteries (PCA), right and left vertebral arteries and basilar artery. The results were evaluated by an experienced neurologist blinded to all the clinical data of the study population.
Follow-Up of Stroke Recurrence and Post-stroke Mortality
Patients admitted to the ASU were followed up using Clinical Management System (CMS), a territory-wide and networked clinical records system which was utilized by the Hospital Authority throughout all government hospitals in Hong Kong. All study patients who were discharged from the hospital were followed up regularly till June 1, 2016 for ischemic stroke recurrence and post-stroke mortality. Deaths classified as due to stroke causes were death from stroke that occurred within 7 days after the current stroke or another stroke during follow-up period. A search of the patient's serial CMS records was performed to identify any development of recurrent ischemic stroke or related mortality across the Hong Kong government hospital system.
Clinical Data and Stroke Subtypes
Clinical data of demographic characteristics, stroke risk factors, and stroke etiology of the patients were retrieved from the data-collection sheets of stroke registry collected on entry and obtained by record linkage to the CMS. Demographic characteristics included age and sex. Stroke risk factors for each patient were documented regarding the following: hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, ischemic heart disease, two or more ischemic stroke history and ever smoking. The stroke etiology (TOAST, Trial of ORG 10172 in Acute Stroke Treatment criteria) (13) was classified as large-artery atherosclerosis, small-vessel occlusion, cardioembolism, other determined etiology, undetermined etiology, and two or more causes identified.
Statistical Analysis
Given the skewed distribution of the IAC Agatston score we applied a natural log-transformation after 1 was added to the non-transformed values to deal with zero calcium scores [Ln (IAC + 1.0)]. Cox proportional hazards regression models were used to investigate the association of the presence of IAC and IAC Agatston scores with stroke recurrence and post-stroke mortality. In the first model we show the crude, unadjusted effect estimates. In the second model we adjusted all analyses for age, sex, hypertension, diabetes mellitus, atrial fibrillation, hyperlipidaemia, ischemic heart diseases, two or more ischemic stroke history and ever smoking. Next, we analyzed the association of IAC Agatston score with stroke subtypes (large-artery atherosclerosis, small-vessel occlusion, cardioembolism). Finally, we used Kaplan-Meier curves and log-rank tests for estimation of stroke recurrence and post-stroke mortality for patient groups with higher (>=median score) or lower (< median score) IAC Agatston scores. A significance level of 0.05 was used for all analyses. The SPSS software SPSS (version 16.0, IBM SPSS Statistics, Chicago, U.S.A.) was employed for this study.
Results
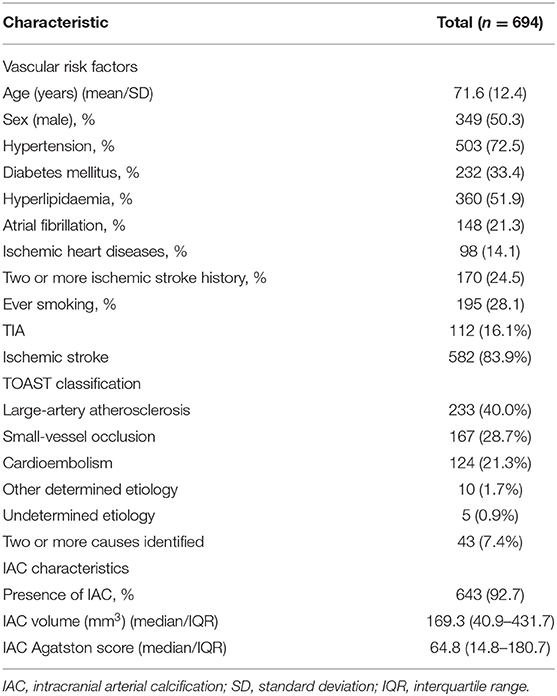
The baseline characteristics of the study population are provided in Table 1. The mean age of the patients was 72 years (age range: 28–101 years) and 49.7% were females. The most prevalent stroke-etiology was large-artery atherosclerosis (40.0%, 233/582). Small-vessel occlusion was found in 167 patients (28.7%), cardioembolic stroke in 124 patients (21.3%), other determined etiology in 10 patients (1.7%), and cryptogenic causes in 5 patients (0.9%). Two or more causes were identified in 43 patients (7.4%). IAC was found in 643 patients (92.7%) (Table 2).
Stroke Recurrence and Post-stroke Mortality
During a median follow-up period of 8.8 years (interquartile range 3.2–11.1 years), 156 patients (22.5%) suffered a recurrent stroke and 84 (12.1%) died within 7 days after the initial stroke.
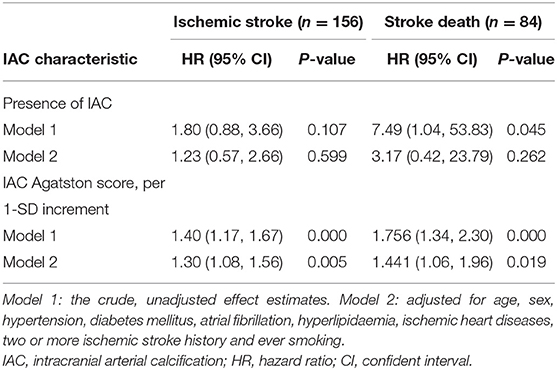
Table 3 shows the associations between IAC characteristics and risks of stroke recurrence. We found that IAC Agatston score was related to a higher risk of recurrent stroke independent of cardiovascular risk factors (HR per 1-SD increase in IAC, 1.30; 95% CI, 1.08–1.56, p = 0.005) (Table 3, model 2).
For the associations between IAC characteristics and risks of post-stroke mortality, we found that IAC Agatston score was independently related to a higher risk of post-stroke mortality (HR per 1-SD increase in IAC, 1.44; 95% CI, 1.06–1.96) (Table 3, model 2).
Stroke Recurrence in Patients With Stroke of Different Etiological Subtypes or TIA
We found 133 (22.9%) recurrence stroke in 582 stroke patients, and 23 (20.5%) in 112 TIA patients (Table 4). Among the 133 recurrence cases, 54 (23.2%) were found in patient with index large-artery atherosclerotic stroke, 34 (20.4%) in patient with index small-vessel occlusive stroke, and 27 (21.8%) with index cardioembolic stroke.
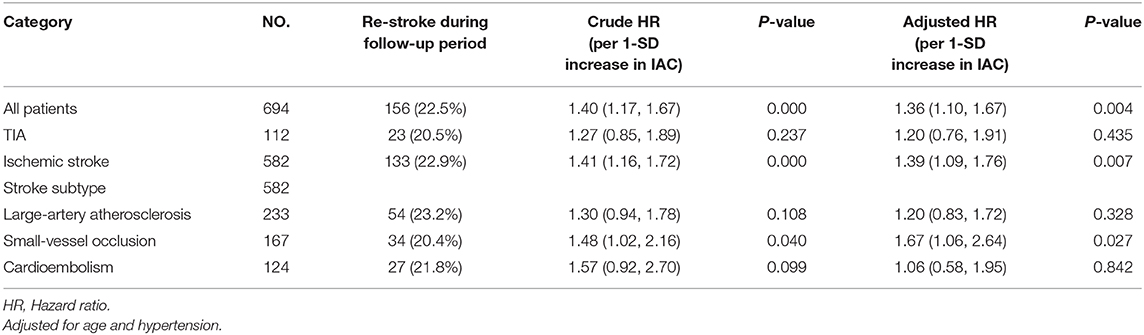
Table 4. Cox proportional hazard model for predictive value of IAC Agatston score on patient groups with index TIA or different major stroke subtypes.
For patients with index small-vessel occlusive stroke, IAC Agatston score was found to be associated with a higher risk of stroke recurrence (HR per 1-SD increase in IAC, 1.67; 95% CI, 1.06–2.64, p = 0.027) independent of age and hypertension.
The Kaplan–Meier Risk of Stroke Recurrence and Post-stroke Mortality
The Kaplan-Meier curves and log-rank tests showed significantly higher rates of stroke recurrence (P = 0.001) and lower rates of post-stroke survival (P < 0.001) in patients with higher IAC Agatston score (Figure 1). In this group, the risk of developing recurrent stroke was 9.2% at 1 year, 21.0% at 5 years, and 25.6% at 10 years. While for patients with lower IAC Agatston score, the risk was much lower, with 6.9% at 1 year, 13.5% at 5 years, and 18.7% at 10 years.
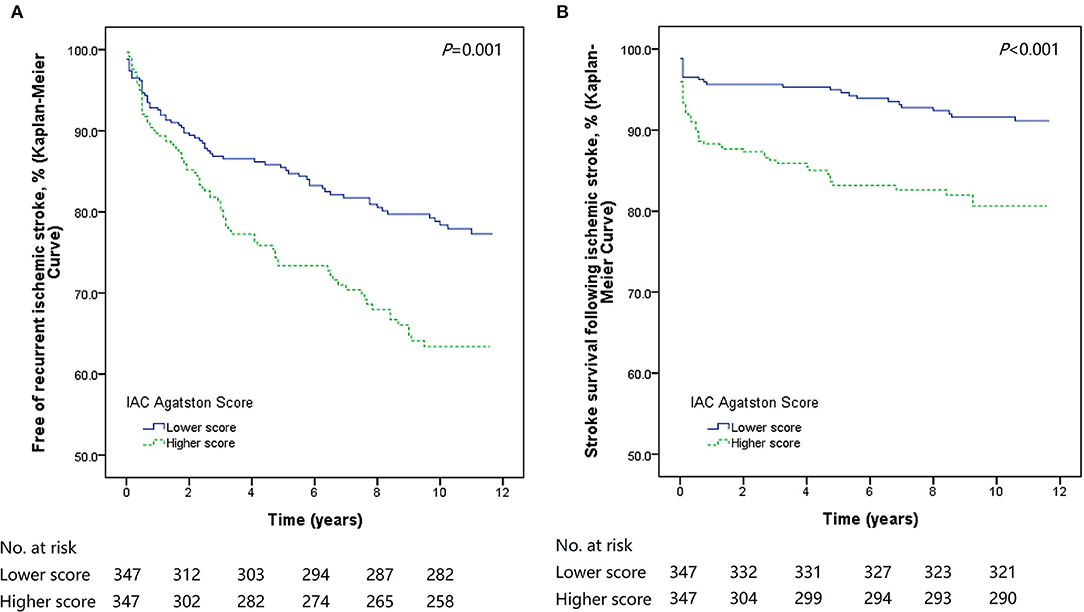
Figure 1. Kaplan-Meier survival plots of stroke recurrence and stroke death. Patients with higher IAC Agatston score had significantly poorer outcomes (recurrent stroke or stroke death) than those with lower score. (A) Kaplan-Meier survival plot for patients free of recurrent stroke. (B) Kaplan-Meier survival plot for stroke survival.
Discussion
In this cohort study, we found IAC was associated with the risk of recurrent stroke and post-stroke mortality during over 10 years of follow-up. In addition, index small-vessel occlusive stroke patients who had higher IAC score were particularly vulnerable for another stroke.
In the term of IAC impact on ischemic event risk, Mak et al. (5) in a prospective study involving 60 Chinese stroke patients failed to verify the association between IAC and stroke recurrence (14). Different from the study involving Asians, a French research group in 2011 followed up 302 stroke patients over 1 year and demonstrated that a visual IAC score might constitute a risk factor of total future major clinical events (15). Similarly, Ovesen et al. (16) found that the severity of IAC (graded as the number of calcified cerebral arteries) predicted an increased risk of total recurrent ischemic events in a Danish population. However, in these early studies the direct association between IAC and ischemic stroke was not shown. One explanation might be that, IAC was qualitatively assessed with visual scales in these studies, but not quantified, which failed to fully reflect the IAC characteristics. Recently with a quantified software, the Rotterdam Study–a large population-based cohort study in a general community—established the IICA calcification volume to be a major risk factor for a first stroke in a White population (6). Symptomatic intracranial atherosclerotic stenoses patients have recently been found to experience more cerebrovascular events, event under best prevention management, compared to asymptomatic ones with similar vascular risk factors (17). In this study, we concerned about the stroke population who had a higher incidence of IAC. With a quantitative-evaluated IAC Agatston score reflecting both calcium volume and density information, our study demonstrated the association between IAC and recurrent ischemic stroke. The important values of IAC Agatston score may help high risk patient's stratification and management optimization in clinical practice.
In the field about prognostic value of IAC on specific stroke subtypes, little was known. A cross-sectional subgroup analysis based on the Rotterdam Study showed that compared to lacunar ischemic strokes (small vessel disease), non-lacunar strokes is associated with a larger aortic arch calcification volume (18), which confirmed extent arterial calcifications differ on different etiologies. Different to this study, we longitudinally followed up the stroke patients. Our study found that among different stroke etiological subtypes, IAC was independently related to stroke recurrence in patients with index small-vessel occlusive stroke. This finding implicated that IAC play various roles on different causes of stroke and small-vessel occlusive stroke patients with more severe IAC may be particularly vulnerable for another stroke.
For arterial calcification detected on CT, there are now three recognized patterns: intimal calcification which is indicative of a proxy for intracranial atherosclerosis documented in our previous histological study, medial calcification described by Mönckeberg, and internal elastic lamina calcification which is associated with stiffening of the arterial wall and increased pulse pressure (19–23). This study quantitatively evaluated IAC on CT, which include all there above patterns. Although the latter two patterns are currently considered non-atherosclerotic calcification, they could contribute to increased mechanical stress on atherosclerotic plaques, and effect the plaque sequelae potentially. This may be one explanation for the association between quantitatively-evaluated IAC and subsequent events. Besides, association between extent of arterial calcification and stroke prognosis in different ethnicities would also be an interesting point worthy of future attention.
This study presents some strengths. First, consecutive patients are studied on a prospective registry. Second, we use quantitative methods to evaluate arterial calcification, which contain both calcium volume and density information, and reflect the severity of IAC more objectively and authentically than visual scales. Third, we evaluate IAC on non-contrast head CT, which is non-invasive and easily acquired in general hospitals. Finally, this is a very long-term study and the majority of patients are successfully followed up. On the other hand, some limitations exist in this study, including overestimation of extent of vascular calcification in CT imaging, the absence of stroke severity on admission, the absence of treatment for stroke, monocentric study, and the small sample size especially among different stroke subtypes. Yet, as a preliminary study it can provide considerable value for reference in future clinical research.
Conclusion
The quantitatively-evaluated IAC relates to long-term stroke recurrence and vascular mortality. Among stroke subtypes, IAC is associated with recurrent stroke among small-vessel occlusive patients, which indicates choric calcification detected in large cerebral arteries may have potential effects on the cerebrovascular beds extending to small vessels.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XW: conceived and designed the study, collection of data, imaging analysis, data analysis and interpretation, and manuscript writing. XC: conception and design, data interpretation, reviewed and edited the manuscript, and final approval of manuscript. DB: data interpretation, reviewed, and edited the manuscript. LR: data interpretation. TL: collection of clinical data. WC and JA: imaging analysis. LW: conception and design and financial support. All authors: read and approved the manuscript.
Funding
This work was supported by Health and Medical Research Fund (HMRF) (Project Code: 11120161) and Hospital Clinical Research Fund (Project Code: 20193357023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
2. Wagner TH, Lo AC, Peduzzi P, Bravata DM, Huang GD, Krebs HI, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke. (2011) 42:2630–2. doi: 10.1161/STROKEAHA.110.606442
3. Weber R, Weimar C, Diener HC. Medical prevention of stroke and stroke recurrence in patients with TIA and minor stroke. Expert Opin Pharmacother. (2009) 10:1883–94. doi: 10.1517/14656560903048934
4. Chen XY, Lam WW, Ng HK, Fan YH, Wong KS. The frequency and determinants of calcification in intracranial arteries in Chinese patients who underwent computed tomography examinations. Cerebrovasc Dis. (2006) 21:91–7. doi: 10.1159/000090206
5. Mak HK, Wong CW, Yau KK, Wong WM, Gu J, Khong PL, et al. Computed tomography evaluation of intracranial atherosclerosis in Chinese patients with transient ischemic attack or minor ischemic stroke–its distribution and association with vascular risk factors. J Stroke Cerebrovasc Dis. (2009) 18:158–63. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.011
6. Bos D, Portegies ML, van der Lugt A, Bos MJ, Koudstaal PJ, Hofman A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam Study. JAMA Neurol. (2014) 71:405–11. doi: 10.1001/jamaneurol.2013.6223
7. Wu XH, Chen XY, Fan YH, Leung TW, Wong KS. High extent of intracranial carotid artery calcification is associated with downstream microemboli in stroke patients. J Stroke Cerebrovasc Dis. (2016) 26:442–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.007
8. Wu X, Wang L, Zhong J, Ko J, Shi L, Soo Y, et al. Impact of intracranial artery calcification on cerebral hemodynamic changes. Neuroradiology. (2018) 60:357–63. doi: 10.1007/s00234-018-1988-2
9. Wu XH, Chen XY, Wang LJ, Wong KS. Intracranial artery calcification and its clinical significance. J Clin Neurol. (2016) 12:253–61. doi: 10.3988/jcn.2016.12.3.253
10. Leung T, Leung H, Soo YO, Mok VC, Wong KS. The prognosis of acute symptomatic seizures after ischaemic stroke. J Neurol Neurosurg Psychiatry. (2017) 88:86–94. doi: 10.1136/jnnp-2015-311849
11. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. (2006) 31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015
12. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. (1990) 15:827–32. doi: 10.1016/0735-1097(90)90282-T
13. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
14. Ratinov G. Extradural intracranial portion of carotid artery; a clinicopathologic study. Arch Neurol. (1964) 10:66–73. doi: 10.1001/archneur.1964.00460130070010
15. Bugnicourt JM, Leclercq C, Chillon JM, Diouf M, Deramond H, Canaple S, et al. Presence of intracranial artery calcification is associated with mortality and vascular events in patients with ischemic stroke after hospital discharge: a cohort study. Stroke. (2011) 42:3447–53. doi: 10.1161/STROKEAHA.111.618652
16. Ovesen C, Abild A, Christensen AF, Rosenbaum S, Hansen CK, Havsteen I, et al. Prevalence and long-term clinical significance of intracranial atherosclerosis after ischaemic stroke or transient ischaemic attack: a cohort study. BMJ Open. (2013) 3:e003724. doi: 10.1136/bmjopen-2013-003724
17. Fischer U, Hsieh-Meister K, Kellner-Weldon F, Galimanis A, Yan X, Kaesmacher J, et al. Symptomatic and asymptomatic intracranial atherosclerotic stenosis: 3 years' prospective study. J Neurol. (2020) 267:1687–98. doi: 10.1007/s00415-020-09750-2
18. van Dijk AC, Fonville S, Zadi T, van Hattem AM, Saiedie G, Koudstaal PJ, et al. Association between arterial calcifications and nonlacunar and lacunar ischemic strokes. Stroke. (2014) 45:728–33. doi: 10.1161/STROKEAHA.113.003197
19. Yang WJ, Zheng L, Wu XH, Huang ZQ, Niu CB, Zhao HL, et al. Postmortem study exploring distribution and patterns of intracranial artery calcification. Stroke. (2018) 49:2767–9. doi: 10.1161/str.49.suppl_1.WMP44
20. Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. (2009) 4:1892–900. doi: 10.2215/CJN.04320709
21. Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. (2014) 35:1515–25. doi: 10.1093/eurheartj/ehu163
22. Vos A, Van Hecke W, Spliet WG, Goldschmeding R, Isgum I, Kockelkoren R, et al. Predominance of nonatherosclerotic internal elastic lamina calcification in the intracranial internal carotid artery. Stroke. (2016) 47:221–3. doi: 10.1161/STROKEAHA.115.011196
Keywords: intracranial arterial calcification, stroke recurrence, stroke, atherosclerosis, Agatston score, post-stroke mortality
Citation: Wu X, Bos D, Ren L, Leung TW, Chu WC-W, Wong LKS, Abrigo J and Chen XY (2020) Intracranial Arterial Calcification Relates to Long-Term Risk of Recurrent Stroke and Post-stroke Mortality. Front. Neurol. 11:559158. doi: 10.3389/fneur.2020.559158
Received: 05 May 2020; Accepted: 31 August 2020;
Published: 09 October 2020.
Edited by:
Yasushi Takagi, Tokushima University, JapanReviewed by:
Mirjam R. Heldner, University Hospital Bern, SwitzerlandYasuhisa Kanematsu, Tokushima University, Japan
Copyright © 2020 Wu, Bos, Ren, Leung, Chu, Wong, Abrigo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Yan Chen, ZmlvbmEuY2hlbkBwb2x5dS5lZHUuaGs=
 Xiaohong Wu
Xiaohong Wu Daniel Bos
Daniel Bos Lijie Ren
Lijie Ren Thomas Wai-hong Leung
Thomas Wai-hong Leung Winnie Chiu-Wing Chu
Winnie Chiu-Wing Chu Lawrence Ka Sing Wong4
Lawrence Ka Sing Wong4 Xiang Yan Chen
Xiang Yan Chen