
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 03 December 2020
Sec. Headache and Neurogenic Pain
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.557415
This article is part of the Research Topic Orofacial Functions: From Neural Mechanisms to Rehabilitation View all 50 articles
 Kanokporn Bhalang1*
Kanokporn Bhalang1* Beat Steiger2
Beat Steiger2 Nenad Lukic3
Nenad Lukic3 Aleksandra Zumbrunn Wojczyńska3
Aleksandra Zumbrunn Wojczyńska3 Ray S. Hovijitra4
Ray S. Hovijitra4 Dominik A. Ettlin3,5,6
Dominik A. Ettlin3,5,6Introduction: Orofacial pain features may negatively influence a person's well-being and vice versa. Some aspects of well-being can be measured with axis II instruments that assess patients' psychosocial and behavioral status. The aim of this study was to investigate associations between pain features and psychosocial variables as indicators of well-being.
Materials and Methods: Seven hundred ninety-nine anonymized datasets collected using the Web-based Interdisciplinary Symptom Evaluation (WISE) of patients reporting to the Interdisciplinary Orofacial Pain Unit, University of Zurich, between March 19, 2017 and May 19, 2019, were analyzed. Pain features including intensity, number of locations, impact, and duration were evaluated. Psychometric measures assessed pain-related catastrophizing and disability, illness perception, distress, anxiety, depression, injustice experience, dysmorphic concerns, and insomnia.
Results: Most patients were between 30 and 59 years old (58.3%), female (69.8%), working (66.0%), and experienced pain for more than 6 months (68.5%). Pain intensities were higher in women than men and higher in disabled than working patients. Scores indicating elevated stress and depression were also observed in disabled patients. The sample prevalence rates of clinically relevant axis II instrument scores were as follows: Graded Chronic Pain Scale for the Head (GCPS-H), 27%; Patient Health Questionnaire 4 (PHQ4), 21%; PHQ9, 21%; Pain Catastrophizing Scale (PCS), 20%; General Anxiety Disorder 7 (GAD7), 15%; Insomnia Severity Index (ISI), 15%; Injustice Experience Questionnaire (IEQ), 14%; GCPS for the Body (GCPS-B), 13%; PHQ for Stress (PHQstr), 6%; and Dysmorphic Concern Questionnaire (DCQ), 2%. Noteworthy results of correlation analysis of the clinically relevant axis II scores and pain measures were as follows: the PHQstr had moderate associations (0.34–0.43) with the sum of pain intensity at rest and during function, number of pain locations, and typical pain intensity. The IEQ scores were moderately associated with typical pain intensity at 0.39. The DCQ scores were moderately associated with pain extension at 0.41.
Conclusions: Moderate correlations of certain pain and well-being measures were found in patients reporting clinically relevant stress, injustice experience, and dysmorphic concern, all of which reflect impaired well-being. PHQ4 is suitable for routine distress screening in the clinical setting.
Pain is a complex, multidimensional construct that includes features such as intensity (at rest and during movements), spread, symmetry, chronicity, and impact of pain. Analogous to pain in any other body region, orofacial pain (OFP) negatively influences well-being and vice versa (1). Well-being can be conceptualized as a spectrum, with happiness, minimal distress, and high well-being at one end and elevated depression, anxiety, and low well-being at the other (2). Diverse psychometric instruments exist for indirectly measuring well-being, e.g., the Hospital Depression and Anxiety Scale, the General Health Questionnaire, the Perceived Stress Scale, and the Positive and Negative Affect Schedule (3). Accordingly, a well-being proxy measure may consist of a diverse set of psychometric instruments that offer information on a patient's psychological and behavioral status. From a pain management perspective, well-being proxy measures, such as anxiety, depression, catastrophizing, and others, are all relevant in influencing treatment outcomes in patients experiencing OFP and temporomandibular disorders (TMDs) (4–7). Another important negative influence on well-being is unemployment, resulting in feelings of worry, insecurity, and stress due to changes in the patient's financial situation (8, 9).
Pain and well-being are interrelated. Hence, a dual-axis diagnostic system has been proposed for evaluating patients experiencing OFP and TMD (10). This system differentiates physical diagnoses (which can be grouped into axis I) from the patients' psychosocial and behavioral status (known as axis II) reflecting their well-being. Notably, axis I diagnoses are categorical in nature, such as temporomandibular joint disc displacement or arthritis, while axis II construct measures have ordinal values. According to an international expert panel, axis II instruments aim at assessing the psychosocial and behavioral status (including pain-related disability) (11). For the purpose of this paper, we refer to pain as a symptom and not as a diagnosis or psychological status per se. Sleep has been proposed as an additional axis II construct because a strong relationship between self-assessed sleep disturbances, OFP, and TMD has been observed (12, 13). Perceived injustice, anger, illness perception, catastrophization, and dysmorphophobia are additional potential moderators of the pain–to–well-being relationship (14–17).
The Web-based Interdisciplinary Symptom Evaluation (WISE) is an online tool based on self-reports that assists clinicians in the comprehensive assessment of patients with OFP or TMD (18). WISE records sex, age, and employment status. This evaluation further combines a description of pain features with widely available in-depth psychometric measures. The automatically generated summary report supports clinicians in identifying case complexity and disability levels. WISE thus facilitates resource planning for the initial consultation, such as allocation of time and appropriate healthcare specialists. Anonymized WISE data can be extracted for research purposes.
Using anonymous data collected by the WISE tool, the aim of this study was to correlate pain features with other axis II construct measures reflecting well-being in patients experiencing OFP of mixed etiologies. Further, we analyzed whether known confounders such as sex, age, employment status, and pain duration influenced psychometric scores and pain features.
This study included 799 anonymized WISE datasets of patients reporting to the Interdisciplinary Orofacial Pain Unit, Center of Dental Medicine, University of Zurich, Switzerland, between March 19, 2017 and May 19, 2019. The spectrum of axis I diagnoses encountered in this unit has been reported elsewhere (16). Patients completed the WISE questionnaire prior to their first clinical appointment. Anonymized data were retrieved from a server located at Hof University of Applied Science, Germany, and exported in csv format for statistical analysis. According to Swiss law, researchers can use strictly anonymized data and do not require approval by an ethics committee. Only fully completed WISE datasets of patients reporting OFP were analyzed, given that the subjects consented to the use of their anonymized data for research. Data related to non-painful symptom reports were excluded.
The WISE system is a Web-based instrument for interdisciplinary subject-tailored symptom evaluation in patients experiencing OFP and TMD (18). WISE combines a symptom-oriented checklist with validated in-depth questionnaires, also referred to as case finding instruments. The questionnaires are presented when the checklist scores exceed threshold values and thus indicate a burden related to the screening item. Graphical maps offer patients the opportunity to indicate their pain location, intensity, and duration in various defined oral and cranial tissues as well as other body regions. In addition to pain measures, several other psychometric instruments are integrated. Only German and English versions of the WISE were completed. German versions of the applied measures are available. The following variables from the WISE datasets were used in this study.
Pain features were captured by detailed interactive pain maps of the head and neck and front/back view maps of the rest of the body. Pain locations were differentiated by 93 selectable anatomical areas across the entire body and 72 areas across the head and neck region (Figure 1). Patients marked painful areas by clicking on a defined area and reported the typical pain intensity at rest and upon movement. Both were reported for the last 4 weeks on an 11-point numeric rating scale (NRS) for pain. Different pain intensities at rest were represented by gradients of red, and different pain intensities upon movement were captured by graded widths of anatomical green borders.
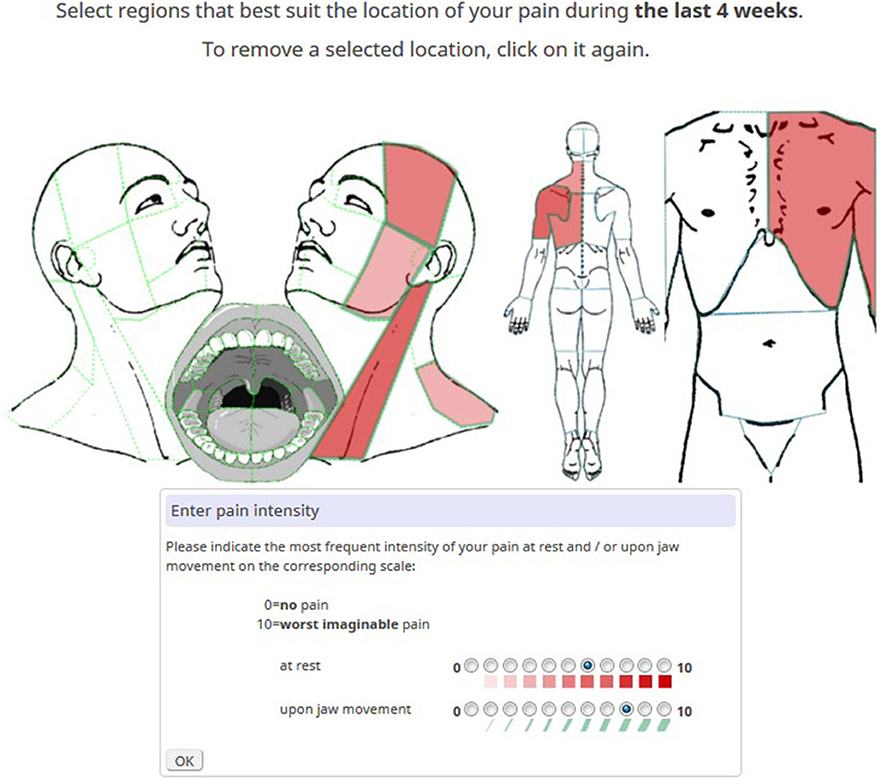
Figure 1. Example of a pain diagram with marked pain locations. The most intense red hue signifies the highest pain intensity in the sternocleidomastoid muscle. The broadest green border reflects the highest pain intensity upon jaw movement in the masseter area.
Using the same NRS, typical (PI-typ) and maximum (PI-max) pain intensities of the chief complaint were additionally reported. Pain extension for the body (P-ext-B) was defined as the total number of pain locations across the entire body and pain extension for the head (P-ext-H) was defined as the number of pain locations across the head and neck region (left part of Figure 1). Pain impact at rest and pain impact upon movement were calculated as the sum scores of pain intensities across the head and neck region at rest (∑-PI-rest-H) and upon movement (∑-PI-move-H), respectively. The duration of OFP (P-dur) as a measure of chronicity was grouped by intervals: <3 months, 3–6 months, 6 months−2 years, 2–5 years, >5 years.
The Brief Illness Perception Questionnaire (B-IPQ) (19–21) assesses cognitive and emotional representations of illness and health threat. Eight questions covering different aspects of illness perception were rated on an NRS ranging from 0 to 10. The maximum score is 80. No cutoff score has been reported for this questionnaire. Test–retest reliability of the single items of the B-IPQ ranged from 0.48 to 0.70; a coefficient alpha value was not reported.
The Dysmorphic Concern Questionnaire (DCQ) (22, 23) assesses excessive preoccupation with an imagined or a real minimal defect in appearance that is associated with a significant impact on psychosocial functioning. The questionnaire consists of seven items that cover different aspects of dysmorphic concern. Each item is rated on an ordinal scale ranging from 0 to 3, resulting in a maximum sum score of 21. A cutoff score of nine indicates a possible body dysmorphic disorder. The following coefficient alpha for the DCQ has been reported: 0.85; test–retest reliability was not reported.
The Graded Chronic Pain Scale (GCPS) (24) consists of three pain intensity scales ranging from “no pain” (=0) to “pain as bad as it could be” (=10) assessing current, worst, and average pain intensity for the last 30 days. The disability days report the number of days being kept from daily activities by pain for the last 3 months. The number of days determines a disability score ranging from 0 to 3. Three additional scales measure the interference by pain during (1) daily activities, (2) recreational, social, and family activities, and (3) working ability on a scale from “no interference” (=0) to “unable to carry on any activities” (=10) for the last 30 days. The average value of these three disability scales yields a disability score ranging from 0 to 3. Combining the pain activity interference score with the disability day score results in a disability sum score ranging from 0 to 6. Two separate disability scores due to pain in the head (GCPS-H) or the body (GCPS-B) were calculated. Scores ≥3 are considered as high disability and severely limiting. The following coefficient alpha has been reported: 0.71 for TMD pain; test–retest reliability has not been reported.
The General Anxiety Disorder 7 (GAD7) (25) assesses general anxiety in primary care patients. Seven items cover different aspects of general anxiety. For the question “Over the last 2 weeks, how often have you been bothered by the following problems? “items are scored on a four-point scale from not at all” (=0), “several days” (=1), “half of the days” (=2), and “nearly every day” (=3). Summary scores range from 0 to 21 and indicate anxiety levels of “none/minimal” (0–4), “mild” (5–9), “moderate” (10–14), or “severe” (>14). The following coefficient alpha has been reported: 0.92; for test–retest reliability: 0.83.
The Injustice Experience Questionnaire (IEQ) (26–28) assesses injustice experienced due to accidents, injuries, or maltreatment. Twelve items reflect the frequency of thoughts, beliefs, and emotions associated with injury. They are rated on a scale with the following response options: “never” (=0), “rarely” (=1), “sometimes” (=2), “often” (=3), to “all the time” (=4). The maximum score is 48. Scores ≥18 indicate the need for professional evaluation. The following coefficient alpha has been reported: 0.92; for test–retest reliability: 0.90.
The Insomnia Severity Index (ISI) (29, 30) screens for sleep disorders by measuring the severity of insomnia problems, sleep-related satisfaction, and interference on a scale of “none” (=0), “mild” (=1), “moderate” (=2), “severe” (=3), and “very severe” (=4) for the items falling asleep, staying asleep, and waking up too early over the past 2 weeks. On a scale from “very satisfied” (=0), “satisfied” (=1), “moderately satisfied” (=2), “dissatisfied” (=3), to “very dissatisfied” (=4), the patient indicates how satisfied/dissatisfied he/she perceives his/her current sleep pattern. On three additional scales from “not at all” (=0), “a little” (=1), “somewhat” (=2), “much” (=3), to “very much” (=4), the patient is asked how much he/she considers his/her sleep problem to interfere with daily activities, how noticeable the interference by his/her sleep problem is to others, and how worried/distressed the patient is because of his/her sleep problem. The maximum score is 28, with scales of “none” (0–7), “subthreshold” (8–14), “moderate” (15–21), or “severe” (>21). The following coefficient alpha has been reported: 0.74; test–retest reliability was not reported.
The Pain Catastrophizing Scale (PCS) (31, 32) assesses catastrophizing thoughts and corresponding behavior. The questionnaire has 13 items that are scored on a five-point ordinal scale [“not at all” (=0), “to a slight degree” (=1), “to a moderate degree” (=2), “to a great degree” (=3), or “all the time” (=4)]. Scores can also be calculated for three subscales of helplessness, maximizing, and ruminating. The maximum value is 52 for the entire questionnaire. The corresponding cutoff values are 13 for helplessness, five for maximizing, 13 for ruminating, and 30 for the entire questionnaire. These cutoff values correspond to the 75th percentile. The following coefficient alpha has been reported: 0.87; for test–retest reliability: 0.75.
The Patient Health Questionnaire 4 (PHQ4) (33) screens for anxiety and depression. This questionnaire consists of two subscales, GAD2 (items one and two of the GAD7) and PHQ2 (items one and two of the PHQ9). Items are scored on an ordinal scale ranging from 0 to 3 using the same labels as the GAD7. Scores can be calculated for the two subscales (maximum score = 6) and overall (maximum score = 12). Scores ≥6 for the total score and three for the sub-scores indicate expert evaluation referral. The following coefficient alpha has been reported: 0.87; for test–retest reliability: 0.81.
The PHQ9 (34, 35) assesses the severity of depression. Nine items covering different aspects of depression are scored on an ordinal scale ranging from 0 to 3 using the same labels as the GAD7. Summary scores range from 0 to 27, indicating depression levels of “none/minimal” (0–4), “mild” (5–9), “moderate” (10–14), “moderately severe” (15–19), or “severe” (>19). A cutoff score range of 8–11 has been recommended for expert evaluation referral. The following coefficient alpha has been reported: 0.89; for test–retest reliability: 0.84.
The Patient Health Questionnaire for Stress (PHQstr) (34, 36) is a 10-item subscale of the Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire that addresses psychosocial stress burden. Items are scored on an ordinal scale ranging from “not at all” (=0), “a little” (=1), to “a lot” (=2). Summary scores range from 0 to 20 indicating the degree of psychosocial stress burden by the following levels: “none/minimal” (0–4), “mild” (5–9), “medium” (10–14), or “severe” (>14). No coefficient alpha nor test–retest reliability was reported.
The data were analyzed using SPSS (Version 22, Armonk, New York). Descriptive statistics were used to characterize the datasets. Spearman rank correlation was used for correlation analysis. To determine the effect size, small (0.1 < r < 0.3), moderate (0.3 < r < 0.5), and strong (r ≥ 0.5) were used for the different association levels (37). Analysis of variance was used with a Bonferroni or Tamhane correction for post hoc testing depending on whether the variances were equal or not. A significance level of ≤0.05 was considered statistically significant.
Most patients were female (N = 558; 69.8%), with a female-to-male ratio of 2.3:1. Among the patients, 73.6% (N = 588) were 20–59 years old, 66.0% were working (N = 527), and 84.4% suffered from pain for more than 3 months (N = 675) (Table 1).
The descriptive statistics of various pain measures are shown in Table 2. On average, patients had 8.69 pain sites across the entire body and 5.54 pain sites across the head region. The mean typical pain intensity was 4.91, and the mean maximum pain intensity was 7.10. With an average of 33.4, the intensity sum score at rest was lower compared with upon movement (38.2).
We determined the influence of patient characteristics (sex, age, employment status, and pain duration) on the mean of the assessed pain features. Sex had a significant influence on PI-typ, PI-max (Figure 2), and ∑-PI-rest-H, with women reporting higher mean values. Effects of age were found for P-ext; patients aged 30–39 years indicated more pain locations compared with older patients (>60 years old). Employment status demonstrated significant effects on all pain-related measures. Disabled patients significantly indicated more pain locations compared to employed patients, patients in training, or retired patients. Working patients presented more pain locations compared to retired patients. The highest typical and maximum pain intensities were reported by patients without a job and by disabled patients (Figure 2). The ∑-PI-rest-H was highest in disabled patients. Pain duration had significant effects on all pain-related measures, except for PI-typ and PI-max. The number of pain locations, ∑-PI-rest-H, and ∑-PI-move-H increased with the duration of pain.
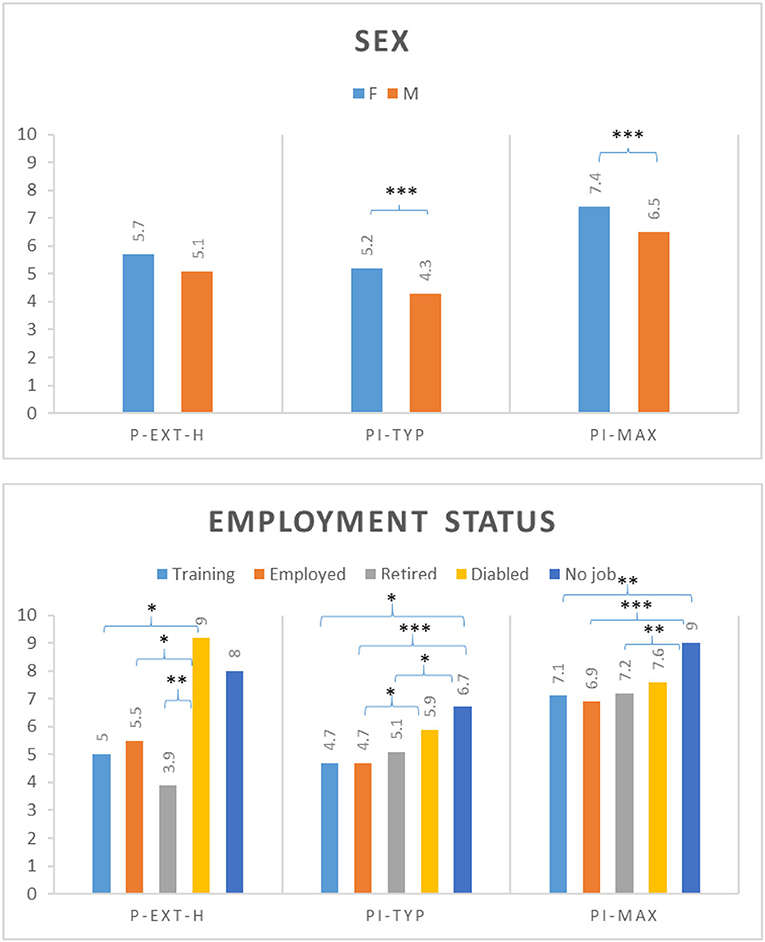
Figure 2. Analysis of variance between selected patient characteristics (sex, employment status) and selected pain features (P-ext-H, number of pain locations across the head and neck region; PI-typ, typical pain intensity; PI-max, maximum pain intensity); Post hoc tests with Bonferroni correction or Tamhane correction (*P ≤ 0.05 **P ≤ 0.01 ***P ≤ 0.001).
The number of patients who completed one of the case finding questionnaires ranged from 216 (GAD7) to 799 (PCS, PHQ4, and PHQstr). The percentage of patients reaching a clinically relevant score (cutoff) ranged from 5.1% (DCQ) to 73.7% (PHQ9) (Table 3).
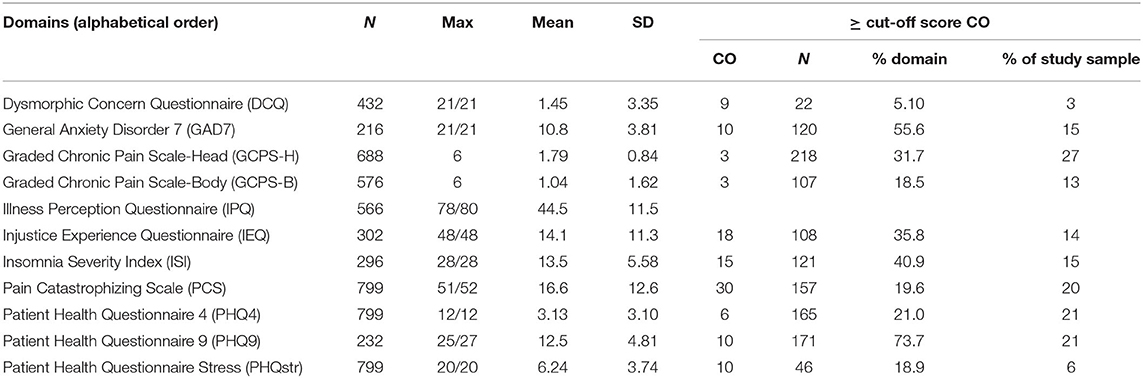
Table 3. Scores of the axis II psychometric measures [Note that ≥ cutoff score (CO) indicates clinical relevance].
The analysis of variance of the selected mean axis II domain scores grouped by selected patient characteristics (age group and employment status) is shown in Figure 3. There were no significant effects of age on axis II measures, except that older patients (>60 years) had lower PHQstr scores than those aged 30–59 years, and patients in the middle age groups had a higher PHQ4 score than those in the younger age groups and in older patients. Employment status had a significant effect on several measures. GCPS-H scores were higher among disabled patients or patients with no job than among trainees, working, or retired patients; GCPS-B scores were higher in disabled patients than in trainees, working, or retired patients. PCS and PHQ4 values were higher in disabled patients or patients with no job compared with trainees, working, or retired patients. PHQ9 scores were higher in disabled than in retired or working patients, and PHQstr scores were higher in disabled patients than in trainees, working, or retired patients. Pain duration had no significant effect on psychosocial variables except for PHQ4 and PHQstr, where patients experiencing pain longer than 5 years had higher mean values compared with patients with a pain duration of <3 months.
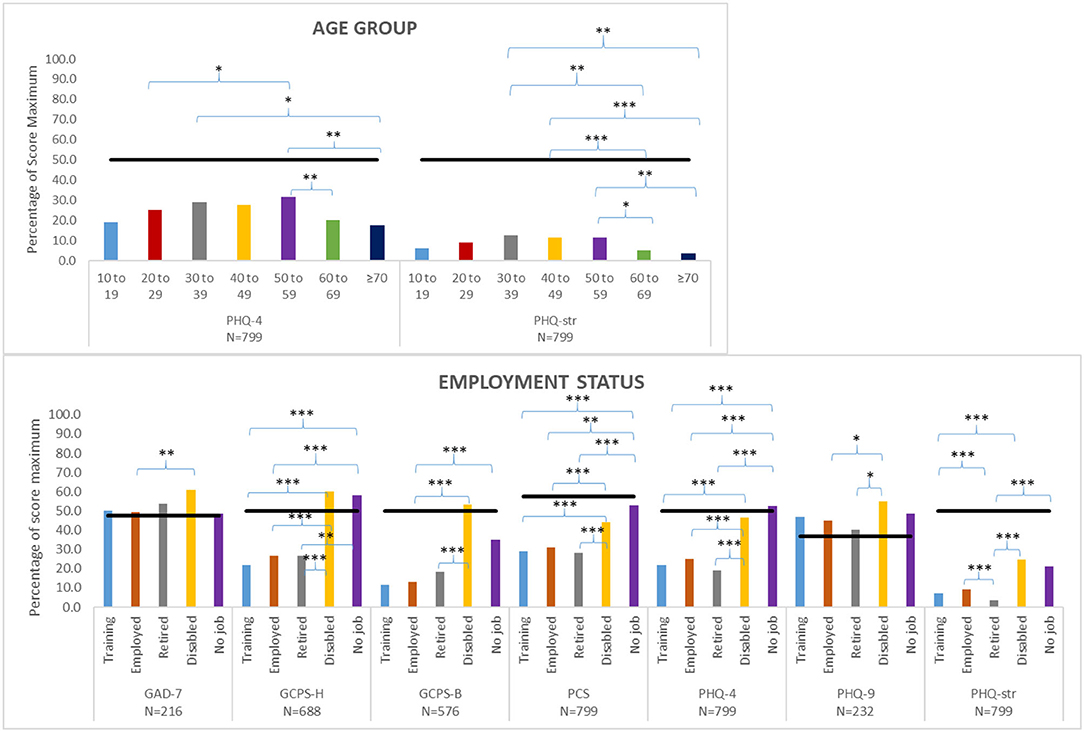
Figure 3. Analysis of variance between selected patient characteristics (age group, employment status) and selected axis II measures [Patient Health Questionnaire 4 (PHQ4), Patient Health Questionnaire Stress (PHQstr), General Anxiety Disorder 7 (GAD7), Graded Chronic Pain Scale-Head (GCPS-H), Graded Chronic Pain Scale-Body (GCPS-B), Pain Catastrophizing Scale (PCS), Patient Health Questionnaire 9 (PHQ9)]; Post hoc tests with Bonferroni correction or Tamhane correction (*P ≤ 0.05 **P ≤ 0.01 ***P ≤ 0.001). Maximum scores are listed in Table 3. Black bars indicated cutoff scores of the respective psychometric instrument.
For the correlations between various pain features and axis II measures (Figure 4A), the following five pain features showed moderate correlation effects (r ≥ 0.30; P < 0.50) with other axis II measures: (1) ∑-PI-rest-H with PCS, PHQ4, PHQstr, GAD7, IPQ, GCPS-H, and GCPS-B; (2) ∑-PI-move-H with PCS, PHQ4, GCPS-H, and GCPS-B; (3) PI-max with PCS, IPQ, and GCPS-H; (4) PI-typ with PCS and GCPS-H; and (5) P-ext-B with PHQ4 and PHQstr. Pain duration showed only weak or no correlations with all pain measures. All other axis II measures (IEQ, ISI, and DCQ) only had weak or no correlations with pain features.
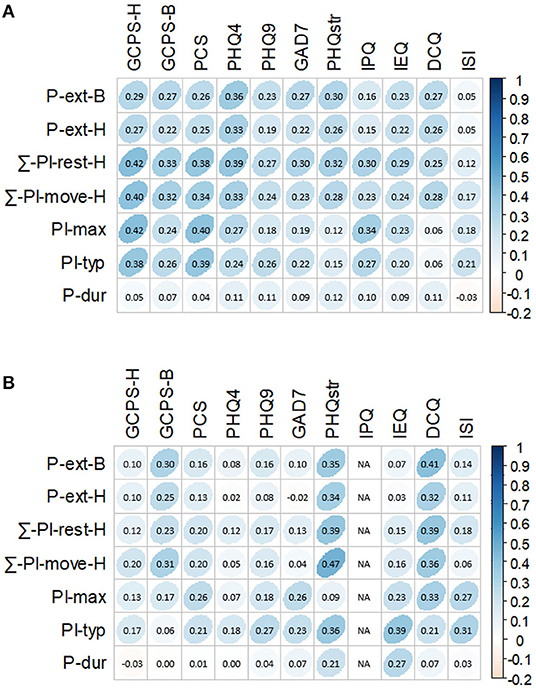
Figure 4. Correlations between scores of pain measures and scores of axis II measures as proxies for well-being [Pain measures number of pain locations across the entire body (P-ext-B), number of pain locations across the head and neck region (P-ext-H), summary score of pain intensity at rest across the head and neck region (∑-PI-res-H), summary score of pain intensity upon movement across the head and neck region (∑-PI-move-H), maximum pain intensity (PI-max), typical pain intensity (PI-typ), and pain duration (P-dur) and axis II measures Graded Chronic Pain Scale-Head (GCPS-H), Graded Chronic Pain Scale-Body (GCPS-B), Pain Catastrophizing Scale (PCS), Patient Health Questionnaire 4 (PHQ4), Patient Health Questionnaire 9 (PHQ9), General Anxiety Disorder 7 (GAD7), Patient Health Questionnaire Stress (PHQstr), Illness Perception Questionnaire (IPQ), Injustice Experience Questionnaire (IEQ), Dysmorphic Concern Questionnaire (DCQ), and Insomnia Severity Index (ISI); Spearman rank correlation was used for the analysis (P = 0.05), and darker blue shades represent higher correlations]. (A) Correlations between scores of pain measures and all scores of axis II measures. (B) Correlations between scores of pain measures and scores above clinical relevance of axis II measures.
From Figure 4B, when correlation analyses were performed after dichotomizing questionnaire scores into above and below cutoff values, we found that PHQstr scores above nine (N = 46) significantly correlated with levels of P-ext, PI-typ, ∑-PI-rest-H, and ∑-PI-move-H (r = 0.34–0.47; P < 0.05). GCPS-B scores above cutoff level (N = 107) significantly correlated with P-ext-B and ∑-PI-move-H (r = 0.30 and 0.31, respectively; P < 0.001). DCQ scores above cutoff level (N = 22) moderately correlated (r = 0.32–0.41) with P-ext, PI-max, ∑-PI-rest-H, and ∑-PI-move-H. IEQ scores above cutoff level (N = 93) significantly correlated with PI-typ (r = 0.39; P < 0.001). ISI scores above 14 (N = 121) significantly correlated with PI-typ (r = 0.31; P < 0.001).
The aim of this study was to further understand the pain–to–well-being relationship in patients experiencing OFP. Thus, the relationships between various pain features and other psychometric measures as indicators of well-being were analyzed. Furthermore, pain features and psychometric scores were analyzed with regard to patient characteristics, such as gender, age group, employment status, and pain duration.
Anonymized data were derived from a large clinical cohort of 799 patients who completed the WISE between March 19, 2017 and May 19, 2019. This comprehensive evaluation tool is routinely used in the Orofacial Pain Unit of the Center of Dental Medicine, University of Zurich, to plan the resources for the initial consultation, such as allocation of time and appropriate healthcare specialists. Table 1 indicates that the gender proportion and age distribution matched those reported in other studies on patients seeking care for OFP (16, 38–40). Three quarters of our sample were in training or working, while 6.5% of patients were disabled. Most patients were considered chronic pain sufferers since more than 80% of them experienced pain longer than 3 months (41).
On average, patients reported pain in ~9 of possible 93 locations as defined in the diagrams in Figure 1, indicating that the majority experienced locoregional pain. On the 11-point numerical pain scale, the reported mean pain intensity was moderate (4.9), with a mean maximum intensity of 7.1. As expected, pain was more intense upon movement compared to at rest (Table 2). Pain intensities were higher in women than men and higher in disabled than working patients (Figure 2). As was previously reported, pain is generally more prevalent in females in the head and neck areas (42, 43). Somewhat counterintuitively, pain extension decreased from middle to old age as was reported in fibromyalgia patients (44). This may be due to the observation that OFP origins shift across age from myogenic to neurogenic (e.g., trigeminal neuralgia) and the latter being typically a localized pain (45, 46). This may also explain why working subjects indicated more pain locations than those retired. Recent findings do not indicate that OFP mediates the relationship between socioeconomic inequalities (linked to disability) and the impacts of oral conditions on daily life (47).
Table 3 summarizes the axis II questionnaire scores serving as proxies for well-being. The PHQ4 is part of the WISE checklist and was therefore completed by all patients. All patients were also asked to complete the PCS due to relevant associations of pain catastrophizing with pain-related disability, number of painful body sites, and pain-related impact (7). The sample prevalence rates of clinically relevant axis II instrument scores (above cutoff) in descending order were as follows: GCPS-H, 27%; PHQ4 and PHQ9, 21%; PCS, 20%; GAD7, 15%; ISI, 15%; IEQ, 14%; GCPS-B, 13%; PHQstr, 6%; and DC, 3%. No cutoff value has been reported for the IPQ. The GCPS can predict the healthcare utilization cost for chronic OFP patients (48). Approximately one in four patients experienced moderate or severe impairment due to craniofacial pain (and 13% due to bodily pain). Almost as many had PHQ scores that warrant further evaluation for depression, whereas 15% experienced symptoms characteristic of a GAD. The 20% of patients reporting pain catastrophizing scores above 29 might have an elevated risk of delayed pain recovery (49). Pain catastrophizing was demonstrated to mediate the effects of distress in OFP patients, which was attributed to the helplessness component of the PCS (50). Due to the sample overlap, the prevalence of moderate and severe grades of insomnia (15%) confirms the 16% we previously reported (13). A novel finding of this study is that approximately one in seven patients (14%) perceived injustice of a clinically relevant level. It is noteworthy that patients concerned about injustices in the treatment they receive are vulnerable to greater emotional distress, prolonged work disability, invalidating or stigmatizing reactions of others, and poor pain-related outcomes (51–53). According to Sullivan (54), blame cognitions may have an impact on feelings of anger and revenge motives that warrant interventions to alter the individual's perceptions of the offender. Although perceived stress has been reported to predict the incidence of painful TMDs, the number of patients (6%) reporting medium to severe stress on the PHQstr was rather low in this sample, likely due to the broad variety of pain origins (16, 18, 29). And only 2% reached clinically relevant DCQ scores. Health anxiety questionnaire was reported to have good association with OFP and other related syndromes (55) and was found to be a strong predictor of chronic OFP (56). However, we only observed moderate correlation between GAD7 score and ∑-PI-rest-H in our subjects. The inclusion of patients experiencing various types of disorders in this study is justified by the pain associations with axis II psychological and psychosocial variables independent of pain origin (57).
Women not only experienced significantly more intense pain than men (Figure 2) but also suffered significantly severer impairment (data not shown). A multitude of mechanisms has been proposed to explain sex differences in pain and emotional processing, including the effects of sex hormones, differences in endogenous opioid function, cognitive/affective influences, coping patterns, and contributions of social factors such as stereotypic gender roles (58, 59). In our sample, PHQ4 and PHQstr scores were highest in 50–59-year-old subjects, which is consistent with a Japanese study reporting PHQ scores being low in young adulthood, increasing in middle age (peaking during age 50–59), and then decreasing again in older age (60). The relationship between employment-related factors and chronic pain lacks adequate research (61). Our findings that being disabled and/or unemployed was negatively associated with various axis II instrument scores emphasize the need for routine collection of information on employment status to optimize clinical care and future research (62). It is noteworthy that daily activities are negatively influenced not only by pain itself but also by pain-related fear (63).
As the structure of the WISE instrument is modular, not all 799 patients completed each axis II instrument or each pain-related questionnaire. However, everyone was requested to complete questions related to pain locations, intensities, and duration. None of the calculated correlations was strong (r ≥ 0.5). The fact that the PCS, PHQ4, and PHQstr were completed by all patients and the GCPS-H by nearly 90% of them largely explains their highest correlation levels with pain intensity variables and pain extension for the entire sample (Figure 4A). Such effects reversed when only cases with clinically relevant scores (above cutoff) were analyzed (Figure 4B). Our data thus support the routine use of PHQ4 as a screener for anxiety and depression in the clinical setting, as recommended by international expert panels (11). PEG, a three-item scale measuring pain intensity (P), interference with enjoyment of life (E), and interference with general activity (G), is another tool available for initial pain patient evaluation (64). Psychological comorbidity has been shown to influence patients' illness perceptions by means of pain ratings, treatment-seeking behavior, and treatment adherence, as well as recovery after surgical procedures (65–67).
The well-being of patients is more likely compromised when we defined compromised well-being by axis II scores at or above cutoff values. Figure 4B reveals that patients experiencing widespread pain and high sum intensity scores suffered high distress levels (PHQstr scores ≥10; N = 46; r = 0.34–0.47; all P-values <0.05). This confirms previous findings that self-reported stress independent of its origin (pain or non-pain-related) can initiate or exacerbate the impact of TMDs, chronic pain, and disability (68, 69). However, further research is needed to clarify the amount, timing, severity, and type of stress that is needed to contribute, maintain, and exacerbate persistent pain (70). While stress is often comorbid with anxiety, depression, as well as pain catastrophizing and persistence, clinically relevant scores of these domains did not significantly correlate with any pain measure in our study (6, 71–73). The low number of patients experiencing dysmorphic features (DCQ scores ≥9; N = 22) makes it difficult to interpret correlations with pain features. Still, this may be an interesting topic for further exploration.
A new and highly significant finding was that typical pain intensity moderately correlated with high scores on the IEQ (scores >17; N = 93). The IEQ has proven to be a good predictor for delayed healing in patients experiencing pain and post-traumatic stress (6, 74, 75). However, we are unaware of the use of this questionnaire in the OFP domain, except for its German-language validation (28). Notably, patients perceiving injustice also had pain for a longer duration compared to subjects with lower IEQ scores (r = 0.27; P < 0.001).
Although patients with OFP report sleep disturbances disproportionately more often compared with the general population (13), clinically relevant insomnia only associated with typical pain intensity at a moderate level, which is in line with previous observations (ISI scores >15; N = 121) (76). This finding indicates that other psychosocial variables likely contribute more to insomnia than the selected pain features, and it supports the notion that sleep impairment is more likely a predictor of OFP than pain is of sleep impairment (77). Surprisingly to us, pain duration did not correlate with any of the axis II measures employed in our study, except for a weak correlation with perceived injustice.
A key difference of this study as compared to the OPPERA studies (78) is that our sample is a pure clinical sample and therefore more likely represents a clinical population that most dentists encounter in their practices. Taken together, our results support Schiffman et al. (11) for the use of GCPS and PHQ4 as screening tools in clinical practice; however, for comprehensive assessments of orofacial patients' well-being, not only PHQ9 and GAD7 but also PHQstr, DCQ, IEQ, and ISI should be utilized by specialists to obtain a more in-depth evaluation of these patients.
Although it was not the main objective of this study, we also explored the correlations between various axis II instruments (data not shown) and, interestingly, found that IPQ and IEQ strongly correlated with PCS and PHQ4 (r = 0.55–0.61; P < 0.001).
Since this is a cross-sectional study, we cannot draw definite causal conclusions regarding the relationship between pain and well-being. The WISE pain drawing allows patients to report on pain intensities in every region marked on the body diagram. When a person experienced pain in multiple locations, the limitation to a single numeric value for the typical (PI-typ) and maximum (PI-max) pain intensity characteristic of his/her chief complaint may have made the choice difficult. We did not attempt to capture psychological factors that influence pain intensity and distribution on pain drawings (79, 80), although we are aware of their importance in understanding the somatic awareness present in chronic pain conditions (81). Since the DC/TMD axis II measures currently do not cover some of the biopsychosocial aspects of interest in this study, which included a broad variety of OFP complaints beyond TMDs, we have incorporated several additional psychometric instruments in our data analyses. Most patients in this study had moderate pain intensities, which may explain why no strong correlation levels were detected in our analyses. The low prevalence of DCQ likely explains the lack of correlation between the DCQ and the assessed pain dimensions. Nevertheless, the fact that the correlation between dysmorphic concern and pain has not previously been reported warrants further investigations. This study did not examine the presence of additional painful comorbidities, although patients experiencing prolonged painful TMDs are at increased risk of suffering from other painful conditions (82). Furthermore, we did not employ a specific instrument for measuring well-being, such as the Warwick–Edinburgh Mental Well-Being Scale (83). Rather, our well-being proxy measures included the set of axis II constructs listed in the Materials and Methods section.
Due to the modular structure of the WISE that questions patients according to their symptom burden, a variable number of the 799 patients completed the axis II instruments. Pain intensities were higher in women than men and highest in patients with disabilities or no job. The latter group also had the highest scores for pain-related disability, pain catastrophizing, stress, and depression. Older patients (60+ years) had significantly lower stress scores and were less likely to report symptoms of anxiety or depression. When correlating pain and well-being measures, moderate associations were observed in patients reporting clinically relevant stress, insomnia severity, injustice experience, and dysmorphic concern. Surprisingly, compromised well-being in the form of anxiety, depression, and pain catastrophizing (clinically relevant scores of GAD7, PHQ9, and PCS) did not correlate with any of the pain measures. The results of this study support the clinical usefulness of the DC/TMD core assessment instruments, but also suggest measuring of sleep, catastrophizing, injustice experience, and dysmorphic concern as important dimensions for patients suffering from OFP symptoms. Further studies are needed to determine how pain and well-being influence each other, i.e., to clarify whether there are direct causal influences or influences mediated by other variables not investigated in this study. Preferably this would be related to a theoretical framework about relations between OFP, well-being, psychiatric disorders, and socioeconomic status.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
All authors listed have made substantial, direct and intellectual contribution to the work, and approved it for publication.
KB travel was supported by the Chulalongkorn University Office of International Affairs Scholarship for Short-term Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Prof. Martin Tyas, Dr. Joao Ferreira, Dr. Kevin Tompkins, and Dr. Vera Colombo for manuscript editing. The translation of the WISE in various languages was kindly sponsored by the Digital Society Initiative of the University of Zurich.
1. Ohrbach R, Durham J. Biopsychosocial aspects of orofacial pain. In: Farah CS, et al. editors. Contemporary Oral Medicine. Cham, Switzerland: Springer Nature (2019). p. 1797–818. doi: 10.1007/978-3-319-72303-7_37
2. Johnson J, Wood AM. Integrating positive and clinical psychology: viewing human functioning as continua from positive to negative can benefit clinical assessment, interventions and understandings of resilience. Cognitive Ther Res. (2016) 41:1–15. doi: 10.1007/s10608-015-9728-y
3. Hall LH, Johnson J, Watt I, Tsipa A, O'Connor DB. Healthcare staff wellbeing, burnout, and patient safety: A systematic Review. PLoS ONE. (2016) 11:e0159015. doi: 10.1371/journal.pone.0159015
4. Von Korff M, Dunn KM. Chronic pain reconsidered. Pain. (2008) 138:267–76. doi: 10.1016/j.pain.2007.12.010
5. Fillingim RB, Slade GD, Greenspan JD, Dubner R, Maixner W, Bair E, et al. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: findings from the OPPERA study. Pain. (2018) 159:2403–13. doi: 10.1097/j.pain.0000000000001348
6. Durham J, Raphael KG, Benoliel R, Ceusters W, Michelotti A, Ohrbach R. Perspectives on next steps in classification of oro-facial pain - part 2: role of psychosocial factors. J Oral Rehabil. (2015) 42:942–55. doi: 10.1111/joor.12329
7. Miller VE, Poole C, Golightly Y, Barrett D, Chen DG, Ohrbach R, et al. Characteristics associated with high-impact pain in people with temporomandibular disorder: a cross-sectional study. J Pain. (2019) 20:288–300. doi: 10.1016/j.jpain.2018.09.007
8. Winkelmann R. Unemployment and happiness. IZA World Labor. (2014) 94:1–10. doi: 10.15185/izawol.94
9. Hiswåls AS, Marttila A, Mälstam E, Macassa G. Experiences of unemployment and well-being after job loss during economic recession: results of a qualitative study in east central Sweden. J Public Health Res. (2017) 6:995. doi: 10.4081/jphr.2017.995
10. Dworkin SF, LeReche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. (1992) 6:301–55.
11. Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network and orofacial pain special interest group. J Oral Facial Pain Headache. (2014) 28:6–27. doi: 10.11607/jop.1151
12. Rener-Sitar K, John MT, Pusalavidyasagar SS, Bandyopadhyay D, Schiffman EL. Sleep quality in temporomandibular disorder cases. Sleep Med. (2016) 25:105–12. doi: 10.1016/j.sleep.2016.06.031
13. Meira EC, Lukic N, Wojczynska A, Steiger B, Guimarães AS, Ettlin EA. Insomnia in patients seeking care at an orofacial pain Unit. Front Neurol. (2019) 10:542. doi: 10.3389/fneur.2019.00542
14. Yakobov E, Suso-Ribera C, Vrinceanu T, Adams H, Sullivan MJ. Trait perceived injustice is associated with pain intensity and pain behavior in participants undergoing an experimental pain induction procedure. J Pain. (2019) 20:592–9. doi: 10.1016/j.jpain.2018.11.007
15. Sommer I, Lukic N, Rössler W, Ettlin DA. Measuring anger in patients experiencing chronic pain - A systematic review. J Psychosom Res. (2019) 125:109778. doi: 10.1016/j.jpsychores.2019.109778
16. Galli U, Ettlin DA, Palla S, Ehlert U, Gaab J. Do illness perceptions predict pain-related disability and mood in chronic orofacial pain patients? A 6-month follow-up study. J Eur J Pain. (2010) 14:550–8. doi: 10.1016/j.ejpain.2009.08.011
17. Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. (2001) 17:52–64. doi: 10.1097/00002508-200103000-00008
18. Ettlin DA, Sommer I, Brönnimann B, Maffioletti S, Scheidt J, Hou MY, et al. Design, construction, and technical implementation of a web-based interdisciplinary symptom evaluation (WISE) - a heuristic proposal for orofacial pain and temporomandibular disorders. J Headache Pain. (2016) 17:77. doi: 10.1186/s10194-016-0670-5
19. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. (2006) 60:631–7. doi: 10.1016/j.jpsychores.2005.10.020
20. Gaab J, Bunschoten SL, Sprott H, Ehlert U. Psychometric evaluation of a German translation of the illness perception questionnaire. In: Paper Presented at the 62nd Annual Scientific Meeting of the American Psychosomatic Society (APS). Orlando, USA (2004).
21. Gaab J, Latanzia-Bunschoten S, Sprott H. Illness Perception Questionnaire. In: Begel, Wirtz, Zwingmann, Hrsg. Kompendium: Diagnostische Verfahren in der Rehabilitation. Göttingen: Hogrefe Verlag (2008). S. 109–111.
22. Mancuso SG, Knoesen NP, Castle DJ. The Dysmorphic Concern Questionnaire: a screening measure for body dysmorphic disorder. Aust N Z J Psychiatry. (2010) 44:535–42. doi: 10.3109/00048671003596055
23. Schieber K, Kollei I, de Zwaan M, Martin A. The Dysmorphic Concern Questionnaire in the German general population: psychometric properties and normative data. Aesth Plast Surg. (2018) 42:1412–20. doi: 10.1007/s00266-018-1183-1
24. Visscher CM, Baad-Hansen L, Durham J, Goulet JP, Michelotti A, Roldán Barraza C, et al. Benefits of implementing pain-related disability and psychological assessment in dental practice for patients with temporomandibular pain and other oral health conditions. JADA. (2018) 149:422–31. doi: 10.1016/j.adaj.2017.12.031
25. Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical Care. (2008) 46:266–74. doi: 10.1097/MLR.0b013e318160d093
26. Sullivan MJL, Adams H, Horan S, Maher D, Boland D, Gross R. The role of perceived injustice in the experience of chronic pain and disability: scale development and validation. J Occup Rehabil. (2008) 18:249–61. doi: 10.1007/s10926-008-9140-5
27. Niederstrasser N, Steiger B, Welsch K, Hartmann S, Nilges P, Ljutow A, et al. Deutsche transkulturelle Übersetzung des Injustice Experience Questionnaire [German transcultural translation of the Injustice Experience Questionnaire]. Schmerz. (2018) 32:442–8. doi: 10.1007/s00482-018-0329-z
28. Steiger B, Welsch K, Niederstrasser N, Hartmann S, Nilges P, Ljutow A, et al. Validierung der deutschen Übersetzung des Injustice Experience Questionnaire (IEQ) in 5 ambulanten Schmerzbehandlungseinrichtungen [Validation of the German-language version of the Injustice Experience Questionnaire (IEQ) in five outpatient clinics]. Schmerz. (2019) 33:106–15. doi: 10.1007/s00482-018-0345-z
29. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/S1389-9457(00)00065-4
30. Gerber M, Lang C, Lemola S, Colledge F, Kalak N, Holsboer-Trachsler E, et al. Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: results from three cross-sectional studies. BMC Psychiatry. (2016) 31:174. doi: 10.1186/s12888-016-0876-8
31. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. (1995) 7:524–32. doi: 10.1037/1040-3590.7.4.524
32. Meyer K, Sprott H, Mannion AF. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res. (2008) 64:469–78. doi: 10.1016/j.jpsychores.2007.12.004
33. Löwe B, Wahl I, Rose M, Spitzer C, Glaesmer H, Wingenfeld K, et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. (2010) 122:86–95. doi: 10.1016/j.jad.2009.06.019
34. Gräfe K, Zipfel S, Herzog W, Löwe B. Screening for psychiatric disorders with the patient health questionnaire (PHQ). Res Ger Validation Study Diagnostica. (2004) 50:171–81. doi: 10.1026/0012-1924.50.4.171
35. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
36. Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J. Validity and utility of the PRIME-MD Patient Health Questionnaire in assessment of 3000 obstetric-gynecologic patients: The PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol. (2000) 183:759–69. doi: 10.1067/mob.2000.106580
37. Gignac GE, Szodorai ET. Effect size guidelines for individual differences researchers. Pers Individ Differ. (2016) 102:74–8. doi: 10.1016/j.paid.2016.06.069
38. Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and socio-demographic predictors of its development: the OPPERA prospective cohort study. J Pain. (2013) 14 (Suppl. 2):T20–32. doi: 10.1016/j.jpain.2013.07.014
39. Goldfarb EV, Seo D, Sinha R. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress. (2019) 11:100177. doi: 10.1016/j.ynstr.2019.100177
40. Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. (2019) 86:421–32. doi: 10.1016/j.biopsych.2019.04.028
41. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
42. Shaefer JR, Khawaja SN, Bavia PF. Sex, gender, and orofacial pain. Dent Clin North Am. (2018) 62:665–82. doi: 10.1016/j.cden.2018.06.001
43. Slade GD, Rosend RD, Ohrbach R, Greenspan JD, Fillingimh RB, Parisien M, et al. Anatomical selectivity in overlap of chronic facial and bodily pain. Pain Rep. (2019) 4:e729. doi: 10.1097/PR9.0000000000000729
44. Cronan TA, Serber ER, Walen HR, Jaffe M. The influence of age on fibromyalgia symptoms. J Aging Health. (2002) 14:370–84. doi: 10.1177/08964302014003004
45. Okeson JP. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. Chicago: Quintessence Publishing Company, Inc (1996).
46. De Toledo IP, Conti Réus J, Fernandes M, Porporatti AL, Peres MA, Takaschima A, et al. Prevalence of trigeminal neuralgia: a systematic review. J Am Dent Assoc. (2016) 147:570–76. doi: 10.1016/j.adaj.2016.02.014
47. Joury E, Bernabe E, Gallagher JE, Marcenes W. Burden of orofacial pain in a socially deprived and culturally diverse area of the United Kingdom. Pain. (2018) 159:1235–43. doi: 10.1097/j.pain.0000000000001203
48. Durham J, Shen J, Breckons M, Steele J, Araújo-Soares V, Exley C, et al. Healthcare cost and impact of persistent orofacial pain. J Dent Res. (2016) 95:1147–54. doi: 10.1177/0022034516648088
49. Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. (2010) 468:798–806. doi: 10.1007/s11999-009-0963-y
50. Jang HH, Kim ME, Kim HK. Pain catastrophizing mediates the effects of psychological distress on pain interference in patients with orofacial pain: a cross-sectional study. J Oral Facial Pain Headache. (2018) 32:409–17. doi: 10.11607/ofph.2067
51. De Ruddere L, Goubert L, Stevens MAL, Deveugele M, Craig KD, Crombez G. Healthcare professional reactions to patient pain: impact of knowledge about medical evidence and psychosocial influences. J Pain. (2014) 15:262–9. doi: 10.1016/j.jpain.2013.11.002
52. Cano A, Williams AC. Social interaction in pain: reinforcing pain behaviors or building intimacy? Pain. (2010) 149:9–11. doi: 10.1016/j.pain.2009.10.010
53. Scott W, Yu L, Patel S, McCracken LM. Measuring stigma in chronic ain: preliminary investigation of instrument psychometrics, correlates, and magnitude of change in a prospective cohort attending interdisciplinary treatment. J Pain. (2019) 20:1164–75. doi: 10.1016/j.jpain.2019.03.011
54. Sullivan MJL. User manual for the Injustice Experience Questionnaire (IEQ). (2008). Available online at: http://sullivan-painresearch.mcgill.ca/pdf/ieq/IEQManual.pdf (accessed April 1, 2020).
55. Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain–results of the North Cheshire oro-facial pain prospective population study. Pain. (2010) 149:354–9. doi: 10.1016/j.pain.2010.02.040
56. Aggarwal VR, McBeth J, Zakrzewska JM, Lunt M, Macfarlane GJ. The epidemiology of chronic syndromes that are frequently unexplained: do they have common associated factors? Int J Epidemiol. (2006) 35:468–76. doi: 10.1093/ije/dyi265
57. Gustin SM, Wilcox SL, Peck CC, Murray GM, Henderson LA. Similarity of suffering: equivalence of psychological and psychosocial factors in neuropathic and non-neuropathic orofacial pain patients. Pain. (2011) 152:825–32. doi: 10.1016/j.pain.2010.12.033
58. Fillingim R. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. (2017) 158(Suppl. 1):S11–8. doi: 10.1097/j.pain.0000000000000775
59. El-Shormilisy N, Strong J, Meredith PJ. Associations among gender, 6oping patterns and functioning for individuals with chronic pain: a systematic review. Pain Res Manag. (2015) 1:48–55. doi: 10.1155/2015/490610
60. Tomitaka S, Kawasaki Y, Ide K, Akutagawa M, Ono Y, Furukawa TA. Stability of the distribution of Patient Health Questionnaire-9 scores against age in the general population: data from the National Health and Nutrition Examination survey. Front Psychiatry. (2018) 9:390. doi: 10.3389/fpsyt.2018.00390
61. Teasell RW, Bombardier C. Employment-related factors in chronic pain and chronic pain disability. Clin J Pain. (2001) 17:S39–45. doi: 10.1097/00002508-200112001-00010
62. Rajamani S. Representation of occupational information across resources and validation of the occupational data for health model. J Am Med Inform Assoc. (2015) 25:197–205. doi: 10.1093/jamia/ocx035
63. Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage. (2009) 37:863–72. doi: 10.1016/j.jpainsymman.2008.05.009
64. Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. (2009) 24:733–8. doi: 10.1007/s11606-009-0981-1
65. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. (2003) 163:2433–45. doi: 10.1001/archinte.163.20.2433
66. du Fort GG, Newman SC, Bland RC. Psychiatric comorbidity and treatment seeking: sources of selection bias in the study of clinical populations. J Nerv Ment Dis. (1993) 181:467–74. doi: 10.1097/00005053-199308000-00001
67. Häggman-Henrikson B, Ekberg EC, Ettlin DA, Michelotti A, Durham J, Goulet JP, et al. Mind the gap: a systematic review of implementation of screening for psychological comorbidity in dental and dental hygiene education. J Dent Educ. (2018) 82:1065–76. doi: 10.21815/JDE.018.104
68. Sanders AE, Akinkugbe AA, Fillingim RB, Ohrbach R, Greenspan JD, Maixner W, et al. Causal mediation in the development of painful temporomandibular disorder. J Pain. (2017) 18:428–36. doi: 10.1016/j.jpain.2016.12.003
69. Hall AM, Kamper SJ, Maher CG, Latimer J, Ferreira ML, Nicholas MK. Symptoms of depression and stress mediate the effect of pain on disability. Pain. (2011) 152:1044–51. doi: 10.1016/j.pain.2011.01.014
70. Rodero B, Luciano JV, Montero-Marín J, Casanueva B, Palacin JC, Gili M, et al. Perceived injustice in fibromyalgia: psychometric characteristics of the injustice experience questionnaire and relationship with pain catastrophising and pain acceptance. J Psychosom Res. (2012) 73:86–91. doi: 10.1016/j.jpsychores.2012.05.011
71. Lunde CE, Sieberg CB. Walking the tightrope: a proposed model of chronic pain and Stress. Front Neurosci. (2020) 14:270. doi: 10.3389/fnins.2020.00270
72. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. (2003) 106:127–33. doi: 10.1016/S0304-3959(03)00301-4
73. Reiter S, Eli I, Mahameed M, Emodi-Perlman A, Friedman-Rubin P, Reiter MA, et al. Pain catastrophizing and pain persistence in temporomandibular disorder patients. J Oral Facial Pain Headache. (2018) 32:309–20. doi: 10.11607/ofph.1968
74. Sullivan MJL, Thibault P, Simmonds MJ, Milioto M, Cantin A-P, Velly A. Pain, perceived injustice and the persistence of post-traumatic stress symptoms during the course of rehabilitation for whiplash injuries. Pain. (2009) 145:325–31. doi: 10.1016/j.pain.2009.06.031
75. van Leeuwen WF, van der Vliet QMJ, Janssen SJ, Heng M, Ring D, Vranceanu A-M. Does perceived injustice correlate with pain intensity and disability in orthopaedic trauma patients? Injury. (2016) 47:1212–6. doi: 10.1016/j.injury.2016.02.018
76. Wei Y, Blanken TF, Van Someren EJW. Insomnia really hurts: effect of a bad night's sleep on pain increases with insomnia severity. Front Psychiatry. (2018) 9:377. doi: 10.3389/fpsyt.2018.00377
77. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. (2013) 14:1539–52. doi: 10.1016/j.jpain.2013.08.007
78. Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. (2016) 95:1084–92. doi: 10.1177/0022034516653743
79. Egloff N, Cámara RJ, von Känel R, Klingler N, Marti E, Ferrari MG. Pain drawings in somatoform-functional pain. BMC Musculoskelet Disord. (2012) 13:257. doi: 10.1186/1471-2474-13-257
80. Boudreau SA, Badsberg S, Christensen SW, Egsgaard LL. Digital pain drawings: assessing touch-screen technology and 3D body schemas. Clin J Pain. (2016) 32:139–45. doi: 10.1097/AJP.0000000000000230
81. Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, et al. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain. (2013) 14:T75–90. doi: 10.1016/j.jpain.2013.06.009
82. Nguyen TT, Vanichanon P, Bhalang K, Vongthongsri S. Pain duration and intensity are related to coexisting pain and comorbidities present in temporomandibular disorder pain patients. J Oral Facial Pain Headache. (2018) 33:205–12. doi: 10.11607/ofph.2088
Keywords: injustice, stress, well-being, orofacial pain, dysmorphic
Citation: Bhalang K, Steiger B, Lukic N, Zumbrunn Wojczyńska A, Hovijitra RS and Ettlin DA (2020) The Pain–to–Well-Being Relationship in Patients Experiencing Chronic Orofacial Pain. Front. Neurol. 11:557415. doi: 10.3389/fneur.2020.557415
Received: 07 May 2020; Accepted: 26 October 2020;
Published: 03 December 2020.
Edited by:
Limor Avivi-Arber, University of Toronto, CanadaReviewed by:
Harold Avila, Indiana University, United StatesCopyright © 2020 Bhalang, Steiger, Lukic, Zumbrunn Wojczyńska, Hovijitra and Ettlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanokporn Bhalang, Y3VkZW50NzBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.