
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 18 September 2020
Sec. Sleep Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.549908
This article is part of the Research Topic Sleep and Circadian Disruption in Critically Ill Patients View all 8 articles
Delirium is a syndrome characterized by acute brain failure resulting in neurocognitive disturbances affecting attention, awareness, and cognition. It is highly prevalent among critically ill patients and is associated with increased morbidity and mortality. A core domain of delirium is represented by behavioral disturbances in sleep-wake cycle probably related to circadian rhythm disruption. The relationship between sleep, circadian rhythm and intensive care unit (ICU)-acquired delirium is complex and likely bidirectional. In this review, we explore the proposed pathophysiological mechanisms of sleep disruption and circadian dysrhythmia as possible contributing factors in transitioning to delirium in the ICU and highlight some of the most relevant caveats for understanding the relationship between these complex phenomena. Specifically, we will (1) review the physiological consequences of poor sleep quality and efficiency; (2) explore how the neural substrate underlying the circadian clock functions may be disrupted in delirium; (3) discuss the role of sedative drugs as contributors to delirium and chrono-disruption; and, (4) describe the association between abnormal sleep-pathological wakefulness, circadian dysrhythmia, delirium and critical illness. Opportunities to improve sleep and readjust circadian rhythmicity to realign the circadian clock may exist as therapeutic targets in both the prevention and treatment of delirium in the ICU. Further research is required to better define these conditions and understand the underlying physiologic relationship to develop effective prevention and therapeutic strategies.
Delirium is a syndrome characterized by acute brain failure typically arising over hours or few days that leads to a change in mental state (1–3). It primarily results in disturbances in attention (the ability of directing, focusing, sustaining, and shifting attention) and awareness of the environment (3). Prevalence can be as high as 80% in elderly patients receiving mechanical ventilation (4, 5). Delirium is associated with significant morbidity and mortality in the intensive care unit (ICU). Risk factors for delirium include acute illness, coma, more severe illness, emergency surgery, polytrauma, and mechanical ventilation; patient characteristics that are related to a higher risk of developing delirium include age and chronic conditions (e.g., dementia, hypertension) (6, 7). Environmental characteristics specific to the ICU and related to sleep and circadian rhythm disruption (8) may worsen symptoms. Such factors include the lack of normal variability in light-dark cycle (9), noise (10), the use of mechanical ventilation (11, 12), and need for continuous infusions of sedative drugs (13). The available literature suggests that there may be a close relationship between delirium, sleep, circadian rhythm, and critical illness, however, no causal pathway has been yet clearly described or the directionality of the relationship understood.
Sleep architecture changes throughout an individual's lifespan to support proper development and physiological function (14, 15). Normal sleep architecture varies among individuals but is made up of cycles of rapid eye movement (REM) and the 3 stages of non-REM (NREM) sleep; as an example, “normal” sleep in a healthy adult might be made up of 2–5% stage 1, 45–55% stage 2, 3–15% stage 3, or slow wave sleep and 20–25% REM (16). Transition from wake to sleep onset occurs within 10–20 min and the first period of REM typically occurs within 90–120 min. Poor sleep is associated with both neuropsychological and cognitive impairment (17). In a study by Zhou et al. (18) induced sleep deprivation in 13 young healthy men (mean age 23 yrs.) was associated with impairment in both attention and psychomotor vigilance. Further, in a study of 66 healthy adult volunteers chronic sleep deprivation was associated with increased reaction time to visual stimulus; experienced impairment was dose-dependent and variable time to recovery to baseline cognition was seen between individuals once normal sleep restored (19).
Poor sleep is common in the ICU (20, 21). Critically ill patients experience increases in stages 1 and 2 sleep with frequent arousals and awakenings. Further, they are less likely to transition into stage 3 or slow wave sleep or REM sleep (22–24). Poor sleep has been associated with delirium and other outcomes such as length of stay and long-term cognitive impairment (25–28). Additionally, there is a general believe among ICU clinicians that poor sleep is a risk factor for delirium as shown in a recent global survey, 97% of 1,223 ICU physicians and nurses (29). Moreover, the 2018 Clinical Practice Guidelines for Pain, Agitation, and Delirium (PAD), from the Society of Critical Care Medicine recommended using a sleep-promoting, multicomponent protocol in critically ill adults based on the pooled analysis of three observational before-after studies demonstrating an overall reduction in the prevalence of delirium when sleep promoting interventions were used (30, 31). Although a direct relationship between ICU delirium and sleep has yet to be shown, in long-term follow-up, total duration of ICU delirium was found by Altman et al. (32) to be significantly associated with increased sleep disturbance at long-term follow-up (mean 5 months after hospital discharge).
Two primary processes, the homeostatic process (Process S) interacts with the Process C, controlled by the circadian pacemaker to control the normal sleep-wake cycle, with time courses regulated from physiological and behavioral variables (33). The suprachiasmatic nucleus (SCN) acts as a central pacemaker coordinating daily physiological and behavioral cycles. The SCN is a neural network that entrains peripheral cellular clocks across the body achieving circadian control of behavior, neuroendocrine and autonomic signals in target tissues (34).
The circadian rhythm appears to be disrupted commonly in critically ill patients (35–40). It is hypothesized that chronodisruption may be associated with ICU-acquired delirium. Alterations in the structure and function of specific neural networks represent potential substrates for this relationship. Among patients with impaired consciousness during vegetative or comatose states resting state functional magnetic resonance imaging (MRI) showed a reduction in connectivity of posteromedial and anteromedial cortices as well as temporoparietal junctions with worsening impairment in consciousness (41). The posteromedial cortex, including the posterior cingulate cortex is responsible for maintenance of wakefulness and consciousness (42). In 22 medical inpatients with delirium, Choi et al. (43) found abnormalities in functional connectivity of the posterior cingulate cortex, intralaminar thalamic nuclei, and mesencephalic networks, including the nucleus basalis and the ventral tegmental area, part of the ascending reticular activating system (ARAS), during episodes of delirium. Interestingly, abnormal resting state connectivity of the SCN, as assessed by functional MRI, has been identified by Kyeong et al. (44) as a possible substrate for chronodisruption in 34 delirious patients admitted to various inpatient units for various medical conditions. In this study, abnormal resting state connectivity between the SCN, cortical nodes including the posteromedial/anteromedial cortices, and temporoparietal junctions, and subcortical regions was seen in delirious patients (44).
An important hallmark of delirium is decreased activity of the ARAS (5): a neuronal network located in the brainstem that controls wake, sleep and dreaming states through connections with the SCN and releasing acetylcholine during the individual's active phase (45). In delirium, due to transient hypoxic states, the depletion of acetylcholinergic projections and the dopaminergic overproduction from the ARAS result in alertness and attention disturbances (5, 46). Normal circadian distribution of sleep-wakefulness states has been attributed to the intimate relationship between the SCN and cholinergic neurons within the ARAS such as the locus coeruleus (47, 48). However, despite consistently described alterations in melatonin metabolism in critically ill patients (36, 40, 49, 50), abnormalities in circadian connectivity seen on neuroimaging studies were not associated with measured melatonin levels.
Abnormal sleep architecture (20), circadian dysrhythmia (8), and delirium (51–53) are all common in the ICU. Disturbance in sleep-wake cycle, alertness and altered sensorium often have multiple possible etiologies and likely share attributable pathophysiological mechanisms (See Figure 1).
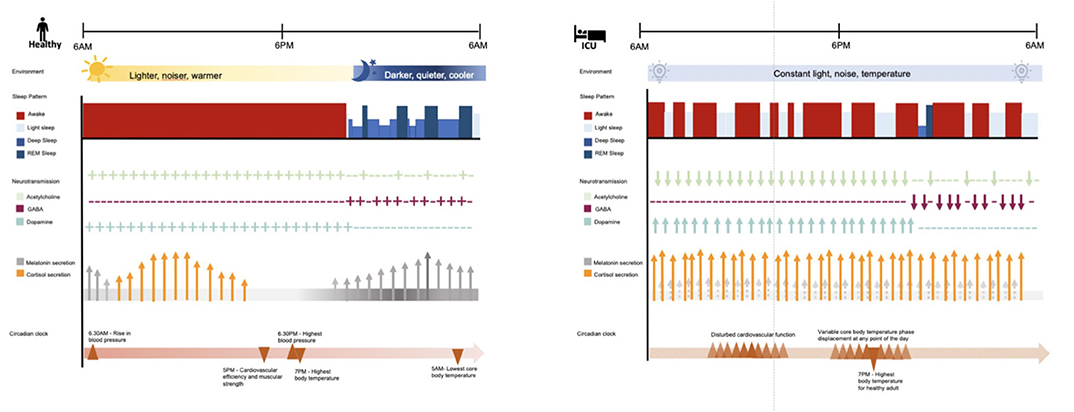
Figure 1. Comparison of sleep and circadian rhythms in healthy adult and adult with critical illness in the ICU. In a healthy adult, the circadian clock is synced to the daily external cycle of changing light, sound, and temperature. The sleep stage of a healthy adult occurs during the night and is composed of 2–5% light sleep, 48–70% deep sleep, 20–25% rapid eye movement (REM) sleep. REM sleep reoccurs in cycles of 90–120 min. Acetylcholine is predominantly discharged during wakefulness and REM sleep; while GABAergic activity is predominant during deep sleep. Dopaminergic activity promotes alertness and reduces sleep. Melatonin secretion starts around 9.30 pm (dim light melatonin onset) and stops around 7.30 am, peaking around 3 am. Cortisol secretion starts in the early morning, peaks around 10 am in correspondence to the time of highest alertness, and keeps declining gradually throughout the day, and the night. Overall, these processes coordinate physiological functions including cardiovascular functions and temperature. In contrast, the intensive care unit (ICU) constant artificial environment disrupts the daily cycle of circadian functions. In the ICU critically ill patients, compared to healthy adults, present equal to normal sleep time in the course of 24 h but the majority of it consists of light sleep; 50% of sleep time is distributed during the day and disturbed by frequent arousals. For instance, benzodiazepine decrease sleep latency, slow wave sleep (SWS) and REM sleep duration and frequency; propofol suppresses SWS EEG bursts; opioids alter REM sleep; while, dexmedetomidine improve stage 2 and sleep efficiency by shifting 75% of sleep to nighttime. In ICU patients, the acute stress environment has been associated with decreased GABAergic and cholinergic transmission, and increased dopaminergic transmission, impaired melatonin secretion and increased cortisol production, along with displacement of physiological functions normally coordinated by the circadian clock. These disturbances have been associated with symptoms of delirium. ICU, intensive care unit; REM, rapid eye movement; GABA, gamma-aminobutyric acid.
The upregulation of GABA-A receptors, either through an increase in synthesis of endogenous GABA agonists or stimulation from exogenous GABA agonists, has been implicated in delirium (54, 55). GABA-ergic agents are thought to destabilize neurons preventing sleep transition (55), and upregulate inhibitory tone in the central nervous system contributing to neural connectivity and cognitive disintegration (56). Both benzodiazepines and propofol, commonly administered sedatives in the ICU, mediate their effect by modulating the effects of GABA. Zaal et al. (13) demonstrated that continuous infusion as compared to intermittent bolus dosing was associated with delirium; suggesting that higher doses or greater exposure is an important factor in transitioning to delirium. Although the interpretation of sleep in the ICU can be difficult in sedated patients, benzodiazepines typically increase total sleep time through prolonging stage 2 NREM, while suppressing both stage 3 NREM or slow wave sleep and REM (57). Propofol has been shown to worsen overall sleep architecture; specifically, suppressing REM sleep (58).
In contrast to the potentially deleterious effects of GABA agonists, the use of the alpha-2 agonist dexmedetomidine has been shown to be relatively effective in decreasing the daily prevalence of delirium in mechanically ventilated ICU patients (59). Dexmedetomidine is a highly selective alpha-2 agonist that facilitates both sedation and analgesia, without much respiratory depression. It appears to be particularly effective in lowering the daily prevalence and duration of delirium when compared with benzodiazepines, such as lorazepam, the comparator in the randomized, double-blind MENDS trial (60). The daily prevalence of delirium in dexmedetomidine-treated patients was also significantly lower when compared to a midazolam group in the randomized, double-blind SEDCOM trial (61). Recently, the prophylactic use of nocturnal dexmedetomidine was shown to reduce the incidence of delirium in the SKY-DEX trial (62). In the DahLIA, placebo-controlled trial (63), 74 mechanically ventilated patients with agitation and delirium were randomized to dexmedetomidine or placebo; patients treated with dexmedetomidine had increased ventilator-free hours at 7 days (median, 145 vs. 128 h) and faster resolution of their delirium symptoms (median, 23 vs. 40 h). The effect of dexmedetomidine on delirium prevention may be mediated both through its reduction in glutamate release, as glutamate toxicity has been previously associated with the development of delirium (64), but also through its decreased GABA receptor modulation or cholinergic receptor activity when compared to other commonly used sedatives (65). Furthermore, dexmedetomidine through the stimulation of alpha-2 receptors and resultant inhibition of noradrenergic neurons in the locus coeruleus and disinhibition of GABA neurons in the ventrolateral preoptic nucleus it may promote more natural sleep in the ICU environment (66).
Disturbed melatonergic activity is also implicated in delirium pathogenesis. Risk factors for delirium including pre-existing cognitive impairment, old age, and psychotropic medication use are all associated with impaired melatonergic function. Melatonin deficiency and abnormal secretion contribute to impairment of the sleep-wake cycle. Perras et al. (67) report that the normal response of melatonin secretion to changes in light and darkness is impaired in critically ill patients suggesting a dysregulation of the mechanism of melatonin secretion or a shift in the circadian clock phase in the SCN. Altered urinary levels of the melatonin metabolite 6-sulfatoxymelatonin (6-SMT) has been reported in delirious patients as compared to patients who were not delirious; levels were elevated in hypoactive patients and lower in hyperactive patients (68). Urinary 6-SMT exhibited loss of circadian rhythmicity with no daytime decline in septic patients (36), a frequently present in critical illness. Recently, Li et al. (69) measured plasma levels of melatonin, TNF-α, IL-6 and messenger RNA of the circadian genes Cry-1 and Per-2 for 24-h in septic and non-septic ICU patients (n = 22). Altered circadian rhythm of melatonin secretion, reduced expression of Cry-1 and Per-2, and elevated levels of TNF-α and IL-6 were again seen in patients with sepsis (69).
Hypothalamic-pituitary-adrenal-stress axis (HPA) dysregulation might also be related to incident delirium and circadian dysrhythmia and is intimately related to the SCN and melatonergic functions. Elevated plasma cortisol levels are associated with increased delirium risk after both cardiac- (70) and non-cardiac surgery (71). Pearson et al. (72) found that in post-operative patients over the age of 60 years with acute hip fracture, cortisol CSF levels were elevated in those who developed delirium as compared to those who did not. It has been postulated that aberrant stress responses related to disrupted function in the limbic-HPA axis and its interaction with the inflammatory response may be responsible for the increased risk of delirium with age as well as pre-existing cognitive impairment (73). In a cohort of ICU patients with severe sepsis and septic shock (n = 140), plasma cortisol level between 6 and 12 h post hemodynamic stabilization (odds ratio [OR]: 2.3, 95% CI 2.0–3.2; p = 0.02) and the combination of older age and plasma cortisol level (OR: 1.0, 95% CI 1.0–1.9; p = 0.04) were associated with increase delirium risk (74).
Numerous opportunities exist to improve sleep and re-entrain circadian rhythm in the ICU. Easily modifiable risk factors associated with disruption of sleep and circadian rhythm such as mechanical ventilation strategies paired with sedation stewardship and multicomponent sleep improvement strategies in the ICU may help to reduce delirium duration or severity and facilitate recovery from critical illness (75) (See Table 1).
Relationship between mechanical ventilation, sleep disturbances and circadian dysrhythmia are not well-understood as they are complex and likely associated with variation in the administration of sedative agents and subsequent patient-ventilator asynchrony management (8). However, this relationship strongly influences the weaning process as sleep disturbances and delirium are associated with greater duration of mechanical ventilation and prolonged ICU length of stay (78–80). An opportunity must therefore exist for intervention with the aim of modifying ventilator settings and practices for improving sleep and circadian rhythmicity.
Sedation is commonly used to help patients tolerate mechanical ventilation. The 2018 PADIS Clinical Guidelines recommend the use of light levels of sedation (Richmond Agitation Sedation Score of −2 to +1) during invasive mechanical ventilation (30). This inevitably results in patients breathing spontaneously during assisted ventilation and resultant abnormal patient-ventilator interactions named asynchronies. The typical reaction of physicians to the presence of asynchronies involves the administration of additional sedation boluses or increasing the dose of sedative infusions which can significantly affect sleep architecture, circadian rhythmicity and delirium risk. However, not all asynchronies are “improved” (81) with sedation (82, 83). Therefore, an understanding of the mechanisms leading to the experienced asynchrony and personalized adjustment of ventilator settings (81) might spare a patient from further exposure to sedative agents, possibly mitigating further risk of sleep and circadian rhythm disruption. The titration of pressure support settings during non-invasive ventilation by adjusting the amount of support to meet the needs of the patient has previously been shown to decrease the number of asynchronies and improve sleep architecture in patients with chronic neuromuscular diseases (84).
Caution however needs to be exercised as over-assistance during pressure support can alternately lead to central apneas with resultant poor sleep, characterized by frequently awakenings and arousals leading to greater sleep fragmentation (85, 86). Ventilatory support in excess to a patient's metabolic need results in hyperventilation where CO2 levels decrease below the apnea threshold (87). To eliminate over-assistance, which can also lead to other adverse outcomes such as disuse diaphragmatic atrophy (88), the amount of ventilatory support needs to be decreased (89). Alternatively, assist-controlled modes with a back-up respiratory rate can also be used to prevent apnea events which has been shown to reduce sleep fragmentation (86). Using assist-controlled modes however does not eliminate the risk of disuse diaphragmatic atrophy and might create the false impression that patients are not ready for liberation, potentially delaying extubation. Proportional modes of ventilation [i.e., proportional assist-ventilation [PAV+] and neurally adjust ventilatory assist [NAVA]] can be used to deliver pressure proportional to the patient's instantaneous efforts in terms of amplitude and timing which avoids over- and under-assistance and improves synchrony. The use of these modes has systematically been shown to decrease asynchronies (82, 90, 91) and improve sleep quality in cross-over studies of small size (92, 93).
Recently a review by Flannery investigated whether interventions targeted at improving sleep in the ICU were associated with reductions in ICU delirium (94). Six of the ten identified studies demonstrated a statistically significant reduction in the incidence of ICU delirium associated with sleep intervention. Unfortunately, although sleep interventions seem to be a promising approach for improving delirium and related outcomes (e.g., ICU length of stay) conclusions are limited by confounding, variable methodology and bias issues. Further study is therefore needed. Based on available data, potentially effective interventions might include a combination of dexmedetomidine (62), oral melatonin (95), and cognitive behavioral sleep therapy (96) using modified polysomnography to quantify sleep quality and quantity (25, 78) and understand to which extent sleep actually affects delirium occurrence.
Despite advances in our understanding of the sleep-wake cycle its association with the underlying mechanism of delirium, and how both influence ICU patient outcome, significant knowledge gaps exist in understanding the relationships between sleep, circadian rhythm and delirium. Hypotheses for mechanistic relationships between the sleep-wake cycle and delirium have largely been derived from studies of non-ICU patients. A better understanding of mechanisms would guide the development of new methods for prevention and treatment that consequently may improve short- and long-term outcomes of ICU survivors.
MW, MD, and IT wrote the first draft and revised the manuscript. All authors revised and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GB declared a shared affiliation, with no collaboration, with three of the authors IT, LB, and MW to the handling Editor.
1. Blazer DG, van Nieuwenhuizen AO. Evidence for the diagnostic criteria of delirium: an update. Curr Opin Psychiatry. (2012) 25:239–43. doi: 10.1097/YCO.0b013e3283523ce8
2. European Delirium A, American Delirium S. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. (2014) 12:141. doi: 10.1186/s12916-014-0141-2
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association (2013)
4. Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. (2015) 350:h2538. doi: 10.1136/bmj.h2538
5. Thom RP, Levy-Carrick NC, Bui M, Silbersweig D. Delirium. Am J Psychiatry. (2019) 176:785–93. doi: 10.1176/appi.ajp.2018.18070893
6. van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. (2009) 13:R77. doi: 10.1186/cc7892
7. Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. (2015) 43:40–7. doi: 10.1097/CCM.0000000000000625
8. Telias I, Wilcox ME. Sleep and circadian rhythm in critical illness. Crit Care. (2019) 23:82. doi: 10.1186/s13054-019-2366-0
9. Smonig R, Magalhaes E, Bouadma L, Andremont O, de Montmollin E, Essardy F, et al. Impact of natural light exposure on delirium burden in adult patients receiving invasive mechanical ventilation in the ICU: a prospective study. Ann Intensive Care. (2019) 9:120. doi: 10.1186/s13613-019-0592-x
10. Simons KS, Verweij E, Lemmens PMC, Jelfs S, Park M, Spronk PE, et al. Noise in the intensive care unit and its influence on sleep quality: a multicenter observational study in Dutch intensive care units. Crit Care. (2018) 22:250. doi: 10.1186/s13054-018-2182-y
11. Bulic D, Bennett M, Rodgers H, Nourse M, Rubie P, Looi JC, et al. Delirium after mechanical ventilation in intensive care units: the Cognitive and Psychosocial Assessment (CAPA) study protocol. JMIR Res Protoc. (2017) 6:e31. doi: 10.2196/resprot.6660
12. Ely EW, Evans GW, Haponik EF. Mechanical ventilation in a cohort of elderly patients admitted to an intensive care unit. Ann Intern Med. (1999) 131:96–104. doi: 10.7326/0003-4819-131-2-199907200-00004
13. Zaal IJ, Devlin JW, Hazelbag M, Klein Klouwenberg PM, van der Kooi AW, Ong DS, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. (2015) 41:2130–7. doi: 10.1007/s00134-015-4063-z
14. Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. (2011) 98:653–65. doi: 10.1016/B978-0-444-52006-7.00041-1
15. Banks G, Nolan PM, Peirson SN. Reciprocal interactions between circadian clocks and aging. Mamm Genome. (2016) 27:332–40. doi: 10.1007/s00335-016-9639-6
16. Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. (1957) 9:673–90. doi: 10.1016/0013-4694(57)90088-3
17. Honn KA, Hinson JM, Whitney P, van Dongen HPA. Cognitive flexibility: a distinct element of performance impairment due to sleep deprivation. Accid Anal Prev. (2019) 126:191–7. doi: 10.1016/j.aap.2018.02.013
18. Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, et al. Dynamics of neurobehavioral performance variability under forced desynchrony: evidence of state instability. Sleep. (2011) 34:57–63. doi: 10.1093/sleep/34.1.57
19. Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. (2003) 12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x
20. Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. (2015) 191:731–8. doi: 10.1164/rccm.201411-2099CI
21. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. (2013) 17:R46. doi: 10.1186/cc12565
22. Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. (2000) 117:809–18. doi: 10.1378/chest.117.3.809
23. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. (2001) 163:451–7. doi: 10.1164/ajrccm.163.2.9912128
24. Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. (2003) 167:708–15. doi: 10.1164/rccm.2201090
25. Drouot X, Roche-Campo F, Thille AW, Cabello B, Galia F, Margarit L, et al. A new classification for sleep analysis in critically ill patients. Sleep Med. (2012) 1:7–14. doi: 10.1016/j.sleep.2011.07.012
26. Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. (2013) 41:263–306. doi: 10.1097/CCM.0b013e3182783b72
27. Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit: executive summary. Am J Health Syst Pharm. (2013) 70:53–8. doi: 10.1093/ajhp/70.1.53
28. Wilcox ME, McAndrews MP, Van J, Jackson JC, Pinto R, Black SE, et al. Sleep fragmentation and cognitive trajectories after critical illness. Chest. (2020). doi: 10.1016/j.chest.2020.07.036. [Epub ahead of print].
29. Kamdar BB, Knauert MP, Jones SF, Parsons EC, Parthasarathy S, Pisani MA, et al. Perceptions and practices regarding sleep in the Intensive Care Unit. a survey of 1,223 critical care providers. Ann Am Thorac Soc. (2016) 13:1370–7. doi: 10.1513/AnnalsATS.201601-087OC
30. Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
31. Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Executive summary: clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:1532–48. doi: 10.1097/CCM.0000000000003259
32. Altman MT, Knauert MP, Murphy TE, Ahasic AM, Chauhan Z, Pisani MA. Association of intensive care unit delirium with sleep disturbance and functional disability after critical illness: an observational cohort study. Ann Intensive Care. (2018) 8:63. doi: 10.1186/s13613-018-0408-4
33. Borbely AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. (2016) 25:131–43. doi: 10.1111/jsr.12371
34. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. (2012) 35:445–62. doi: 10.1146/annurev-neuro-060909-153128
35. Guo X, Kuzumi E, Charman SC, Vuylsteke A. Perioperative melatonin secretion in patients undergoing coronary artery bypass grafting. Anesth Analg. (2002) 94:1085–91. doi: 10.1097/00000539-200205000-00006
36. Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. (2002) 30:536–40. doi: 10.1097/00003246-200203000-00007
37. Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. (2004) 48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x
38. Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. (1999) 317:278–81. doi: 10.1016/S0002-9629(15)40528-2
39. Verceles AC, Silhan L, Terrin M, Netzer G, Shanholtz C, Scharf SM. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med. (2012) 38:804–10. doi: 10.1007/s00134-012-2494-3
40. Madrid-Navarro CJ, Sanchez-Galvez R, Martinez-Nicolas A, Marina R, Garcia JA, Madrid JA, et al. Disruption of circadian rhythms and delirium, sleep impairment and sepsis in critically ill patients. Potential therapeutic implications for increased light-dark contrast and melatonin therapy in an ICU environment. Curr Pharm Des. (2015) 21:3453–68. doi: 10.2174/1381612821666150706105602
41. Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. (2010) 133:161–71. doi: 10.1093/brain/awp313
42. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. (2006) 129:564–83. doi: 10.1093/brain/awl004
43. Choi SH, Lee H, Chung TS, Park KM, Jung YC, Kim SI, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. (2012) 169:498–507. doi: 10.1176/appi.ajp.2012.11060976
44. Kyeong S, Choi SH, Eun Shin J, Lee WS, Yang KH, Chung TS, et al. Functional connectivity of the circadian clock and neural substrates of sleep-wake disturbance in delirium. Psychiatry Res. (2017) 264:10–12. doi: 10.1016/j.pscychresns.2017.03.017
45. Hut RA, van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behav Brain Res. (2011) 221:466–80. doi: 10.1016/j.bbr.2010.11.039
46. Hughes CG, Pandharipande PP, Thompson JL, Chandrasekhar R, Ware LB, Ely EW, et al. Endothelial activation and blood-brain barrier injury as risk factors for delirium in critically Ill patients. Crit Care Med. (2016) 44:e809–17. doi: 10.1097/CCM.0000000000001739
47. Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. (2001) 4:732–8. doi: 10.1038/89522
48. Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev. (2005) 49:429–54. doi: 10.1016/j.brainresrev.2005.01.005
49. Boyko Y, Holst R, Jennum P, Oerding H, Nikolic M, Toft P. Melatonin secretion pattern in critically Ill patients: a pilot descriptive study. Crit Care Res Pract. (2017) 2017:7010854. doi: 10.1155/2017/7010854
50. Maas MB, Lizza BD, Abbott SM, Liotta EM, Gendy M, Eed J, et al. Factors disrupting melatonin secretion rhythms during critical illness. Crit Care Med. (2020) 48:854–61. doi: 10.1097/CCM.0000000000004333
51. Arumugam S, El-Menyar A, Al-Hassani A, Strandvik G, Asim M, Mekkodithal A, et al. Delirium in the intensive care unit. J Emerg Trauma Shock. (2017) 10:37–46. doi: 10.4103/0974-2700.199520
52. Grover S, Ghosh A, Sarkar S, Desouza A, Yaddanapudi LN, Basu D. Delirium in intensive care unit: phenomenology, subtypes, and factor structure of symptoms. Indian J. (2018) 40:169–77. doi: 10.4103/IJPSYM.IJPSYM_274_17
53. Mori S, Takeda JR, Carrara FS, Cohrs CR, Zanei SS, Whitaker IY. Incidence and factors related to delirium in an intensive care unit. Rev Esc Enferm USP. (2016) 50:587–93. doi: 10.1590/S0080-623420160000500007
54. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. (2013) 21:1190–222. doi: 10.1016/j.jagp.2013.09.005
55. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. (2018) 33:1428–57. doi: 10.1002/gps.4823
56. Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. (2011) 77:140–3. doi: 10.1016/j.mehy.2011.03.048
57. Weinhouse GL, Watson PL. Sedation and sleep disturbances in the ICU. Anesthesiol Clin. (2011) 29:675–85. doi: 10.1016/j.anclin.2011.09.007
58. Kondili E, Alexopoulou C, Xirouchaki N, Georgopoulos D. Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study. Intensive Care Med. (2012) 38:1640–6. doi: 10.1007/s00134-012-2623-z
59. Reade MC, O'Sullivan K, Bates S, Goldsmith D, Ainslie WR, Bellomo R. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open-label trial. Crit Care. (2009) 13:R75. doi: 10.1186/cc7890
60. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. (2007) 298:2644–53. doi: 10.1001/jama.298.22.2644
61. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. (2009) 1:489–99. doi: 10.1001/jama.2009.56
62. Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-dose nocturnal dexmedetomidine prevents ICU delirium. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. (2018) 197:1147–56. doi: 10.1164/rccm.201710-1995OC
63. Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. (2016) 315:1460–8. doi: 10.1001/jama.2016.2707
64. Pandharipande PP, Morandi A, Adams JR, Girard TD, Thompson JL, Shintani AK, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. (2009) 35:1886–92. doi: 10.1007/s00134-009-1573-6
65. Huang R, Hertz L. Receptor subtype and dose dependence of dexmedetomidine-induced accumulation of [14C]glutamine in astrocytes suggests glial involvement in its hypnotic-sedative and anesthetic-sparing effects. Brain Res. (2000) 873:297–301. doi: 10.1016/S0006-8993(00)02525-7
66. Hipp DM, Ely EW. Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurother. (2012) 9:158–75. doi: 10.1007/s13311-011-0102-9
67. Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. (2007) 33:1954–8. doi: 10.1007/s00134-007-0769-x
68. Balan S, Leibovitz A, Zila SO, Ruth M, Chana W, Yassica B, et al. The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci. (2003) 15:363–6. doi: 10.1176/jnp.15.3.363
69. Li CX, Liang DD, Xie GH, Cheng BL, Chen QX, Wu SJ, et al. Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Mol Med Rep. (2013) 7:1117–22. doi: 10.3892/mmr.2013.1331
70. Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. (2010) 36:2081–9. doi: 10.1007/s00134-010-2004-4
71. Shi CM, Wang DX, Chen KS, Gu XE. Incidence and risk factors of delirium in critically ill patients after non-cardiac surgery. Chin Med J. (2010) 123:993–9. doi: 10.3760/cma.j.issn.0366-6999.2010.08.004
72. Pearson A, de Vries A, Middleton SD, Gillies F, White TO, Armstrong IR, et al. Cerebrospinal fluid cortisol levels are higher in patients with delirium versus controls. BMC Res Notes. (2010) 3:33. doi: 10.1186/1756-0500-3-33
73. Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. (2008) 65:229–38. doi: 10.1016/j.jpsychores.2008.05.019
74. Nguyen DN, Huyghens L, Zhang H, Schiettecatte J, Smitz J, Vincent JL. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. Biomed Res Int. (2014) 2014:712742. doi: 10.1155/2014/712742
75. Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med. (2012) 27:97–111. doi: 10.1177/0885066610394322
76. Campbell AM, Axon DR, Martin JR, Slack MK, Mollon L, Lee JK. Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta-analysis. BMC Geriatr. (2019) 19:272. doi: 10.1186/s12877-019-1297-6
77. Martinez FE, Anstey M, Ford A, Roberts B, Hardie M, Palmer R, et al. Prophylactic Melatonin for Delirium in Intensive Care (Pro-MEDIC): study protocol for a randomised controlled trial. Trials. (2017) 18:4. doi: 10.1186/s13063-016-1751-0
78. Dres M, Younes M, Rittayamai N, Kendzerska T, Telias I, Grieco DL, et al. Sleep and pathological wakefulness at the time of liberation from mechanical ventilation (SLEEWE). A prospective multicenter physiological study. Am J Respir Crit Care Med. (2019) 199:1106–15. doi: 10.1164/rccm.201811-2119OC
79. Rault C, Sangare A, Diaz V, Ragot S, Frat JP, Raux M, et al. Impact of sleep deprivation on respiratory motor output and endurance: a physiological study. Am J Respir Crit Care Med. (2020) 201:976–83. doi: 10.1164/rccm.201904-0819OC
80. Thille AW, Reynaud F, Marie D, Barrau S, Rousseau L, Rault C, et al. Impact of sleep alterations on weaning duration in mechanically ventilated patients: a prospective study. Eur Respir J. (2018) 51:1702465. doi: 10.1183/13993003.02465-2017
81. Pham T, Telias I, Piraino T, Yoshida T, Brochard LJ. Asynchrony consequences and management. Crit Care Clin. (2018) 34:325–41. doi: 10.1016/j.ccc.2018.03.008
82. Vaschetto R, Cammarota G, Colombo D, Longhini F, Grossi F, Giovanniello A, et al. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. (2014) 42:74–82. doi: 10.1097/CCM.0b013e31829e53dc
83. Chanques G, Kress JP, Pohlman A, Patel S, Poston J, Jaber S, et al. Impact of ventilator adjustment and sedation-analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med. (2013) 41:2177–87. doi: 10.1097/CCM.0b013e31828c2d7a
84. Fanfulla F, Delmastro M, Berardinelli A, Lupo ND, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med. (2005) 172:619–24. doi: 10.1164/rccm.200406-694OC
85. Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol. (1998) 85:1929–40. doi: 10.1152/jappl.1998.85.5.1929
86. Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. (2002) 166:1423–9. doi: 10.1164/rccm.200209-999OC
87. Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically Ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med. (2020) 201:20–32. doi: 10.1164/rccm.201903-0596SO
88. Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. (2018) 197:204–13. doi: 10.1164/rccm.201703-0536OC
89. Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med. (2008) 34:1477–86. doi: 10.1007/s00134-008-1121-9
90. Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. (2010) 38:518–26. doi: 10.1097/CCM.0b013e3181cb0d7b
91. Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre PF, et al. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med. (2011) 37:263–71. doi: 10.1007/s00134-010-2052-9
92. Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. (2007) 35:1048–54. doi: 10.1097/01.CCM.0000260055.64235.7C
93. Delisle S, Ouellet P, Bellemare P, Tetrault JP, Arsenault P. Sleep quality in mechanically ventilated patients: comparison between NAVA and PSV modes. Ann Intensive Care. (2011) 1:42. doi: 10.1186/2110-5820-1-42
94. Flannery AH, Oyler DR, Weinhouse GL. The impact of interventions to improve sleep on delirium in the ICU. A systematic review and research framework. Crit Care Med. (2016) 44:2231–40. doi: 10.1097/CCM.0000000000001952
95. Burry L, Scales D, Williamson D, Foster J, Mehta S, Guenette M, et al. Feasibility of melatonin for prevention of delirium in critically ill patients: a protocol for a multicentre, randomised, placebo-controlled study. BMJ Open. (2017) 7:e015420. doi: 10.1136/bmjopen-2016-015420
96. Zhang Y, Su J, Wang J, Tang G, Hu W, Mao J, et al. Cognitive behavioral therapy for insomnia combined with eszopiclone for the treatment of sleep disorder patients transferred out of the intensive care unit: a single-centred retrospective observational study. Medicine. (2018) 97:e12383. doi: 10.1097/MD.0000000000012383
Keywords: ICU acquired delirium, sleep, circadian rhythm, intervention, mechanisms
Citation: Daou M, Telias I, Younes M, Brochard L and Wilcox ME (2020) Abnormal Sleep, Circadian Rhythm Disruption, and Delirium in the ICU: Are They Related? Front. Neurol. 11:549908. doi: 10.3389/fneur.2020.549908
Received: 07 April 2020; Accepted: 18 August 2020;
Published: 18 September 2020.
Edited by:
Sairam Parthasarathy, University of Arizona, United StatesReviewed by:
Gregory M. Brown, University of Toronto, CanadaCopyright © 2020 Daou, Telias, Younes, Brochard and Wilcox. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Elizabeth Wilcox, ZWxpemFiZXRoLndpbGNveEB1dG9yb250by5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.