
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 10 December 2020
Sec. Movement Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.533731
Objectives: This study aimed to investigate the relationship between nutritional status and Parkinson's disease (PD) features.
Methods: The cohort was composed of 556 Parkinson's patients who were admitted to the hospital. Patients were categorized as normal nutrition or at risk of malnutrition/already malnourished. Questionnaires, physical examinations, and biochemical tests were conducted. The relationship between nutrition status and PD was analyzed using t-tests, χ2-tests, and logistic regression models.
Results: The prevalence of malnutrition [defined as a Mini Nutritional Assessment (MNA) score <17] was 39.2%, and 30.3% of patients were at risk of malnutrition (17 ≤ MNA score ≤ 23.5). There was no difference in gender and age between the different nutrition groups (P < 0.05). Patients at risk of malnutrition and those who were malnourished had a longer course of disease, more severe motor symptoms, a higher stage of PD according to the Hoehn and Yahr (H-Y) classification, a lower body mass index (BMI) index, a lower cognitive score, higher levels of depression and anxiety, and more serious non-motor symptoms (P < 0.05) than patients with normal nutrition. There were differences in adenosine deaminase, albumin, phosphorus, chlorine, total protein, and uric acid between the two groups (P < 0.05). High Unified PD Rating Scale (UPDRS-III) scores, high H-Y stages, and dyskinesia were risk factors for malnutrition in PD patients, while high levels of total protein, uric acid, and chlorine were protective factors that led to good nutrition (P < 0.05).
Conclusions: Our results showed that dyskinesia, disease severity, total protein levels, uric acid levels, and chlorine levels were associated with nutritional status among Chinese PD patients. The findings of this study indicate the significance of the early detection and prevention of malnutrition to improve the quality of life of PD patients.
Parkinson's disease (PD) is a chronic degenerative neurodegenerative disease. The incidence of PD is ~2% among individuals over 65 years old in China, accounting for more than 40% of the total number of PD patients in the world. The prevalence increases annually. PD seriously endangers the physical and mental health of elderly people and causes huge economic and social burdens (1).
Some studies have shown that some lifestyle interventions could improve the quality of life of PD patients, such as behavior therapy, sleep improvement, mood improvement, and physical exercise (2–5). However, few studies have examined the effect of nutritional status on the quality of life of patients with PD (6–8). A study showed a negative association between the severity of motor impairment and total fat mass in patients with PD (7). Another study showed that malnutrition in PD patients can cause olfactory and gustatory dysfunction (9). Nutritional risk screening plays an increasingly important role in PD. Therefore, we evaluated the cross-sectional association between nutritional status and PD to better predict the prognosis and development of PD.
A consecutive series of 556 hospitalized PD patients (324 men and 232 women) from the Brain Hospital Affiliated to Nanjing Medical University during the period January 2015–December 2019. These participants conformed to the diagnostic criteria of UK Brain Bank PD, and we excluded patients with other diseases, such as serious heart disease, liver disease, dementia, and kidney disease. Moreover, all participants with confirmed digestive-system diseases or other consumptive diseases, such as infections and tumors, were excluded from this nutritional assessment. This study was approved by the Ethics Committee of the Brain Hospital Affiliated to Nanjing Medical University. We obtained informed consent from the patients.
All participants were divided into three groups based on nutritional status. Questionnaires, physical examinations, and biochemical tests were conducted. The Mini Nutritional Assessment (MNA) is a short valid nutritional screen for elderly people that is recommended by the European Society for Clinical Nutrition and Metabolism (ESPEN) (10). The MNA was used in this study to categorize patients as normal nutritional status, risk of malnutrition, or already malnourished. The final score predicts malnutrition (MNA score 24–30, normal nutritional status; MNA score 17–23.5, at risk of malnutrition; and MNA score <17, already malnourished) (11). The Unified PD Rating Scale part III (UPDRS-III), Hoehn and Yahr (H-Y) stage, and PD Non-Motor Symptoms (PD-NMS) questionnaire were used to assess the motor symptoms, non-motor symptoms, and severity of PD. The Mini-Mental State Examination (MMSE) scale was used to evaluate the cognitive level of PD patients. The Hamilton Depression Rating Scale (HAMD) and Hamilton Anxiety Rating Scale (HAMA) were used to evaluate the depression and anxiety levels of PD patients, respectively. Patients were divided into tremor-dominant type and akinetic-rigid type PD subgroups using part III of the UPDRS (12). Participants were measured for height with a height meter and sitting height with a sitting height meter; they were required to take off shoes and caps before measurements, and the results were accurate to 0.1 cm. Additionally, body weight was measured with an electronic weighing machine; patients were required to wear only single clothes, and the results were accurate to 0.1 kg. The body mass index (BMI) was calculated by dividing body weight (kg) by the square of height (m2). Levodopa equivalent daily dose (LEDD) was calculated according to previously published recommendations (13). Participants were asked to fast, and then, at 6:00 a.m., a total of 5 ml of venous blood was collected for routine blood tests (automatic blood cell analyzer, Myry), biochemical tests, and blood lipid detection (biochemical analyzer, Olympus).
All statistical analyses were performed with SPSS software version 19.0 (SPSS Inc., Chicago, IL). Numerical data are presented as the mean ± SD. Differences between two groups were evaluated with Student's t-test for quantitative data. Differences between two groups were evaluated with the χ2-test for qualitative data. A multivariate analysis using a forward binary logistic regression model with nutrition as the dependent variable and the above significant disease characteristics [duration of the disease, UPDRS-III score, H-Y classification, dyskinesia, MMSE score, HAMA score, HAMD score, PD-NMS score, LEDD, albumin (ALB) level, total protein (TP) level, uric acid (UA) level, phosphorus (P) level, chlorine (CL) level, and adenosine deaminase (ADA) level] as independent covariables was used to explore the potential clinical factors that may be related to nutrition. As BMI and weight were closely associated with nutrition and the MNA assessment, they were not be considered in the logistic analysis. Differences were considered significant at P < 0.05.
A tabular description of the study population based upon the nutritional status of PD patients is presented in Table 1. Overall, 556 participants (324 men and 232 women, which accounted for 58.3 and 41.7%, respectively) aged 36–92 years from our hospital were included in this cross-sectional study. The duration of disease among these patients ranged from 0.3 to 30 years, with an average of 6.34 ± 4.80 years. The UPDRS-III scores of these patients ranged from 2 to 80, with an average of 25.02 ± 12.37. According to the MNA score, 171 patients had normal nutrition status, 167 patients were at risk of malnutrition and 218 patients were malnourished, which accounted for 30.8, 30.0, and 39.2% of the sample, respectively.
Table 2 presents the general clinical data of PD patients at risk of malnutrition, patients who were malnourished and patients with normal nutrition. We found that there was no difference in gender or age between the two groups (P > 0.05). However, patients at risk of malnutrition and those who were malnourished had a longer duration of disease, more severe motor symptoms, a higher stage of PD according to the H-Y classification, a lower BMI, a lower cognitive score, higher levels of depression and anxiety, and more severe NMS.
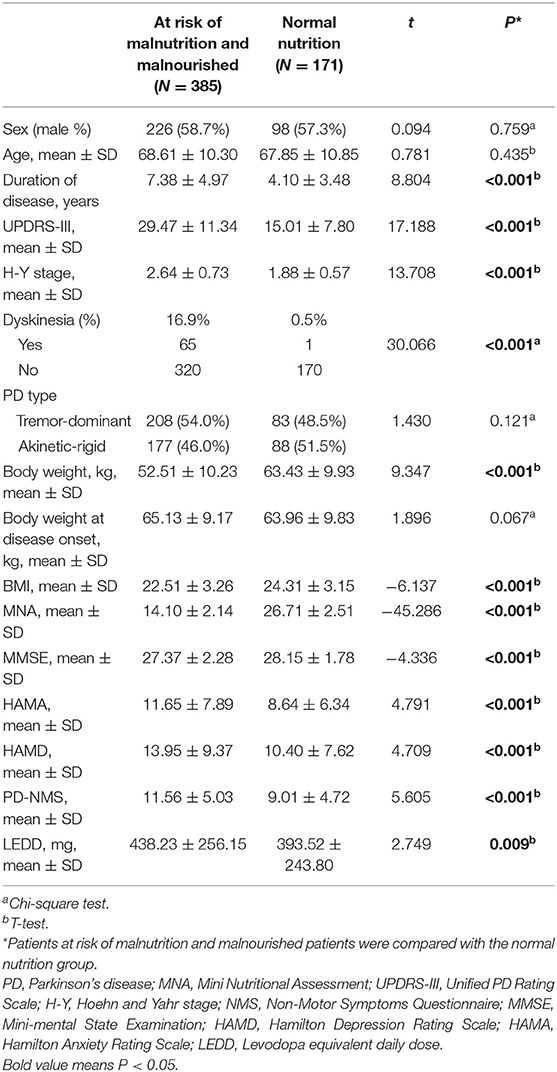
Table 2. The general clinical data of PD patients at risk of malnutrition, malnourished patients, and patients with normal nutrition.
We also presents the biochemical and blood indicators of PD patients at risk of malnutrition, malnourished patients, and patients with normal nutrition (Supplementary Table 1). The results showed that there were differences in ADA, ALB, P, CL, TP, and UA between the two groups (P < 0.05).
Table 3 presents the multivariate analysis of associated factors for malnutrition in PD patients. The nutrition status was taken as the dependent variable (patients at risk of malnutrition and malnourished, patients with normal nutrition). The duration of the disease, UPDRS-III score, H-Y stage, dyskinesia, MMSE score, HAMA score, HAMD score, NMS score, LEDD, ALB level, P level, TP level, UA level, and ADA level were regarded as independent variables. Using the LR forward method, it was concluded that UPDRS-III score, H-Y stage, dyskinesia, and the level of TP, UA, and CL were associated with nutritional status in PD (Figure 1).
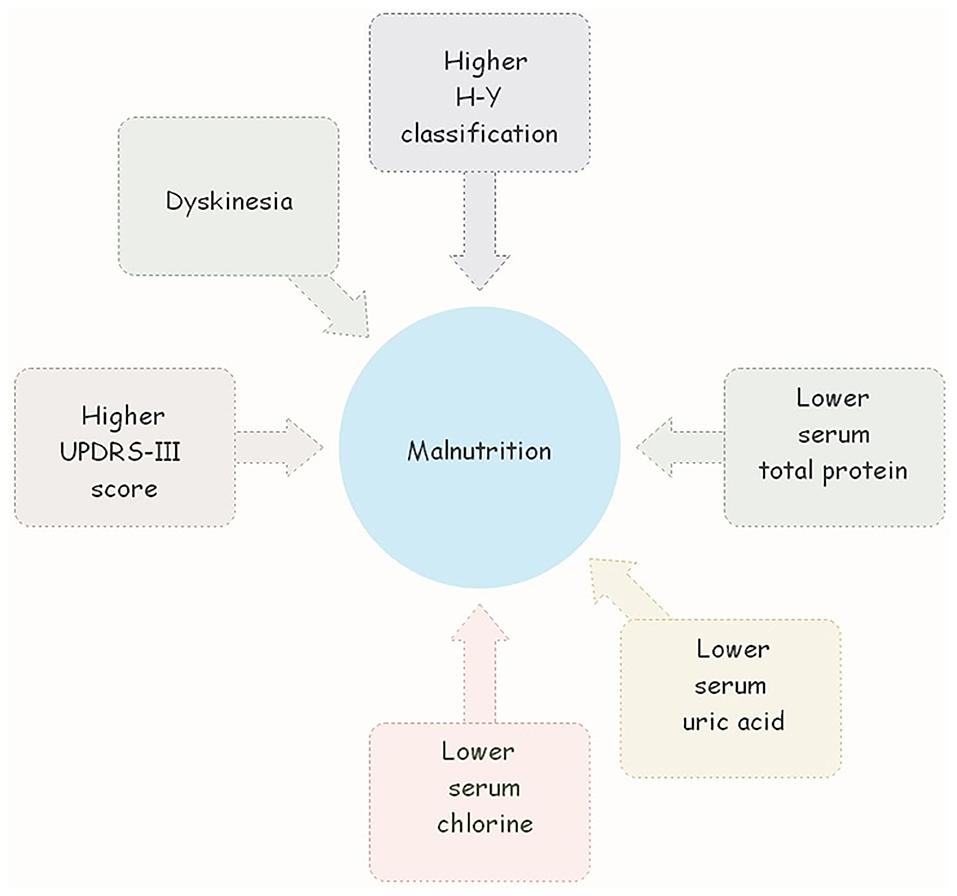
Figure 1. Higer UPDRS-III score, H-Y stage, dyskinesia, and the lower level of TP, UA, and CL were associated with nutritional status in PD.
There are many methods for nutritional risk assessment. Previous studies have shown the risk of malnutrition by using the Short-Form Mini-Nutritional Assessment (MNA-SF), the Nutritional risk screening 2002 (NRS 2002) tool, and the Malnutrition Universal Screening Tool (MUST) (14). Cereda believed that the MNA nutrition screening scale was suitable for elderly patients in different institutions, such as hospitalization, outpatient clinics, and nursing homes. MNA could indicate nutritional risk before the laboratory indicators of malnutrition change and has a strong ability of diagnosis and prediction (15). Therefore, we used the MNA score as a nutritional risk assessment tool in this study. According to the data obtained from our study, 69.2% of our patients had an abnormal nutritional status. In our study, 167 (30.0%) patients were at risk of malnutrition, and 218 (39.2%) patients had malnutrition. These numbers were higher than those in many previous studies (16–18). The fact that the population assessed in our study generally consisted of low-income patients with lower education levels who resided in rural areas may explain the higher malnutrition rates. Due to their low income levels, nutritional intake mostly included carbohydrates.
Risk factors for poor nutrition in adults include older age, living alone, dementia, depression, gastrointestinal dysfunction (such as dysphagia, constipation, and slow gastric emptying), comorbidities and polypharmacy. In PD, these risk factors are more common than in age-matched controls. Factors that are specific to PD may include motor symptoms, NMS, older age at diagnosis, higher LEDD/body weight, depression, dementia, and hallucinations (19, 20). Limited research has been conducted to determine the predictors of malnutrition in Chinese PD patients.
In this study, we found that there were no differences in age and sex between patients at risk of malnutrition, malnourished, and normal nutrition. We also compared the general data, clinical characteristics, and biochemical indicators of the normal nutrition and malnutrition risk groups and found that the duration of disease, motor symptoms, stage of PD, BMI, cognitive scores, depression, and anxiety scores, NMS score, ADA level, ALB level, P level, Cl level, TP level, and UA level differed between the two groups. Then, to reduce bias caused by confounding factors, we used multivariate unconditional logistic regression analysis, which revealed that high UPDRS-III scores, a high H-Y stage, and dyskinesia were risk factors for malnutrition in PD patients, while high levels of TP, UA, and CL were protective factors that led to good nutrition.
Sheard et al. found that a greater UPDRS III score with a higher H&Y stage correlated with lower MNA results (20). We also found that malnourished patients had more pronounced motor symptoms and more severe disease stages. Decreased hand-mouth coordination and difficulties completing fine movements, such as that required for utensils, can be present. Motor symptoms such as bradykinesia, akinesia, rigidity, and tremor can impair functional ability and make it difficult to ambulate, shop, and feed independently.
In our patients with PD who had a poor nutritional status, higher levels of depression, and anxiety were observed. PD is often accompanied by anxiety and depression, which can influence the release of neurotransmitters and hormones in the body and cause intestinal dysfunction, constipation, and nutritional absorption disorders (21). The decrease in enthusiasm for daily activities in patients with PD due to retarded physical motions could also lead to reduced food intake. Reduced food intake, weight loss, and psychiatric disorders could lead to a vicious cycle.
We found that patients with dyskinesia were more likely to suffer from malnutrition, possibly because abnormal exercise leads to an increase in muscle nutrient consumption and eating difficulties. Energy expenditure may be increased by the presence of dyskinesia. Weight loss may result in an increased risk of developing dyskinesia that may, in turn, exacerbate weight loss and the risk of malnutrition.
Our results show that hyperuricemia is a sign of good nutrition, and the reasons may be as follows. Research shows that oxidative stress is a leading factor in the pathogenesis of idiopathic PD, and two intrinsic antioxidative molecules, i.e., bilirubin and UA, are known to protect dopaminergic neurons from oxidative stress in PD (22). Another study showed that the higher UA level group in female PD patients showed a smaller reduction in dopamine transporter uptake in the posterior putamen (23). However, it has been shown that hyperuricemia can also aggravate the development of PD (24). Therefore, how to balance the concentration of UA is a difficult problem at present.
CL is an important negatively charged ion (anion) used to maintain fluid balance. CL ions are the most abundant anions outside the cell. Together with sodium ions, CL ions account for ~80% of the total number of ions that maintain osmotic pressure. It can regulate the volume of extracellular fluid and maintain osmotic pressure. In addition, CL ions have the function of maintaining acid-base balance in body fluids, and excessive intake of CL ions can correct metabolic alkalosis caused by diseases or diuretics. Stomach acid promotes the absorption of vitamin B12 and iron, helps digest food, activates saliva amylase to breakdown starch, and inhibits the growth of microorganisms that enter the stomach with meals. In nerve cells, CL ions stabilize the membrane potential. The relationship between CL and nutrition is still unclear, especially in PD patients. Our research shows that serum CL is closely related to malnutrition. More research is needed to further clarify this correlation.
Adenosine, an important neuromodulator, is capable of attenuating oxidative stress, excitotoxicity, and neuroinflammation (25). On the other hand, adenosine is effective in promoting sleep, improving cognitive function and anti-depressive effects (26). These actions of adenosine have therapeutic implications for motor symptoms and NMS of PD. Several studies indicated that ADA inhibitors protected against neurodegeneration induced by the neurotoxin MPTP (27). In our study, there was a significant increase in ADA in patients at risk of malnutrition and malnourished patients. ADA was included in the logistic regression equation as an independent risk factor. However, ADA was not shown to be an independent predictor of malnutrition in PD patients. The relationship between ADA and nutrition is not fully understood. A study have analyzed if ADA could be considered a functional biochemical parameter in populations at nutritional risk. In this study, the serum ADA activity was analyzed in groups of individuals with altered nutritional status: young adult patients with Nervous Anorexia, overweight children and children suffering cystic fibrosis. The results showed ADA activity statistically significant increased in the in all groups, with respect to their healthy controls (28). The results suggested the importance of including the determination of serum ADA activity in the biochemical evaluation of the nutritional status. Further studies are needed to validate the relationship between ADA and nutrition in PD patients with longitudinal data.
There are many reasons for malnutrition. It was recently reported that the ketogenic diet is a low-carbohydrate and fat-rich diet. Its implementation has a fasting-like effect, which brings the body into a state of ketosis. Due to improper dietary control, PD patients have a ketogenic diet to reduce food intake and cause malnutrition (29). Therefore, we need to pay more attention to the dietary structure of PD patients. On the other hand, some studies have shown that vitamin D deficiency was significantly associated with the risk of PD and closely related to malnutrition in PD patients (30, 31). In future studies, we will pay more attention to the effects of vitamins and trace elements in PD patients to balance macro- and micronutrients. Additionally, owing to the different economic and cultural levels of different PD patients, some patients intake high levels of carbohydrates, which may lead to malnutrition. This phenomenon deserves our attention. We should provide good education and promote healthy nutrition among PD patients. Finally, most of the patients were enrolled from our hospital, so the sample may not be representative of the whole population. Additional general population studies are needed.
The strengths of this paper are the large sample and the evaluation of biochemical parameters. This study, however, has several limitations. It was a retrospective study, so it may be difficult to establish cause-effect associations. Longitudinal studies are needed in the future study. We have explained the selections of variables for the modes above. In the univariate analysis we found that patients with malnutrition showed had higher depression and anxiety scores, lower MMSE score, and a greater non-motor symptoms burden. However, neither of these non-motor symptoms emerged as an independent factor predictive of malnutrition in our study. Possible mechanisms underlying this comorbidity are difficult to interpret. It was a retrospective study, so it may be difficult to establish cause-effect associations. We are conducting a 5-year prospective, multicenter study on the nutritional status of Parkinson's patients. More advanced methods for testing the importance of these factors will be used in the future study.
In summary, PD is a neurodegenerative disease, and malnutrition is its main concomitant symptom in the later stage. High UPDRS-III scores, high H-Y stages, dyskinesia, high levels of TP, UA, and CL were associated with nutritional status among Chinese PD patients. These findings provide evidence that nutritional status assessments should be standard during the treatment of PD patients.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Brain Hospital Affiliated to Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
TY and JD participated in conception and design of the study, acquisition of raw data, analysis of data, drafting the article, and revising the manuscript. ZZ, LiaZ, JZ, and YL participated in conception and design of the study, collection of date, analysis of data, and revising the manuscript. LilZ, JG, YZ, and LiaZ participated in conception, design of the study, and revising the manuscript. All authors contributed to the article and approved the submitted version.
Nanjing Medical Science and Technology Development Foundation (No. QRX17180) and Special Funds of the Jiangsu Provincial Key Research and Development Projects (Grant No. BE2018610) provided the funding for the design of the study, collection and data analysis, and for the development of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the patients and their families for their participation in the study. We would like to acknowledge the hard and dedicated work of all the staff who implemented the evaluation components of the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.533731/full#supplementary-material
1. Zhang L, Dong J, Liu W, Zhang Y. Subjective poor sleep quality in Chinese patients with parkinson's disease without dementia. J Biomed Res. (2013) 27:291–5. doi: 10.7555/JBR.27.20120143
2. Park MS, Choi JY, Song YJ, Choi H, Park EJ, Ji ES. Systematic review of behavioral therapy to improve swallowing functions of patients with parkinson's disease. Gastroenterol Nurs. (2019) 42:65–78. doi: 10.1097/SGA.0000000000000358
3. Gilat M, Coeytaux Jackson A, Marshall NS, Hammond D, Mullins AE, Hall JM, et al. Melatonin for rapid eye movement sleep behavior disorder in parkinson's disease: a randomised controlled trial. Mov Disord. (2019) 35:344–9. doi: 10.1002/mds.27886
4. Assogna F, Pellicano C, Savini C, Macchiusi L, Pellicano GR, Alborghetti M, et al. Drug choices and advancements for managing depression in parkinson‘s disease. Curr Neuropharmacol. (2019) 18:277–87. doi: 10.2174/1570159X17666191016094857
5. Zhu M, Zhang Y, Pan J, Fu C, Wang Y. Effect of simplified Tai Chi exercise on relieving symptoms of patients with mild to moderate parkinson's disease. J Sports Med Phys Fitness. (2019) 60:282–8. doi: 10.23736/S0022-4707.19.10104-1
6. Ongun N. Does nutritional status affect parkinson's disease features and quality of life? PLoS ONE. (2018) 13:e0205100. doi: 10.1371/journal.pone.0205100
7. Pisciotta MS, Fusco D, Grande G, Brandi V, Lo Monaco MR, Laudisio A, et al. Untangling the relationship between fat distribution, nutritional status and parkinson's disease severity. Aging Clin Exp Res. (2019) 32:77–84. doi: 10.1007/s40520-019-01166-x
8. Budrewicz S, Zmarzly A, Raczka D, Szczepanska A, Koziorowska-Gawron E, Slotwinski K, et al. Clinical and nutritional correlations in parkinson's disease: preliminary report. Adv Clin Exp Med. (2019) 28:193–8. doi: 10.17219/acem/76375
9. Roos DS, Oranje OJM, Freriksen AFD, Berendse HW, Boesveldt S. Flavor perception and the risk of malnutrition in patients with parkinson's disease. J Neural Transm. (2018) 125:925–30. doi: 10.1007/s00702-018-1862-8
10. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M, Educational, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/S0261-5614(03)00098-0
11. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. (1999) 15:116–22. doi: 10.1016/S0899-9007(98)00171-3
12. Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. (2005) 76:343–8. doi: 10.1136/jnnp.2003.033530
13. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
14. Raslan M, Gonzalez MC, Dias MC, Nascimento M, Castro M, Marques P, et al. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. (2010) 26:721–6. doi: 10.1016/j.nut.2009.07.010
15. Cereda E. Mini nutritional assessment. Curr Opin Clin Nutr Metab Care. (2012) 15:29–41. doi: 10.1097/MCO.0b013e32834d7647
16. Wang G, Wan Y, Cheng Q, Xiao Q, Wang Y, Zhang J, et al. Malnutrition and associated factors in Chinese patients with parkinson's disease: results from a pilot investigation. Parkinsonism Relat Disord. (2010) 16:119–23. doi: 10.1016/j.parkreldis.2009.08.009
17. Barichella M, Villa MC, Massarotto A, Cordara SE, Marczewska A, Vairo A, et al. Mini nutritional assessment in patients with parkinson's disease: correlation between worsening of the malnutrition and increasing number of disease-years. Nutr Neurosci. (2008) 11:128–34. doi: 10.1179/147683008X301441
18. Uc EY, Struck LK, Rodnitzky RL, Zimmerman B, Dobson J, Evans WJ. Predictors of weight loss in parkinson's disease. Mov Disord. (2006) 21:930–6. doi: 10.1002/mds.20837
19. Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Malnutrition in a sample of community-dwelling people with parkinson's disease. PLoS ONE. (2013) 8:e53290. doi: 10.1371/journal.pone.0053290
20. Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Markers of disease severity are associated with malnutrition in parkinson's disease. PLoS ONE. (2013) 8:e57986. doi: 10.1371/journal.pone.0057986
21. Panarese A, Pesce F, Porcelli P, Riezzo G, Iacovazzi PA, Leone CM, et al. Chronic functional constipation is strongly linked to vitamin D deficiency. World J Gastroentero. (2019) 25:1729–40. doi: 10.3748/wjg.v25.i14.1729
22. Lee DY, Oh M, Kim SJ, Oh JS, Chung SJ, Kim JS. Bilirubin-related differential striatal [18F]FP-CIT uptake in parkinson disease. Clin Nucl Med. (2019) 44:855–9. doi: 10.1097/RLU.0000000000002749
23. Oh YS, Kim JS, Yoo SW, Hwang EJ, Lyoo CH, Lee KS. Gender difference in the effect of uric acid on striatal dopamine in early parkinson's disease. Eur J Neurol. (2019) 27:258–64. doi: 10.1111/ene.14070
24. Csoti I, Dresel C, Hauptmann B, Muller T, Redecker C, Warnecke T, et al. Nutritional aspects in parkinson's disease: disease risk, dietary therapy and treatment of digestive tract dysfunction. Fortschritte Neurol Psychiatrie. (2018) 86:S34–S42. doi: 10.1055/a-0681-6700
25. Min KJ, Kim JH, Jou I, Joe EH. Adenosine induces hemeoxygenase-1 expression in microglia through the activation of phosphatidylinositol 3-kinase and nuclear factor E2-related factor 2. Glia. (2008) 56:1028–37. doi: 10.1002/glia.20676
26. Bortolotto JW, de Melo GM, Cognato GD, Vianna MRM, Bonan CD. Modulation of adenosine signaling prevents scopolamine-induced cognitive impairment in zebrafish. Neurobiol Learn Mem. (2015) 118:113–9. doi: 10.1016/j.nlm.2014.11.016
27. Huang W, Xu Y, Zhang Y, Zhang P. Metabolomics-driven identification of adenosine deaminase as therapeutic target in a mouse model of parkinson's disease. J Neurochem. (2019) 150:282–95. doi: 10.1111/jnc.14774
28. Susana FM, Paula P, Slobodianik N. Dietary modulation of thymic enzymes. Endocr Metab Immune Disord Drug Targets. (2014) 14:309–12. doi: 10.2174/1871530314666140915125248
29. Wlodarek D. Role of ketogenic diets in neurodegenerative diseases (alzheimer's disease and parkinson's disease). Nutrients. (2019) 11:169. doi: 10.3390/nu11010169
30. Zhou ZL, Zhou RZ, Zhang ZQ, Li KP. The association between vitamin d status, vitamin d supplementation, sunlight exposure, and parkinson's disease: a systematic review and meta-analysis. Med Sci Monitor. (2019) 25:666–74. doi: 10.12659/MSM.912840
Keywords: Parkinson's disease, malnutrition, MNA, disease severity, dyskinesia
Citation: Yang T, Zhan Z, Zhang L, Zhu J, Liu Y, Zhang L, Ge J, Zhao Y, Zhang L and Dong J (2020) Prevalence and Risk Factors for Malnutrition in Patients With Parkinson's Disease. Front. Neurol. 11:533731. doi: 10.3389/fneur.2020.533731
Received: 10 February 2020; Accepted: 19 November 2020;
Published: 10 December 2020.
Edited by:
Steven Frucht, Mount Sinai Hospital, United StatesReviewed by:
Yih-Ru Wu, Chang Gung Memorial Hospital, TaiwanCopyright © 2020 Yang, Zhan, Zhang, Zhu, Liu, Zhang, Ge, Zhao, Zhang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, bmV1cm9femhhbmdsaUAxNjMuY29t; Jingde Dong, ZG9uZ2ppbmdkZTE5NzhAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.