
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 20 August 2020
Sec. Stroke
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00910
This article is part of the Research Topic Consequences of the COVID-19 Pandemic on Care for Neurological Conditions View all 77 articles
 Parneet Grewal*†
Parneet Grewal*† Pranusha Pinna†
Pranusha Pinna† Julianne P. Hall
Julianne P. Hall Rima M. Dafer
Rima M. Dafer Tachira Tavarez
Tachira Tavarez Danielle R. Pellack
Danielle R. Pellack Rajeev Garg
Rajeev Garg Nicholas D. Osteraas
Nicholas D. Osteraas Alejandro Vargas
Alejandro Vargas Sayona John
Sayona John Ivan Da Silva‡
Ivan Da Silva‡ James J. Conners‡
James J. Conners‡Background: COVID-19 has been associated with increased risk of venous and arterial thromboembolism including ischemic stroke. We report on patients with acute ischemic stroke and concomitant COVID-19 in a diverse patient population.
Methods: This is a retrospective analysis of patients hospitalized with acute ischemic stroke (AIS) and COVID-19 to our comprehensive stroke center in Chicago, IL, between March 1, 2020, and April 30, 2020. We reviewed stroke characteristics, etiologies, and composite outcomes. We then compared our cohort with historic patients with AIS without COVID-19 admitted in the same time frame in 2019 and 2020.
Results: Out of 13 patients with AIS and COVID-19, Latinos and African-Americans compromised the majority of our cohort (76.8%), with age ranging from 31–80 years. Most strokes were cortical (84.6%) and more than 50% of patients had no identifiable source, and were categorized as embolic stroke of unknown source (ESUS). A trend toward less alteplase administration was noted in the COVID-19 stroke patients compared to the non-COVID group from 2020 and 2019 (7.1 vs. 20.7% p 0.435 and 7.1 vs. 27.2% p 0.178). Endovascular thrombectomy was performed in 3 (23%) patients. Systemic thrombotic complications occurred in 3 (23%) COVID-19 AIS patients. Median National Institutes of Health Stroke Scale and modified Rankin Scale at discharge were 11 (IQR 4–23) and 4 (IQR 3–4), respectively. In the logistic regression model corrected for age and sex, COVID-19 was associated with discharge to mRS > 2 (p 0.046, OR 3.82, CI 1.02–14.3). Eight patients (63.8%) were discharged home or to acute rehabilitation, and two deceased from COVID-19 complications.
Conclusion: AIS in the setting of COVID-19 is associated with worse outcomes, especially among African-American and Latino populations. Large vessel disease with ESUS was common suggesting an increased risk of coagulopathy and endothelial dysfunction as a potential etiology.
The severe acute respiratory distress syndrome virus (SARS-CoV-2) causing the coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China, and has since spread throughout the world at an alarming rate, affecting over 7 million people as of June 7, 2020 (1). Neurologic involvement including stroke has been reported (2, 3). Ischemic strokes in COVID-19 have been associated with poor outcomes but the data are mainly limited to Asian and white populations. Data on the potential increased risk of stroke in COVID-19 has not yet been reported in racially diverse patient populations such as Latinos and African-Americans (4, 5).
In this manuscript, we report clinical and laboratory characteristics along with outcomes of patients with COVID-19 and acute ischemic stroke (AIS) who presented to our comprehensive stroke center in Chicago, IL, between March 1, 2020 and April 30, 2020. Our tertiary care center has been in the epicenter of the outbreak in Chicago in the Midwest US and regularly cares for an underserved and diverse patient population with lower health literacy. To validate our findings, to further identify mechanisms of stroke and outcome variables, we compared our cohort with stroke patients from the same time frame in 2020 along with historical cohort from 2019.
We conducted a retrospective observational analysis of the medical records of all patients admitted to Rush University Medical Center in Chicago, Illinois, United States, between March 1, 2020 and April 30, 2020, with the diagnosis of AIS, confirmed on magnetic resonance imaging (MRI) or computed tomography (CT) and who were positive for COVID-19 with real-time reverse transcriptase polymerase-chain-reaction assay from a nasopharyngeal swab. To compensate for any seasonal or monthly variation in incidence and mortality from AIS, we compared the cohort with a control group of non-COVID-19 AIS patients hospitalized within the matched time frame in 2020. We also compared with a historical cohort from 2019 to control for any changes in the patient population over time. Two different cohorts were used as control to avoid random variation in demographics between pre-COVID and COVID-era.
Demographics and clinical and laboratory data were collected via a review of the electronic medical record system. These included age, gender, ethnicity, pre-existing vascular risk factors, admission vital signs, laboratory values, and National Institutes of Health Stroke Scale (NIHSS) score on admission and at discharge (or at the time of data collection for patients still hospitalized). We divided the patients with COVID-19 AIS into the “COVID” group, defined as patients admitted initially with COVID-19 symptoms then subsequently developing AIS, and the “neuro” group, with patients admitted for AIS as initial symptoms, and tested positive for COVID-19. “COVID” group had more extensive inflammatory and coagulopathy workup. All patients received acute stroke care per the American Heart Association and American Stroke Association guidelines (6).
We used the (TOAST) classification to determine stroke etiology (7). All AIS patients received extensive evaluation including advanced cardiac imaging, hypercoagulability panel, and prolonged cardiac monitoring while admitted inpatient. We further evaluated cryptogenic stroke patients to identify embolic stroke of unknown source (ESUS) etiology according to the published criteria (8). Patients with potential stroke mechanisms thought to be due to hypercoagulable state due to COVID-19 were placed under cryptogenic and/or ESUS mechanisms.
Outcome measures were based on discharge disposition and modified Rankin Scale (mRS) (9). COVID-19 severity was defined as mild, regular, or severe/critical based on the 7th edition of “Novel Coronavirus Pneumonia Diagnosis and Treatment Plan,” with the description as follows: mild, defined as minor clinical symptoms and lack of lung inflammation on imaging; regular, with fever and respiratory tract symptoms, and evidence of visible lung inflammation on imaging; severe, with either shortness of breath, RR more than 30 breaths per minute, or SpO2 <93% at rest on pulse oximetry; and critical, with the need for mechanical ventilation or the presence of shock or combined failure of other organs requiring ICU monitoring (10).
Statistical testing was used to detect in-between group differences and association of individual variables to the pre-selected outcomes. The cohort groups were compared using Student's t-test for parametric continuous variables, MannWhitney U test for non-parametric continuous variables, and Fisher's exact test for dichotomous variables. Logistic regression was used to analyze selected variables (either clinically relevant or with statistical association in the first analysis) in regards to the pre-selected outcome measurements, correcting for confounding factors. All analyses were performed using commercially available SPSS (v. 21, Chicago IL, USA) statistical software. Significance was set at p < 0.05. Data were collected using REDCap, an electronic data capture tool hosted at our institution (11). This research protocol was approved by the Rush University institutional review board.
Between March 1, 2020 and April 30, 2020, ~650 patients were hospitalized with COVID-19, of whom 13 patients had AIS (estimated percentage of 2.0%). The COVID-19 AIS cohort was mostly comprised of Latino (46.1%) and African-American (30.7%) individuals, ages ranging from 31 to 80 years (mean 61.6 years). There were 6 patients in the “COVID” group (47%) and 7 in the “Neuro” group (53%). The average time for diagnosis of AIS in the “COVID” group after the hospitalization was 7.1 ± 5.1 days. Conventional vascular risk factors were common in both with no specific predilection for either the “COVID” or the “Neuro” groups. The three most common risk factors in the COVID-19 AIS cohort were hypertension (69.2%), type 2 diabetes mellitus (DM) (69.2%), and hyperlipidemia (30.7%). The COVID-19 was considered severe or critical in 61.5% (n = 8) divided between the “COVID” (50%) and the “Neuro” (71.4%) groups, and mild and/or regular in 38.5% (n = 5) of patients. Out of the 13 patients, 30.7% (n = 4) patients also had superimposed bacterial infection. Median admission NIHSS was 16 (IQR 4–23) in all the COVID-AIS patients, with higher score of 13 in the “Neuro” group compared to 4.5 in the “COVID” group (Table 1).
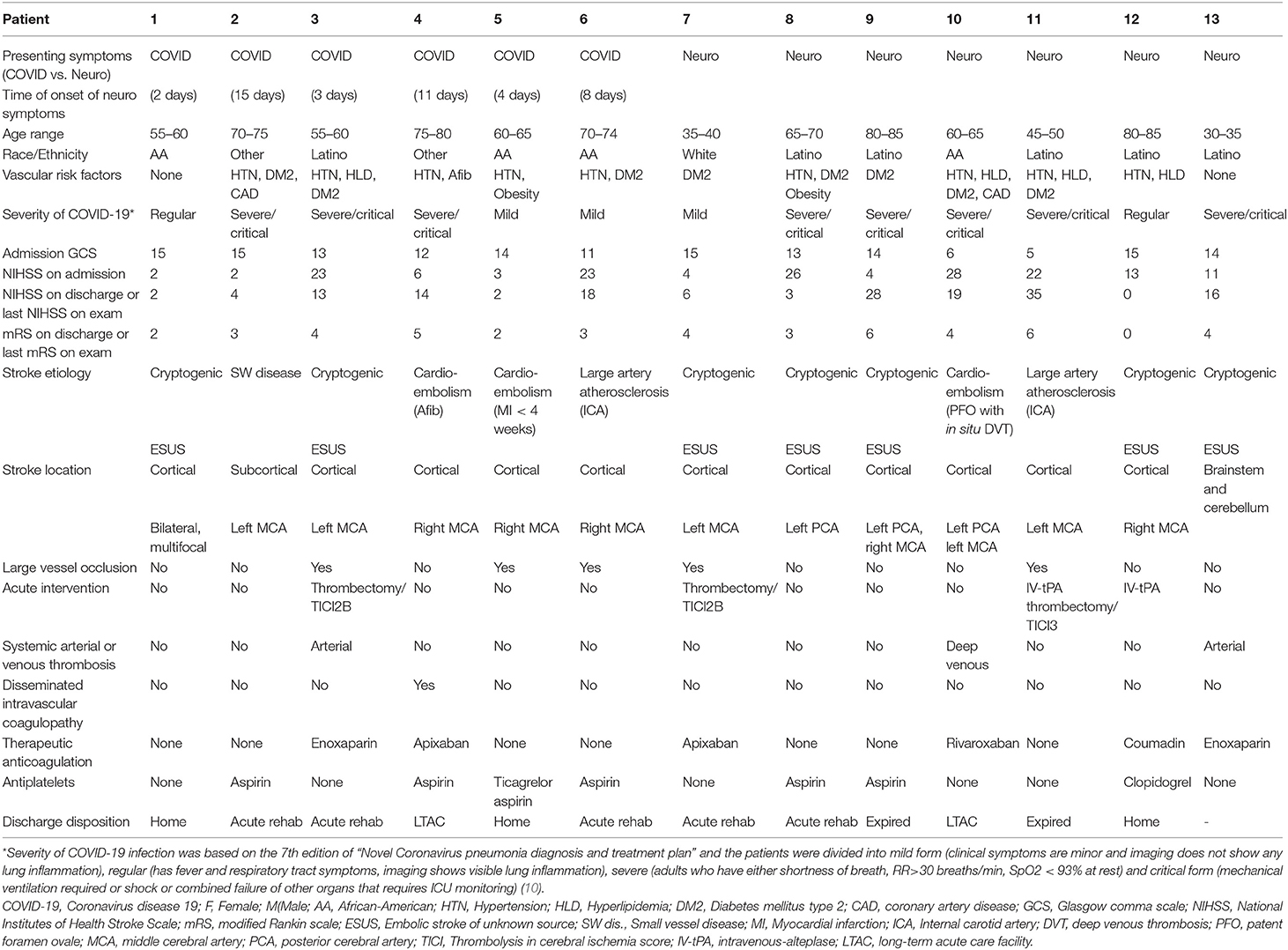
Table 1. Demographics and clinical features of 13 consecutive acute ischemic stroke patients with COVID-19 infection.
Initial vitals and laboratory values are demonstrated in detail in Table 2. Average temperature was 99.6 ± 1.8 degree F, mean arterial pressure was 82.6 ± 38.7 mmHg, heart rate was 90.6 ± 29.4 beats per minute, respiratory rate was 22 ± 8 breaths per minute, and oxygen saturation was 93.3 ± 7.8%. Patients in the “COVID” group were more likely to have multiorgan failure and elevated inflammatory and coagulopathy markers (Table 2).
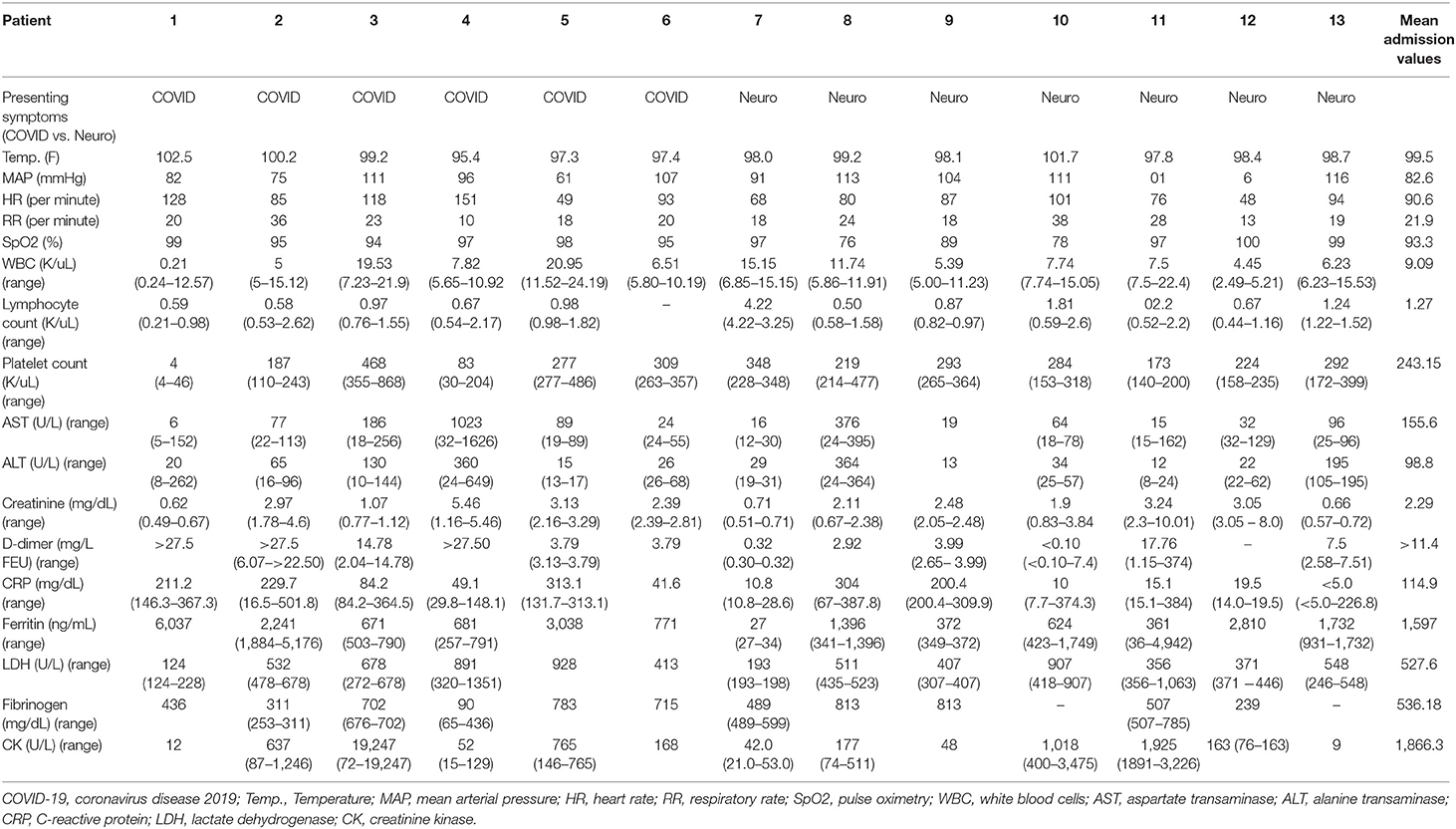
Table 2. Admission vitals and admission laboratory values for acute ischemic stroke patients with COVID-19.
Ischemic strokes were predominantly cortical (84.6%), in the distribution of the middle cerebral artery (76.9%), followed by the posterior cerebral artery (23%). Stroke etiology was classified as cryptogenic and/or ESUS in 53.8% (n = 7), cardioembolic in 23% (n = 3), large artery atherosclerosis in 15.3% (n = 2), and small vessel disease in 7% (n = 1). ESUS was suspected in 71.4% of “Neuro” compared to 33.3% of “COVID.” Overall, 60% (n = 3) of patients with evidence of large vessel occlusion (LVO) underwent endovascular thrombectomy. While the “COVID” group had more LVO (50%), more patient in the “Neuro” group (42.8 vs. 16.6%) received acute stroke interventions, with delays in identification of AIS symptoms in the “COVID” group attributed to masking of symptoms by the COVID-19 systemic manifestations. Therapeutic anticoagulation was initiated in 38.4% (n = 5) patients due to concerns of hypercoagulable state and in 7% (n = 1) due to atrial fibrillation. The median NIHSS at discharge for the COVID AIS cohort was 11 (IQR 4–23), with median mRS of 4 (IQR 3–4). Favorable outcome with discharges to home or to acute rehabilitation facilities was seen in 61.5% (n = 3) Two patients (15.3%) expired from COVID-19 complications, and two (15.3%) required long term facility care. One patient remains hospitalized (Table 1).
Except for DM type 2 which was more prevalent in the COVID-19 AIS group (64.2 vs. 24.5%, p 0.008), patients were overall equally balanced for age, sex and comorbidities with the non-COVID stroke cohorts from 2019 and 2020. The COVID-19 AIS group had more Latinos compared to both historical 2019 and 2020 cohorts (46.1 vs. 9%, p 0.0036 and 46.1 vs. 9.5%, p 0.0075). The percentage of African-American patients in all groups was similar (31 vs. 32%, p 0.989 and 31 vs. 36%, p 0.766). While the median admission NIHSS was not different among the COVID-19 AIS and the non-COVID-19 stroke patients in 2020 and 2019, the discharge NIHSS was significantly higher [11 (IQR 4–23) vs. 3 (IQR 2–13), p 0.036 and 11 (IQR 4–23) vs. 4 (IQR 1–11), p 0.042]. There was a trend toward less alteplase administration in the COVID-19 AIS patients though no statistically significant (7.1 vs. 20.7%, p 0.435 and 7.1 vs. 27.2%, p 0.178). COVID-19 AIS cohort had worse mRS > 2 at discharge (78.5 vs. 47.16%, p 0.047 and 78.5 vs. 40.9%, p 0.010) (Table 3), even after correction for age and sex in a logistic regression model [p 0.046, OR 3.82, (CI 1.02–14.3)].
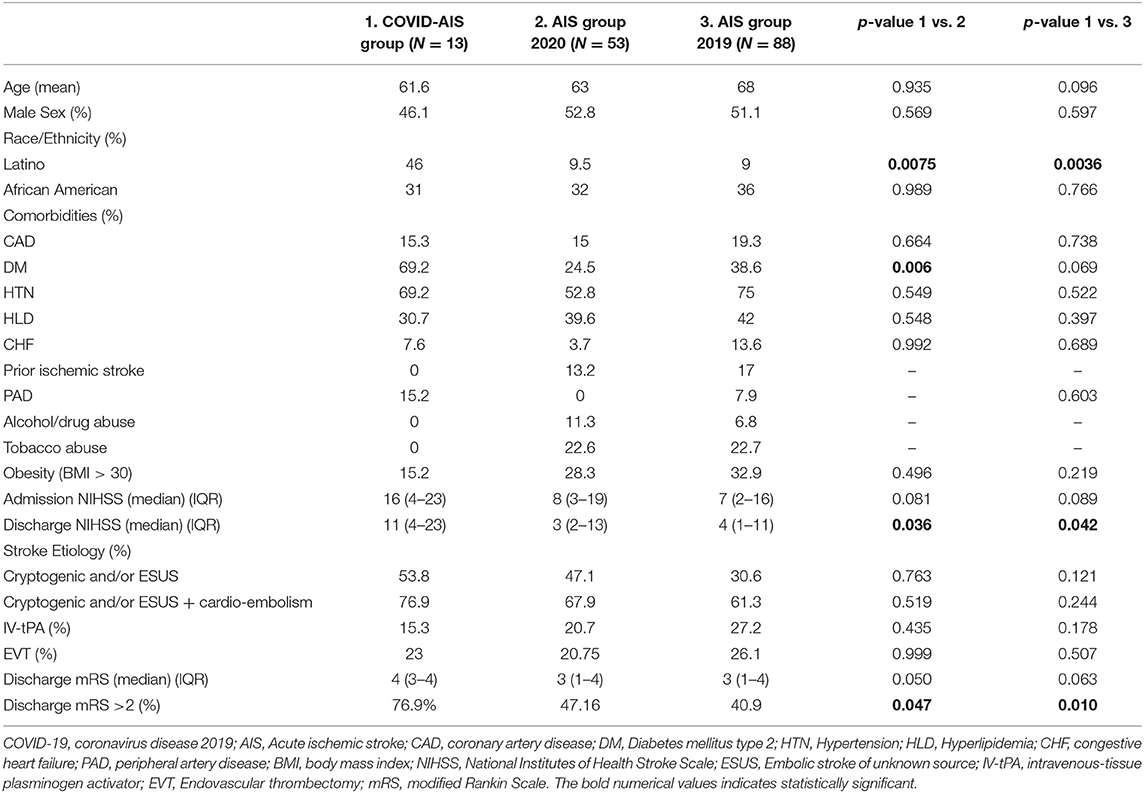
Table 3. Comparison of acute ischemic stroke patients with COVID-19 infection with acute ischemic stroke patients without COVID-19 infection admitted in the same time frame of March 1, 2020–April 30, 2020, and March 1, 2019–April 30, 2019.
In this single-center retrospective observational study, we identified 13 patients with AIS and concomitant COVID-19. Approximately 2.0% of all COVID-19 patients at our institution were diagnosed with AIS, a percentage higher than previously reported in the literature from the USA (12) but similar to that from Wuhan, China (5). The mean age of patients with AIS and COVID-19 was 61.6, without significant sex predilection. Unlike the data emerging out of New York (12), there was a higher percentage of Latinos and African Americans in our cohort (76.8%), highlighting the racial disparity of COVID-19 in our Metropolitan city. Several studies have highlighted the disproportionate burden of this disease on these communities. Social and economic disparities, less access to healthcare along with genetic factors associated with more potent thrombo-inflammatory response may have contributed to higher infection rate and worse outcome in Latinos and African-Americans (13, 14). The majority of our patients had the severe or critical form of COVID-19, which re-iterates the prior published findings of high prevalence of neurological complications seen in this group (2). Also, the trend toward cortical strokes with etiological classification as ESUS reflects the coagulopathy and potential causal link between COVID-19 and stroke (12).
The delay in conventional stroke interventions especially amongst patients who developed AIS while receiving treatment for COVID-19 may be explained by the masking of acute stroke symptoms by the viral illness, delay in stroke symptoms recognition, and/or use of anticoagulation at the time of evaluation.
Several potential mechanisms can lead to a stroke in the setting of COVID-19. Angiotensin-converting enzyme which is the target site of SARS-CoV2 is expressed by cells of the nervous system. This renders the brain at risk of direct endothelial cell infection and diffuse endothelial inflammation (15). COVID-associated coagulopathy which is likely the result of intense inflammatory response, can lead to increased thrombotic complications including ischemic stroke (16). Cardiac involvement is also a prominent feature of COVID-19, leading to stress cardiomyopathy, direct myocardial injury, and arrhythmias with potential increased risk of ischemic stroke (17). Lastly, prolonged hospitalization and dysautonomia may lead to ischemic stroke especially in the setting of septic shock and hypotension (18).
Our study provides a detailed description of patients with COVID-19 and AIS and highlights the racial disparity and poor outcomes associated with this highly contagious viral infection. This study also highlights that despite COVID-19 affecting elderly patients more severely, increased risk of AIS in COVID-19 is independent of age. Comparison with current and historical cohorts suggests a direct causal link of COVID-19 and AIS highlighting the importance of checking for COVID-19 in patients with ESUS and/or cryptogenic stroke mechanisms.
Our study has several limitations with its small size, retrospective approach, and lack of long term follow up and outcome. We also suspect that the incidence of AIS is much higher as many patients with the infection may have succumbed to the disease before identification of the stroke symptoms, or may not have been evaluated by the neurology service, and thus the neurological symptoms may not have been captured.
In summary, ischemic stroke in COVID-19- tend to be more severe, mainly cortical, may occur independent of common vascular risk factors, does not have sex predilection and can affect younger population also. AIS in COVID-19 was more commonly seen in Latino and African American communities by our group, a reflection of the health care disparity and limited access to care among the minority population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Rush University Institutional review board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
PG and PP contributed equally as first authors in the acquisition of data, interpretation of data, and manuscript writing. ID and JC contributed equally as last authors in the interpretation of data and critical revision of the manuscript for intellectual content. JH, RD, TT, DP, RG, NO, AV, and SJ contributed in design and acquisition of data. All authors take full responsibility for the credibility of the data and results of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge our data coordinators, Anjali Asthana MPH, MS, Kelsey Fegan BS, Vikram Patel MSN, RN.
SARS-CoV-2, severe acute respiratory distress syndrome virus; COVID-19, coronavirus disease 19; NIHSS, National Institutes of Health Stroke Scale.
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in china, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, china. JAMA Neurol. (2020) 77:1–9. doi: 10.1001/jamaneurol.2020.1127
3. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe sars-cov-2 infection. N Engl J Med. (2020) 382:2268–70. doi: 10.1056/NEJMc2008597
4. Escalard S, Maïer B, Redjem H, Delvoye F, Hébert S, Smajda S, et al. Treatment of acute ischemic stroke due to large vessel occlusion with covid-19: experience from paris. Stroke. (2020) 51:2540–3. doi: 10.1161/STROKEAHA.120.030574
5. Qin C, Zhou L, Hu Z, Yang S, Zhang S, Chen M, et al. Clinical characteristics and outcomes of covid-19 patients with a history of stroke in Wuhan, China. Stroke. (2020) 51:2219–23. doi: 10.1161/STROKEAHA.120.030365
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
7. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
8. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13:429–38. doi: 10.1016/S1474-4422(13)70310-7
9. Broderick JP, Adeoye O, Elm J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke. (2017) 48:2007–12. doi: 10.1161/STROKEAHA.117.017866
10. Novel Coronavirus Pneumonia Diagnosis Treatment Plan (Provisional 7th ed.). Available online at: https://www.chinalawtranslate.com/en/coronavirus-treatment-plan-7/ (accessed April 30, 2020).
11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81 doi: 10.1016/j.jbi.2008.08.010
12. Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, et al. Sars2-cov-2 and stroke in a new york healthcare system. Stroke. (2020). 51:2002–11. doi: 10.1161/STROKEAHA.120.030335
14. Vepa A, Bae JP, Ahmed F, Pareek M, Khunti K. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab Syndr. (2020) 14:1043–51. doi: 10.1016/j.dsx.2020.06.056
15. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in covid-19. Lancet. (2020) 395:1417–8 doi: 10.1016/S0140-6736(20)30937-5
16. Connors JM, Levy JH. Covid-19 and its implications for thrombosis and anticoagulation. Blood. (2020) 135:2033–40. doi: 10.1182/blood.2020006000
17. Akhmerov A, Marbán E. Covid-19 and the heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
Keywords: acute ischemic stroke, COVID-19, racial disparity, coronavirus, stroke care
Citation: Grewal P, Pinna P, Hall JP, Dafer RM, Tavarez T, Pellack DR, Garg R, Osteraas ND, Vargas A, John S, Da Silva I and Conners JJ (2020) Acute Ischemic Stroke and COVID-19: Experience From a Comprehensive Stroke Center in Midwest US. Front. Neurol. 11:910. doi: 10.3389/fneur.2020.00910
Received: 17 June 2020; Accepted: 15 July 2020;
Published: 20 August 2020.
Edited by:
Tomohisa Nezu, Hiroshima University, JapanReviewed by:
Anna M. Planas, Consejo Superior de Investigaciones Científicas (CSIC), SpainCopyright © 2020 Grewal, Pinna, Hall, Dafer, Tavarez, Pellack, Garg, Osteraas, Vargas, John, Da Silva and Conners. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parneet Grewal, cGFybmVldGdyZXdhbDI5OEB5YWhvby5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.