
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 20 August 2020
Sec. Neurocritical and Neurohospitalist Care
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00899
 Pascale Grzonka1*
Pascale Grzonka1* Marleen C. Scholz1
Marleen C. Scholz1 Gian Marco De Marchis2,3
Gian Marco De Marchis2,3 Kai Tisljar1
Kai Tisljar1 Stephan Rüegg2,3
Stephan Rüegg2,3 Stephan Marsch1,3
Stephan Marsch1,3 Joachim Fladt2
Joachim Fladt2 Raoul Sutter1,2,3
Raoul Sutter1,2,3Objectives: To present a patient with acute hemorrhagic leukoencephalitis (AHLE) and a systematic review of the literature analyzing diagnostic procedures, treatment, and outcomes of AHLE.
Methods: PubMed and Cochrane databases were screened. Papers published since 01/01/2000 describing adult patients are reported according to the PRISMA-guidelines.
Results: A 59-year old male with rapidly developing coma and cerebral biopsy changes compatible with AHLE is presented followed by 43 case reports from the literature including males in 67% and a mean age of 38 years. Mortality was 47%. Infectious pathogens were reported in 35%, preexisting autoimmune diseases were identified in 12%. Neuroimaging revealed uni- or bihemispheric lesions in 65% and isolated lesions of the cerebellum, pons, medulla oblongata or the spinal cord without concomitant hemispheric involvement in 16%. Analysis of the cerebrospinal fluid showed an increased protein level in 87%, elevated white blood cells in 65%, and erythrocytes in 39%. Histology (reported in 58%) supported the diagnosis of AHLE in all cases. Glucocorticoids were used most commonly (97%), followed by plasmapheresis (26%), and intravenous immunoglobulins (12%), without a clear temporal relationship between treatment and the patients' clinical course.
Conclusions: Although mortality was lower than previously reported, AHLE remains a life-threatening neurologic emergency with high mortality. Diagnosis is challenging as the level of evidence regarding the diagnostic yield of clinical, neuroimaging and laboratory characteristics remains low. Hence, clinicians are urged to heighten their awareness and to prompt cerebral biopsies in the context of rapidly progressive neurologic decline of unknown origin with the concurrence of the compiled characteristics. Future studies need to focus on treatment characteristics and their effects on course and outcome.
Acute hemorrhagic leukoencephalitis (AHLE) is an inflammatory disease of the brain, most often affecting the cerebrum, less commonly the cerebellum, the brain stem, or the spinal cord. Weston Hurst was the first to describe this syndrome in 1941, reporting two adults who developed severe and rapidly progressive encephalopathy due to hemorrhagic lesions of the white matter, histologically characterized by perivascular polymorphonuclear infiltrates, small vessel necrosis and demyelination.
AHLE is commonly considered to be a variant of acute disseminated encephalomyelitis (ADEM) (1, 2). While the latter is mainly seen in children, the former is more common in adults. Due to the rarity of the disease and the complex diagnostic workup, AHLE is likely to be underrecognized, and underreported.
The etiology of AHLE is unknown. The initial emergence of flu-like symptoms, however, supports the hypothesis of an autoimmune process on the basis of molecular mimicry promoted by mostly viral or bacterial pathogens. In keeping with this, immunosuppressive therapy, mainly with glucocorticoids, is the mainstay of treatment. Based on case reports and small case series, mortality is reported to be as high as 70% (3, 4).
In order to heighten awareness of AHLE and its clinical context, we present an adult patient with typical features of AHLE and a systematic review of the literature aiming to analyze the diagnostic procedures, treatment options, and outcomes of AHLE.
The digital databases PubMed and Cochrane were screened by two reviewers using predefined search terms in the advanced search mode. The term “acute hemorrhagic leukoencephalitis” was applied as a MESH term as well as in “title/abstract.” We included all papers meeting each of the following criteria: (1) the papers had to be published after 01/01/2000, (2) the papers had to be describing adult patients (age ≥ 18 years), (3) the publication had to be written in English, and (4) the design had to be either case reports, case series or cohort studies. The results are reported according to the PRISMA-guidelines (http://www.prisma-statement.org).
Details regarding search terms as well as in- and exclusion processes are outlined in Figure 1. Data regarding demographics, clinical and neuroradiologic characteristics were extracted.
A 59-year old caucasian male with a history of recurrent pulmonary embolisms, obstructive sleep apnea syndrome, and psoriasis, as well as an unspecific infection of the respiratory tract infection 2 weeks prior to presentation was referred to our tertiary academic medical care center with suspected wake-up stroke. Clinical examination revealed new onset aphasia, right sided central facial and brachial paralysis. No neck stiffness was noted and inflammatory parameters in the peripheral blood were only mildly elevated [white blood cell count (WBC) 10.9 × 109 per liter, C-reactive protein 13 milligram per liter]. Repeat cerebral CT scans were unremarkable, whereas analysis of the cerebrospinal fluid (CSF) revealed a high leukocyte count (1,074 per microliter), an elevated lactate concentration (4.6 mmol per liter), an increased protein level (2.3 gram per liter) and ferritin (85 microgram per liter). Empiric antibiotic and antiviral therapy with ceftriaxone, amoxicillin and aciclovir was initiated. An extensive microbiologic and rheumatologic workup was negative. Cerebral MRI 1 day after first symptoms mainly demonstrated left-hemispheric hyperintense lesions of the white matter (Figure 2; left part of the first row) and an enhancement of the left parieto-occipital regions (Figure 2; fourth row).
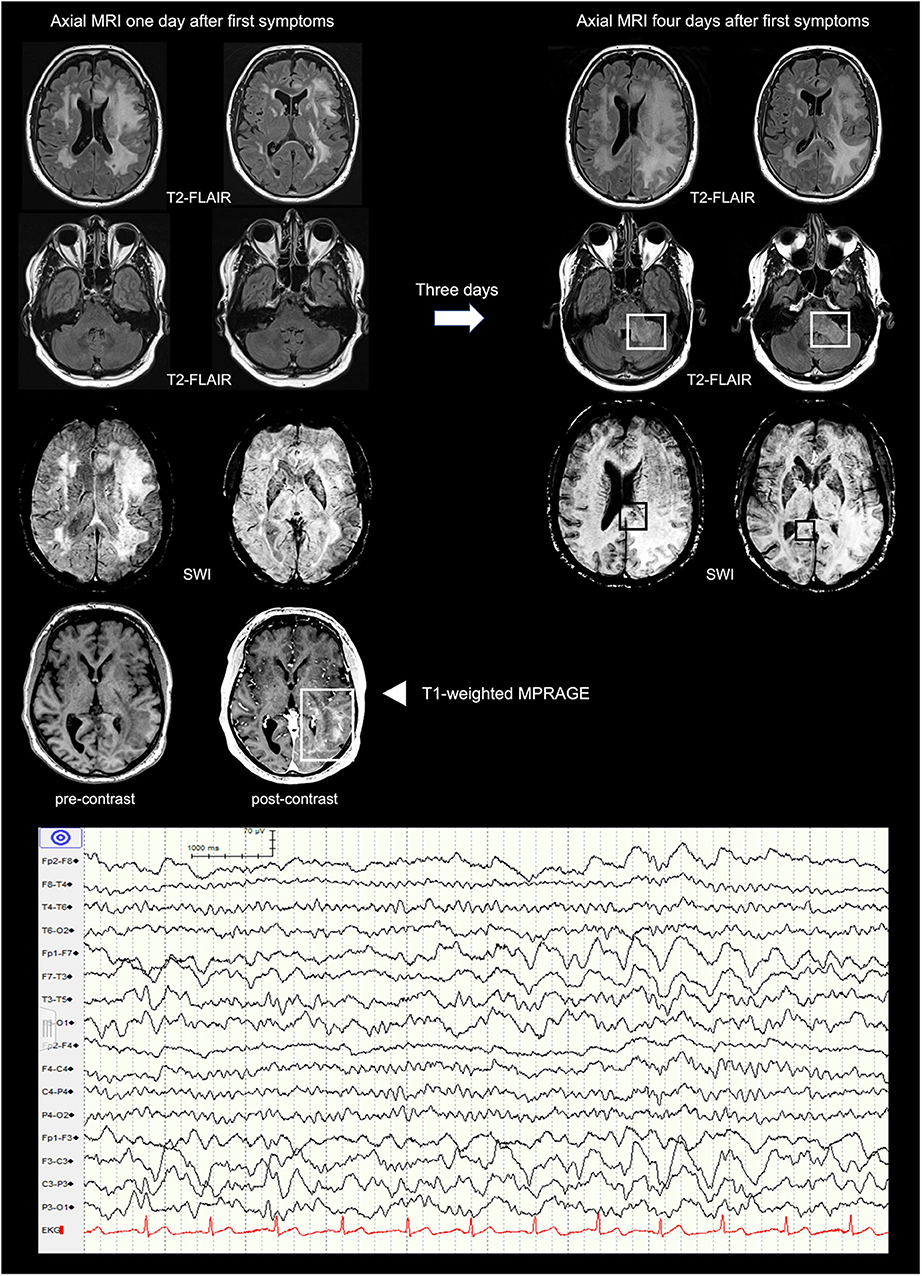
Figure 2. Cerebral MRI presenting the temporal evolution within 3 days (from left to right) and EEG excerpt. First row: axial T2-weighted FLAIR images showing increasing bilateral confluent widespread hyperintensities of the supratentorial white matter predominantly on the left. Second row: axial T2-weighted FLAIR images revealing new hyperintensities of the left cerebellar peduncle. Third row: axial SWI demonstrating subtle and small susceptibility artifacts in the splenium of the corpus callosum. Fourth row: axial pre- and post-contrast T1-weighted MPRAGE showing enhancement of the left parieto-occipital region. FLAIR, Fluid-Attenuated Inversion Recovery; SWI, Susceptibility Weighted Imaging; MPRAGE, Magnetization-Prepared Rapid Acquisition with Gradient Echo.
After a temporary slight improvement, the patient deteriorated rapidly with symptoms evolving to mutism, right sided hemiplegia and finally deep coma with a Glasgow coma scale (GCS) of 3 within 4 days. Repeat cerebral MRI 3 days later revealed a progression of the white matter lesions now involving the cerebellum, the ponto-medullar region, and both hemispheres with cerebral edema resulting in a mild midline shift (Figure 2; right part of the first row). In addition, susceptibility artifacts in the splenium of the corpus callosum and the pedunculus cerebelli were consistent with multiple but subtle micro bleeds. The electroencephalogram (EEG) showed intermittent epileptiform activity in both hemispheres with left fronto-temporal predominance (Figure 2; lower part).
A biopsy of the left frontal lobe showed infiltrates with neutrophilic and eosinophilic granulocytes as well as macrophages, acute focal hemorrhages, and vasculitis-like vessel lesions compatible with AHLE (Figure 3A). Despite intensive immunosuppressive therapy including intravenously administered immunoglobulins (IVIG; 0.4 g per kg body weight for 5 days), high dose glucocorticoids (methylprednisolone 2 gram per day for 3 days followed by tapering) and cyclophosphamide (15 milligram per kilogram body weight), the patient showed no improvement and remained deeply comatose with a loss of protective reflexes. Best supportive care was started 13 days after admission and the patient died shortly after.
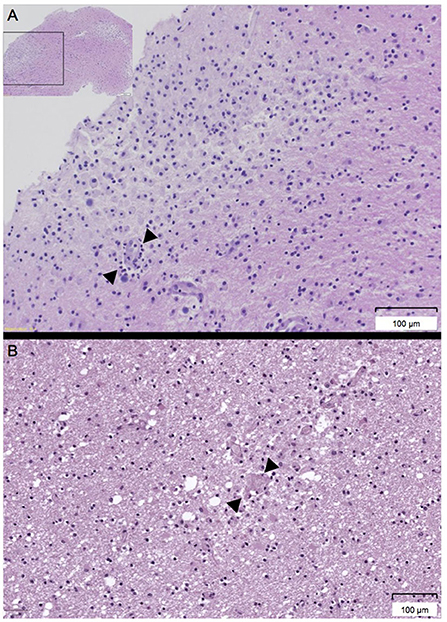
Figure 3. (A,B) Histologic workup of the biopsy of the left frontal lobe and the cerebral autopsy. (A) Histology of the biopsy of the left frontal lobe showing perivascular infiltrates (arrow) of neutrophils, eosinophils, and macrophages (Hemalaun Eosin [HE] stain). (B) Histology of the cerebral autopsy revealing diffuse generalized inflammation and acute hemorrhages (arrow) (Hemalaun Eosin [HE] stain).
Autopsy revealed disseminated, mainly perivascular demyelination with focal hemorrhages (Figure 3B) located in both hemispheres, the corpus callosum and the pons with cortical sparing, compatible with AHLE.
Of the 255 articles screened from the digital databases PubMed and Cochrane, 43 met our inclusion criteria, each contributing one patient. Males were more commonly affected than females (67 vs. 33%) and patients' mean age was 38 years. Detailed data extracted from these reports are outlined in Table 1.
As in our patient described above who initially suffered from a respiratory tract infection, infectious pathogens in the context of AHLE were reported in the literature in 35%, including Staphylococcus epidermidis (6), Epstein Barr virus (EBV) (11, 18, 19), Influenza H1N1 (20, 23, 28), Coxsackie B6 (33), Cytomegalovirus (CMV), Human Herpes virus 6 (HHV-6), Herpes simplex (HSV), (Varicella zoster (VZV) (42, 44), Mumps virus (16), Mycoplasma pneumoniae (8, 15, 17, 34), Plasmodium vivax (29), and Mycobacterium tuberculosis (45).
Symptoms of upper respiratory tract infections without identification of the underlying pathogen were described in 19%. In two patients, AHLE occurred after influenza vaccination (37, 41). As in our patient, who suffered from psoriasis, preexisting autoimmune disease is frequently reported in the literature, such as rheumatoid arthritis (30), inflammatory bowel disease (6, 39), primary sclerosing cholangitis (39), multiple sclerosis (1), and polyarteritis nodosa (19) was present in 12%.
Neuroimaging was performed in 91% of all cases. Uni- or bilateral hemispheric lesions were most frequently reported (in 65%), whereas isolated lesions of the cerebellum, the pons, the medulla oblongata, or the spinal cord without concomitant hemispheric involvement were rare (16%).
CSF analysis was reported in 72%. As seen in the CSF of our patient, the most frequent finding reported in the literature was an increased protein level (87%). WBC was elevated in 65% (of which 50% mainly mononuclear and 40% mainly polymorphonuclear), RBC in 39%.
A histologic diagnostic work-up was performed in 58% of patients (biopsy in 26%, autopsy in 35%, both biopsy and autopsy in 1 patient). In all cases providing histologic work-up, the findings supported the diagnosis of AHLE.
Treatment was described in 79%. Glucocorticoids were the most common immunosuppressive therapy applied (97%), followed by plasmapheresis (26%), and intravenous immunoglobulins (12%). In one patient, the use of cyclophosphamide and rituximab was reported. However, a clear temporal relationship between immunosuppressive therapy and the patients' clinical course could not be established in most case reports.
Overall mortality was 46.5%. Fourteen percentage of patients made a full recovery, whereas 39.5% survived with mild to severe neurological impairment.
Table 2 presents a comparison of clinical, neuroradiologic, and laboratory differences between AHLE and ADEM based on the data of our systematic review and recent case reports and reviews regarding ADEM.
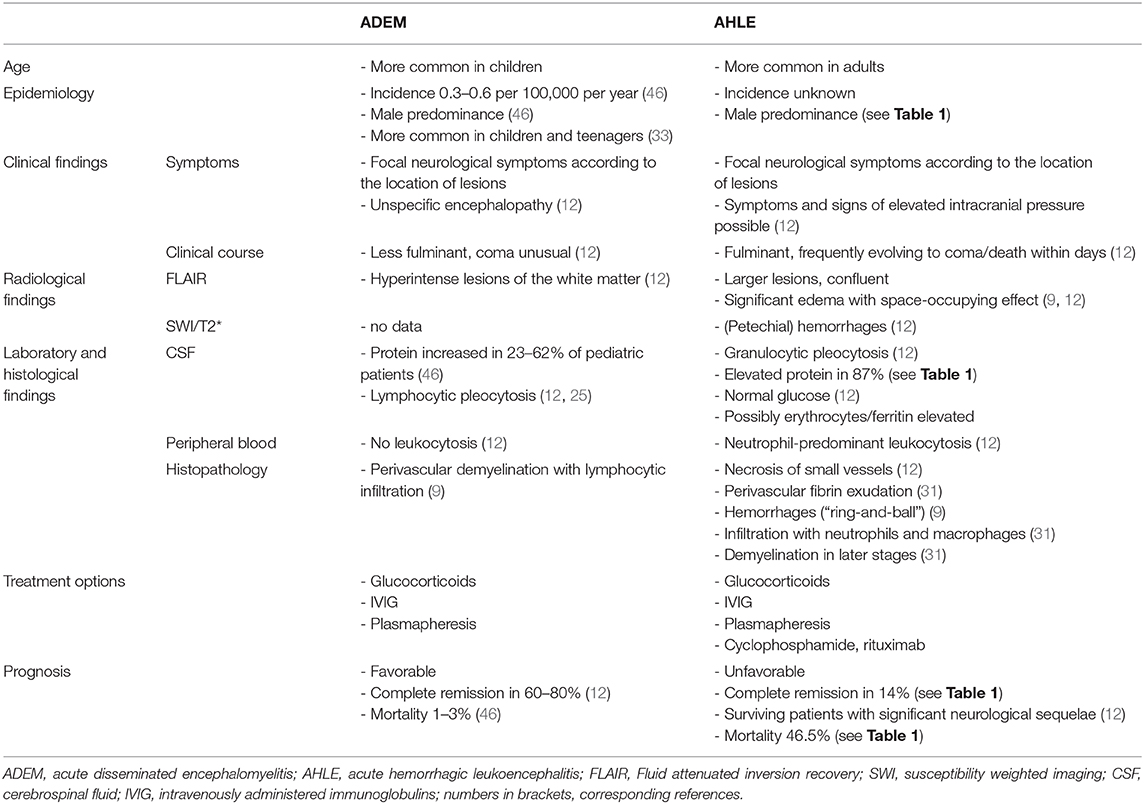
Table 2. Comparison of main clinical, neuroradiologic, and laboratory characteristics between AHLE and ADEM.
AHLE is a rare disease with a rapidly progressive course and prompt recognition is crucial. However, since no evidence-based diagnostic criteria exist, diagnosis is challenging. The limited number of cases described in the literature, likely reflecting the low incidence and/or underreporting, calls for heightened awareness in the context of patients developing acute cerebral inflammation of unknown origin. Since we could not identify formal studies and our insights are based on case reports only, the level of evidence regarding the clinical, neuroimaging, and laboratory characteristics remains very low. However, as mentioned above, our patient presented many of the typical clinical features of AHLE as described in the literature that may assist in the diagnostic workup as follows:
First, a preceding or concomitant infection is reported in more than 50%, with different viruses being the most commonly reported pathogens, for example EBV, mumps, VZV, HSV, HHV-6, and influenza. In 19% of all patients with AHLE, symptoms of a non-specific upper respiratory tract infection without identification of an underlying pathogen are described, as was the case in our patient.
Second, the fact that the illness emerged in a male adult person is typical. In contrast to ADEM, which is more common in children and teenagers (33), AHLE mainly affects adult patients. Moreover, our literature review shows a male preponderance of 67%.
Third, the clinical course with rapid neurological decline eventually leading to coma and death also suggests AHLE. In the literature, mortality is mentioned to be as high as 70% (3, 4). However, in our review of the literature, we found an overall mortality of 46.5%, which is substantially lower. Moreover, 14% of patients made a full recovery and returned to their premorbid neurological baseline (3, 5, 7, 14, 18, 35), and 11% survived with only minor neurological sequelae (2, 12, 15, 23, 38). The reason for the better outcome in this systematic review compared to previous studies (3, 4) remains unclear. The fact that aggressive immunosuppression was described in a large proportion of cases, however, suggests a treatment-related improvement. Unfortunately, if and to what extent aggressive immunosuppression influences outcome cannot be determined by our data and other factors that may play an important role regarding disease control remain to be uncovered.
The most frequently discussed pathomechanistic hypothesis is an autoimmune process promoted by cross reactivity (i.e., molecular mimicry) between human myelin and viral or bacterial antigens, but the exact mechanisms remain to be elucidated (33).
Diagnosis of AHLE mainly relies on neuroimaging, cerebrospinal fluid (CSF) analysis and histopathology. Due to the lack of formal studies, guidelines defining diagnostic algorithms are lacking, and cannot be drawn from current data. Furthermore, most clinical scenarios described in the literature encompass symptoms and signs that may prompt the clinician to suspect ADEM. While single clinical characteristics do not reliably differ between AHLE and ADEM, the concurrence of multiple symptoms, and signs may facilitate the distinction between these two entities. In this context, Table 2 presents a compilation of different symptoms and diagnostic findings that are most discriminative between AHLE and ADEM. However, as the syndromatic appearance and clinical course of AHLE and ADEM are often indistinguishable, treatment options are equal, and the body of evidence regarding the latter is limited for both entities, the need for a reliable differentiation seems questionable.
Brain MRI is crucial and typically reveals confluent white matter lesions with significant edema, space-occupying effects, and petechial hemorrhages (12, 38). The location of the lesions seems highly variable. Uni- or bilateral hemispheric involvement is seen in nearly two third of patients, but other distributive patterns have been described. The most useful aspect on cerebral MRI in differentiating AHLE from ADEM is, however, the presence of intraparenchymal hemorrhages.
While infectious encephalitides are more frequent and may represent an important differential diagnosis to AHLE, their diagnosis is usually less challenging, as multiplex PCR assays and whole genome sequencing approaches in the cerebrospinal fluid allow rapid detection of several infectious pathogens including HSV type 1, the most commonly identified cause of sporadic encephalitis worldwide that mostly affects the temporal lobe and limbic region (47). A study of adult immune competent patients with encephalitis who had temporal lobe abnormalities found that bilateral temporal lobe involvement was associated with lower odds of HSV encephalitis compared to all other etiologies (48). Moreover, patients with Herpes encephalitis were more likely to be older and white, and to present acutely and with fever, seizures, and upper respiratory symptoms. In addition to the bilateral temporal lobe involvement, lesions outside the temporal lobe or limbic region suggested alternative diagnoses and thus may also help to differentiate Herpes encephalitis from AHLE. Early recognition is crucial, as treatment of the former entity is effective with the intravenous administration of aciclovir, as well as screening for and treatment of limbic seizures and status epilepticus—both well-known complication of Herpes encephalitis (49).
Although histopathologic examination was only described in 58% of the cases included in our review, typical features include infiltration with granulocytes and macrophages, necrosis of small vessels and hemorrhages in a “ring and ball” pattern (9, 12, 50). Circumscribed perivascular demyelination seems to occur in cases with a prolonged course of disease, whereas in patients with a fulminant development of AHLE leading to death within 1–2 days from onset, demyelination is usually not (yet) present (31).
Treatment aims at attenuating the autoimmune process believed to cause AHLE, at avoiding secondary neurological damage due to intracranial hypertension and breakdown of the blood-brain-barrier, and at preventing infectious complications, such as pneumonia due to aspiration, that may further promote systemic inflammation.
Although our systematic review of the literature revealed a lower mortality than previously reported, acute hemorrhagic leukoencephalitis remains a life-threatening neurologic emergency with high mortality. Diagnosis is challenging as the level of evidence regarding the diagnostic yield of clinical, neuroimaging, and laboratory characteristics remains very low. Hence, clinicians are urged to heighten their awareness and to prompt cerebral biopsies in the context of rapidly progressive neurologic decline of unknown origin with the concurrence of the compiled characteristics. Future studies need to focus on treatment characteristics and their effects on course and outcome.
Written informed consent was obtained from the patient's family for the publication of any potentially identifiable images or data included in this article.
PG, MS, and RS planned the work, acquired the data, and wrote the manuscript. GD, KT, SR, SM, and JF interpreted the data, revised the manuscript and substantially contributed to the inaugural draft. All authors approved the final submitted version.
GD was supported by the Swiss National Science Foundation; Science Funds [Wissenschaftsfonds] of the University Hospital Basel and University of Basel; Bangerter-Rhyner-Stiftung; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; Fondazione Dr. Ettore Balli; De Quervain research grant; Thermo Fisher GmbH. He received travel and advisory board honoraria by Bayer and speaker honoraria by Medtronic and BMS/Pfizer. SR received unconditional research grants from UCB and CSL Behring. He received honoraria from serving on the scientific advisory boards of Desitin, Eisai, GlaxoSmithKline, Pfizer, and UCB, travel grants from Desitin, speaker fees from UCB and Novartis, compensations from serving as a consultant for Novartis, Sandoz and UCB. He was co-PI of the Swiss National Found Grant #320030_169379/1. He served as president on the board of the Swiss League against Epilepsy and is on the editorial board of EPILEPTOLOGIE and the SWISS EEG BULLETIN. He did not hold any stocks of any pharmaceutical companies or manufacturers of medical devices. JF was supported by the Swiss Heart Foundation. RS received research grants from the Swiss National Foundation (No 320030_169379), the Research Fund of the University Basel, the Scientific Society Basel, and the Bangerter-Rhyner Foundation. He received personal grants from UCB-pharma and holds stocks from Novartis, Roche, and Johnson & Johnson.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PG has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Drs. Stephan Frank and Nikolaus Deigendesch of the Department of Neuropathology for the histopathologic workup.
1. Yildiz O, Pul R, Raab P, Hartmann C, Skripuletz T, Stangel M. Acute hemorrhagic leukoencephalitis (Weston-Hurst syndrome) in a patient with relapse-remitting multiple sclerosis. J Neuroinflammation. (2015) 12:175. doi: 10.1186/s12974-015-0398-1
2. Bonduelle T, Stricker J, Mineo JF, Massri A, Guesdon C, Barroso B, et al. Weston-Hurst syndrome with acute hemorrhagic cerebellitis. Clin Neurol Neurosurg. (2018) 173:118–9. doi: 10.1016/j.clineuro.2018.08.007
3. Duggal N. Acute hemorrhagic leukoencephalitis associated with autoimmune myopathy. J Vasc Interv Neurol. (2014) 7:19–22.
4. Sureshbabu S, Babu R, Garg A, Peter S, Sobhana C, Mittal GK. Acute hemorrhagic leukoencephalitis unresponsive to aggressive immunosuppression. Clin Exp Neuroimmunol. (2017) 2017:63–6. doi: 10.1111/cen3.12361
5. Klein CJ, Wijdicks EF, Earnest FT. Full recovery after acute hemorrhagic leukoencephalitis (Hurst's disease). J Neurol. (2000) 247:977–9. doi: 10.1007/s004150070060
6. Tanser SJ, Walker MB, Hilton DA. Acute haemorrhagic leucoencephalitis complicating sepsis. Anaesth Intensive Care. (2001) 29:54–7. doi: 10.1177/0310057X0102900111
7. Meilof JF, Hijdra A, Vermeulen M. Successful recovery after high-dose intravenous methylprednisolone in acute hemorrhagic leukoencephalitis. J Neurol. (2001) 248:898–9. doi: 10.1007/s004150170076
8. Pfausler B, Engelhardt K, Kampfl A, Spiss H, Taferner E, Schmutzhard E. Post-infectious central and peripheral nervous system diseases complicating Mycoplasma pneumoniae infection. Report of three cases and review of the literature. Eur J Neurol. (2002) 9:93–6. doi: 10.1046/j.1468-1331.2002.00350.x
9. Kuperan S, Ostrow P, Landi MK, Bakshi R. Acute hemorrhagic leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology. (2003) 60:721–2. doi: 10.1212/01.WNL.0000048493.82053.4C
10. Martins HM, Teixeira AL Jr, Lana-Peixoto MA, Lana-Peixoto MA. Acute hemorrhagic leukoencephalitis mimicking herpes simplex encephalitis: case report. Arq Neuropsiquiatr. (2004) 62:139–43. doi: 10.1590/S0004-282X2004000100024
11. Francisci D, Sensini A, Fratini D, Moretti MV, Luchetta ML, Di Caro A, et al. Acute fatal necrotizing hemorrhagic encephalitis caused by Epstein-Barr virus in a young adult immunocompetent man. J Neurovirol. (2004) 10:414–7. doi: 10.1080/13550280490521050
12. Gibbs WN, Kreidie MA, Kim RC, Hasso AN. Acute hemorrhagic leukoencephalitis: neuroimaging features and neuropathologic diagnosis. J Comput Assist Tomogr. (2005) 29:689–93. doi: 10.1097/01.rct.0000173843.82364.db
13. Lee HY, Chang KH, Kim JH, Na DG, Kwon BJ, Lee KW, et al. Serial MR imaging findings of acute hemorrhagic leukoencephalitis: a case report. AJNR Am J Neuroradiol. (2005) 26:1996–9.
14. Alemdar M, Selekler HM, Iseri P, Demirci A, Komsuoglu SS. The importance of EEG and variability of MRI findings in acute hemorrhagic leukoencephalitis. Eur J Neurol. (2006) 13:e1–3. doi: 10.1111/j.1468-1331.2006.01465.x
15. Ryan LJ, Bowman R, Zantek ND, Sherr G, Maxwell R, Clark HB, et al. Use of therapeutic plasma exchange in the management of acute hemorrhagic leukoencephalitis: a case report and review of the literature. Transfusion. (2007) 47:981–6. doi: 10.1111/j.1537-2995.2007.01227.x
16. Kumar RS, Kuruvilla A. Teaching neuroimages: acute hemorrhagic leukoencephalitis after mumps. Neurology. (2009) 73:e98. doi: 10.1212/WNL.0b013e3181c2eee3
17. Catalan M, Naccarato M, Grandi FC, Capozzoli F, Koscica N, Pizzolato G. Acute hemorrhagic leukoencephalitis with atypical features. Neurol Sci. (2009) 30:55–7. doi: 10.1007/s10072-008-0003-9
18. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, et al. Hemorrhagic encephalitis associated with Epstein-Barr virus infection. J Clin Neurosci. (2010) 17:153–4. doi: 10.1016/j.jocn.2009.03.043
19. Befort P, Gaillard N, Roubille C, Quellec AL. Hemorrhagic leukoencephalitis linked to Epstein-Barr virus in an adult patient. Clin Neurol Neurosurg. (2010) 112:829–31. doi: 10.1016/j.clineuro.2010.06.017
20. Fugate JE, Lam EM, Rabinstein AA, Wijdicks EF. Acute hemorrhagic leukoencephalitis and hypoxic brain injury associated with H1N1 influenza. Arch Neurol. (2010) 67:756–8. doi: 10.1001/archneurol.2010.122
21. Abou Zeid NE, Burns JD, Wijdicks EF, Giannini C, Keegan BM. Atypical acute hemorrhagic leukoencephalitis (Hurst's disease) presenting with focal hemorrhagic brainstem lesion. Neurocrit Care. (2010) 12:95–7. doi: 10.1007/s12028-009-9293-x
22. Virmani T, Agarwal A, Klawiter EC. Clinical reasoning: a young adult presents with focal weakness and hemorrhagic brain lesions. Neurology. (2011) 76:e106–9. doi: 10.1212/WNL.0b013e31821d748a
23. Cisse FA, Antoine JC, Pillet S, Jousserand G, Reynaud-Salard M, Camdessanche JP. Acute hemorrhagic leukoencephalopathy associated with influenza A (H1N1) virus. J Neurol. (2011) 258:513–4. doi: 10.1007/s00415-010-5772-4
24. Pinto PS, Taipa R, Moreira B, Correia C, Melo-Pires M. Acute hemorrhagic leukoencephalitis with severe brainstem and spinal cord involvement: MRI features with neuropathological confirmation. J Magn Reson Imaging. (2011) 33:957–61. doi: 10.1002/jmri.22505
25. Lee NK, Lee BH, Hwang YJ, Kim SY, Lee JY, Joo M. Serial computed tomography and magnetic resonance imaging findings of biphasic acute hemorrhagic leukoencephalitis localized to the brain stem and cerebellum. JPN J Radiol. (2011) 29:212–6. doi: 10.1007/s11604-010-0523-0
26. Hashim HZ, Ibrahim NM, Wanyahya N, Tan HJ, Zainun KA, Mohd Ali SA, et al. A case of biopsy proven acute demyelinating encephalomyelitis (ADEM) with haemorrhagic leucoencephalitis. Ann Acad Med Singapore. (2011) 40:197–200.
27. Kao HW, Alexandru D, Kim R, Yanni D, Hasso AN. Value of susceptibility-weighted imaging in acute hemorrhagic leukoencephalitis. J Clin Neurosci. (2012) 19:1740–1. doi: 10.1016/j.jocn.2011.04.034
28. Jeganathan N, Fox M, Schneider J, Gurka D, Bleck T. Acute hemorrhagic leukoencephalopathy associated with influenza A (H1N1) virus. Neurocrit Care. (2013) 19:218–21. doi: 10.1007/s12028-013-9880-8
29. Venugopal V, Haider M. First case report of acute hemorrhagic leukoencephalitis following Plasmodium vivax infection. Indian J Med Microbiol. (2013) 31:79–81. doi: 10.4103/0255-0857.108736
30. dos Santos MP, Martin J, Woulfe J, Lim SP, Chakraborty S. Autopsy-proven acute hemorrhagic leukoencephalitis in an elderly patient. Can J Neurol Sci. (2014) 41:99–102. doi: 10.1017/S031716710001636X
31. Robinson CA, Adiele RC, Tham M, Lucchinetti CF, Popescu BF. Early and widespread injury of astrocytes in the absence of demyelination in acute haemorrhagic leukoencephalitis. Acta Neuropathol Commun. (2014) 2:52. doi: 10.1186/2051-5960-2-52
32. Kitulwatte ID, Kim PJ, Pollanen MS. Acute hemorrhagic leukoencephalomyelitis in a man with viral myocarditis. Forensic Sci Med Pathol. (2015) 11:416–20. doi: 10.1007/s12024-015-9692-6
33. Atherton DS, Perez SR, Gundacker ND, Franco R, Han X. Acute disseminated encephalomyelitis presenting as a brainstem encephalitis. Clin Neurol Neurosurg. (2016) 143:76–9. doi: 10.1016/j.clineuro.2016.02.014
34. Magun R, Verschoor CP, Bowdish DM, Provias J. Mycoplasma pneumoniae, a trigger for Weston Hurst syndrome. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e187. doi: 10.1212/NXI.0000000000000187
35. Prabhakar AT, Kamanahalli R, Sivadasan A, Joseph E, Viggeswarpu S. Non-fatal acute haemorrhagic leukoencephalitis following snake bite: a case report. Trop Doct. (2016) 46:57–9. doi: 10.1177/0049475515577987
36. Nabi S, Badshah M, Ahmed S, Nomani AZ. Weston-Hurst syndrome: a rare fulminant form of acute disseminated encephalomyelitis (ADEM). BMJ Case Rep. (2016) 2016:bcr2016217215. doi: 10.1136/bcr-2016-217215
37. Wu CY, Riangwiwat T, Nakamoto BK. Hemorrhagic longitudinally extensive transverse myelitis. Case Rep Neurol Med. (2016) 2016:1596864. doi: 10.1155/2016/1596864
38. Solis WG, Waller SE, Harris AK, Sugo E, Hansen MA, Lechner-Scott J. Favourable outcome in a 33-year-old female with acute haemorrhagic leukoencephalitis. Case Rep Neurol. (2017) 9:106–13. doi: 10.1159/000472706
39. George IC, Youn TS, Marcolini EG, Greer DM. Clinical reasoning: acute onset facial droop in a 36-year-old pregnant woman. Neurology. (2017) 88:e240–4. doi: 10.1212/WNL.0000000000004030
40. Peerani R, Berggren M, Herath JC. Sudden death of a young man by acute hemorrhagic leukoencephalitis. Acad Forensic Pathol. (2017) 7:487–93. doi: 10.23907/2017.041
41. Sinzobahamvya E, Borrelli S, Rutgers MP, Clause D, Gille M. Acute hemorrhagic leukoencephalitis after seasonal influenza vaccination. Acta Neurol Belg. (2018) 118:127–9. doi: 10.1007/s13760-017-0861-0
42. Mondia MWL, Reyes NGD, Espiritu AI, Pascual VJ. Acute hemorrhagic leukoencephalitis of Weston Hurst secondary to herpes encephalitis presenting as status epilepticus: a case report and review of literature. J Clin Neurosci. (2019) 67:265–70. doi: 10.1016/j.jocn.2019.06.020
43. Ashraf Z, Todnam N, Morgan J, Rojiani AM. 42-year-old man with worsening headache. Brain Pathol. (2019) 29:305–6. doi: 10.1111/bpa.12706
44. An SF, Groves M, Martinian L, Kuo LT, Scaravilli F. Detection of infectious agents in brain of patients with acute hemorrhagic leukoencephalitis. J Neurovirol. (2002) 8:439–46. doi: 10.1080/13550280260422749
45. Chetty KG, Kim RC, Mahutte CK. Acute hemorrhagic leukoencephalitis during treatment for disseminated tuberculosis in a patient with AIDS. Int J Tuberc Lung Dis. (1997) 1:579–81.
46. Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. (2016) 87(9 Suppl. 2):S38–45. doi: 10.1212/WNL.0000000000002825
47. Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. (2010) 10:835–44. doi: 10.1016/S1473-3099(10)70222-X
48. Chow FC, Glaser CA, Sheriff H, Xia D, Messenger S, Whitley R, et al. Use of clinical and neuroimaging characteristics to distinguish temporal lobe herpes simplex encephalitis from its mimics. Clin Infect Dis. (2015) 60:1377–83. doi: 10.1093/cid/civ051
49. Lai CW, Gragasin ME. Electroencephalography in herpes simplex encephalitis. J Clin Neurophysiol. (1988) 5:87–103. doi: 10.1097/00004691-198801000-00003
Keywords: leukoencephalitis, immunosuppressive therapy, outcome, mortality, parainfectious disease
Citation: Grzonka P, Scholz MC, De Marchis GM, Tisljar K, Rüegg S, Marsch S, Fladt J and Sutter R (2020) Acute Hemorrhagic Leukoencephalitis: A Case and Systematic Review of the Literature. Front. Neurol. 11:899. doi: 10.3389/fneur.2020.00899
Received: 13 April 2020; Accepted: 13 July 2020;
Published: 20 August 2020.
Edited by:
Barak Bar, Loyola University Medical Center, United StatesReviewed by:
Enrico Tedeschi, University of Naples Federico II, ItalyCopyright © 2020 Grzonka, Scholz, De Marchis, Tisljar, Rüegg, Marsch, Fladt and Sutter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pascale Grzonka, cGFzY2FsZXN1c2FubmUuZ3J6b25rYUB1c2IuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.