- 1Stroke and Aging Research Group, Clinical Trials, Imaging and Informatics Division, School of Clinical Sciences at Monash Health, Monash University, Melbourne, VIC, Australia
- 2Department of Neurology, Monash Health, Melbourne, VIC, Australia
- 3Departments of Clinical Neuroscience and Radiology, Cummings School of Medicine, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- 4Data 61, CSIRO, Innovation Hub, Docklands, VIC, Australia
- 5Department of Medicine, Peninsula Clinical School, Central Clinical School, Frankston Hospital, Monash University, Melbourne, VIC, Australia
Endovascular clot retrieval, often referred to as mechanical thrombectomy, has transformed the treatment of patients with ischemic stroke based on an underlying large cerebral vessel occlusion, ranging from the extracranial internal carotid artery (ICA) to the M1 (proximal) segment of the middle cerebral artery (MCA). The aim of this study was to evaluate the effect of a progressive occlusion of the extracranial portion of the ICA on the cerebral blood flow either with a conventional guiding catheter or a balloon-guiding catheter, which enables the operator to completely occlude the parent artery by inflating the balloon around the tip of this type of guiding catheter. We evaluated the impact of flow reduction in the ICA in the setting of ipsilateral MCA occlusion given the different configurations of the circle of Willis (CoW). The computer model of cerebral arteries was based on anatomical works by Rhoton (1) and van der Eecken (2). The interactive experimental results are available on the web at https://gntem3.shinyapps.io/ecrsim. In the setting of left MCA occlusion, compensation from the anterior and posterior communicating artery preserved the flow in the left anterior cerebral artery (ACA) but not the left MCA branches. Under selected CoW configurations, such as classic, missing Acom, or missing A1 segment of the ACA and concurrent right ICA occlusion, there was a progressive decrease of flow in the left ACA to a minimum of 78% when the simulated catheter fully occluded the left ICA. Flow collapsed (<10%) in the left ACA and MCA branches under CoW configurations, such as bilateral fetal PCA. In summary, compensatory flow collapsed under certain clot retrieval scenarios and unusual configurations of CoW.
Introduction
Endovascular clot retrieval (ECR) or mechanical thrombectomy transformed treatment of patients with large vessel occlusion (LVO) (3–8). Patients with LVO involving the internal carotid artery (ICA) and middle cerebral artery (MCA) or combined lesions have been successfully treated in these trials. There are still several unanswered questions with clot retrieval: safety of advancing catheter along ICA in setting of ipsilateral MCA occlusion, given the concomitant occlusion of the contralateral ICA. In this setting, there is a hypothetical possibility that the cross-flow across anterior communicating artery (Acom) and the partial protection provided by leptomeningeal anastomoses (LAs) would be insufficient. This question is hard to answer in vivo and can be investigated using a computer simulation model. This study uses a computer model, based on the anatomical works of Rhoton (1) and van der Eecken (2), which was created in our earlier work (9).
The circle of Willis (CoW) is made up of the ICA, the Acom and posterior communicating arteries (Pcom) and the proximal part of the anterior (ACA) and the proximal part of the posterior cerebral arteries (PCA). The MCA exists distal or downstream from the CoW. The CoW network (primary collateral system) protects the brain from the unilateral extracranial ICA occlusion as long as cross-flow within the CoW is not impeded (10–12). However, the CoW may not offer sufficient protection when the site of occlusion is in the MCA or its cortical arterial branches. Flow in the Pcom is usually in the anterior to posterior direction except when there is ICA occlusion (9, 13, 14). On magnetic resonance (MR) angiography, a complete CoW occurs in 36% of normal subjects and 55% of subjects with ICA occlusion (13). Similarly, a complete posterior CoW configuration was observed in 63% with ICA occlusion vs. 47% in normal subjects.
In many studies, the term collateral anastomoses/circulation is used without further specification. In this study, we used the term leptomeningeal anastomosis to describe a secondary collateral system and that exists between selected branches of the MCA, PCA, and ACA (2). The collaterals described on computed tomography angiography after LVO are likely to be due to the work of the LA in conjunction with CoW (15, 16). The contribution of this secondary collateral system has been observed in maps of infarct territory as relative sparing of the posterior and superior parts of the MCA territory (17, 18) and in our earlier work on the computer model of the cerebral circulation (9).
Our earlier work on the computer model of the cerebral circulation was based on a classic configuration with complete CoW (9). In this study, we have extended the model and incorporate other CoW configurations, as well as simulated catheter movement along the ICA. The aim of this study was to evaluate the effect of a progressive occlusion of the extracranial portion of the ICA on the cerebral blood flow either with a conventional guiding catheter or a balloon-guiding catheter, which enables the operator to completely occlude the parent artery by inflating the balloon around the tip of this type of guiding catheter. We evaluated the impact of flow reduction in the ICA in the setting of ipsilateral MCA occlusion given the different configurations of the CoW.
Materials and Methods
Computational Modeling
A network of cylindrical pipes was used to model blood flow within the brain. In the original publication, we created a two-dimensional model of the major cerebral arteries using MATLAB version 5 (The Mathworks Inc., Natick, MA, USA) (9). In this study, we have transformed the MATLAB codes into R codes (R Statistical Foundation) and added the different configurations of the CoW into the model. The model of the cerebral circulation was based on the anatomical works by Rhoton (1) and van der Eecken (2). The named branches of the MCA, ACA, and PCA were empirically drawn down to the fifth branching order. Connections between the fifth-order branches were used to represent the LA. Consistent with previous anatomical descriptions, interhemispheric connections by LA from ACA branches were permitted (1). This model was then converted to a list of nodes and pipes in graph theory package igraph in R (19).
We assumed that flow was laminar within the pipe network representing cerebral arteries and formulated a linked set of equations for the system by imposing mass balance and pressure (energy) balance over the network. Inflow and outflow boundary conditions were imposed on the model. We modeled flow in each of the ICA and the basilar artery (BA) [via the vertebral arteries] as coming off the aortic arch and heart (connection below the CoW). Based on previous works (20, 21), the inflow conditions were set so that 75% of the total flow passed through the two ICAs and 25% through the BA, and a pressure condition of 5 kPa was imposed over the outer boundary. The outflow was modeled as a consequence of the arterial network branching to smaller and smaller capillaries. We included a drainage component at end points of the arterial branches to accommodate this outflow. The set of equations governing volume, flow rate, and pressure over the whole network was solved iteratively. The process was repeated for several different experiments in which successive arteries or combinations of arteries were occluded in the anterior circulation.
Primary Outcome
The primary outcome was maintenance of flow above a theoretical threshold (defined as 30% of baseline flow) in the setting of LVO (22). Baseline flow was defined according to flow on the contralateral side. This definition of ischemic core (30% of relative blood flow to the normal contralateral side) has also been used in clinical trials (23).
Experiments
There are two key experiments:
(1) occlusion of the right ICA and left MCA trunk or branches and
(2) occlusion of left MCA trunk or branches.
Catheter
For each experiment, we simulated the movement of a catheter along the artery or balloon occlusion by imposing stenosis in the ICA. The sensitivity analysis was performed with stenosis ranging from 0, 25, 50, 75, 90, 99, and 100%.
Leptomeningeal Anastomoses
Each experiment was performed with at different interterritory LA. We explored the effect of augmentation of LA by setting the diameter of LA between 0.5 and 1.5 mm. The default LA size was 1 mm in these experiments (9).
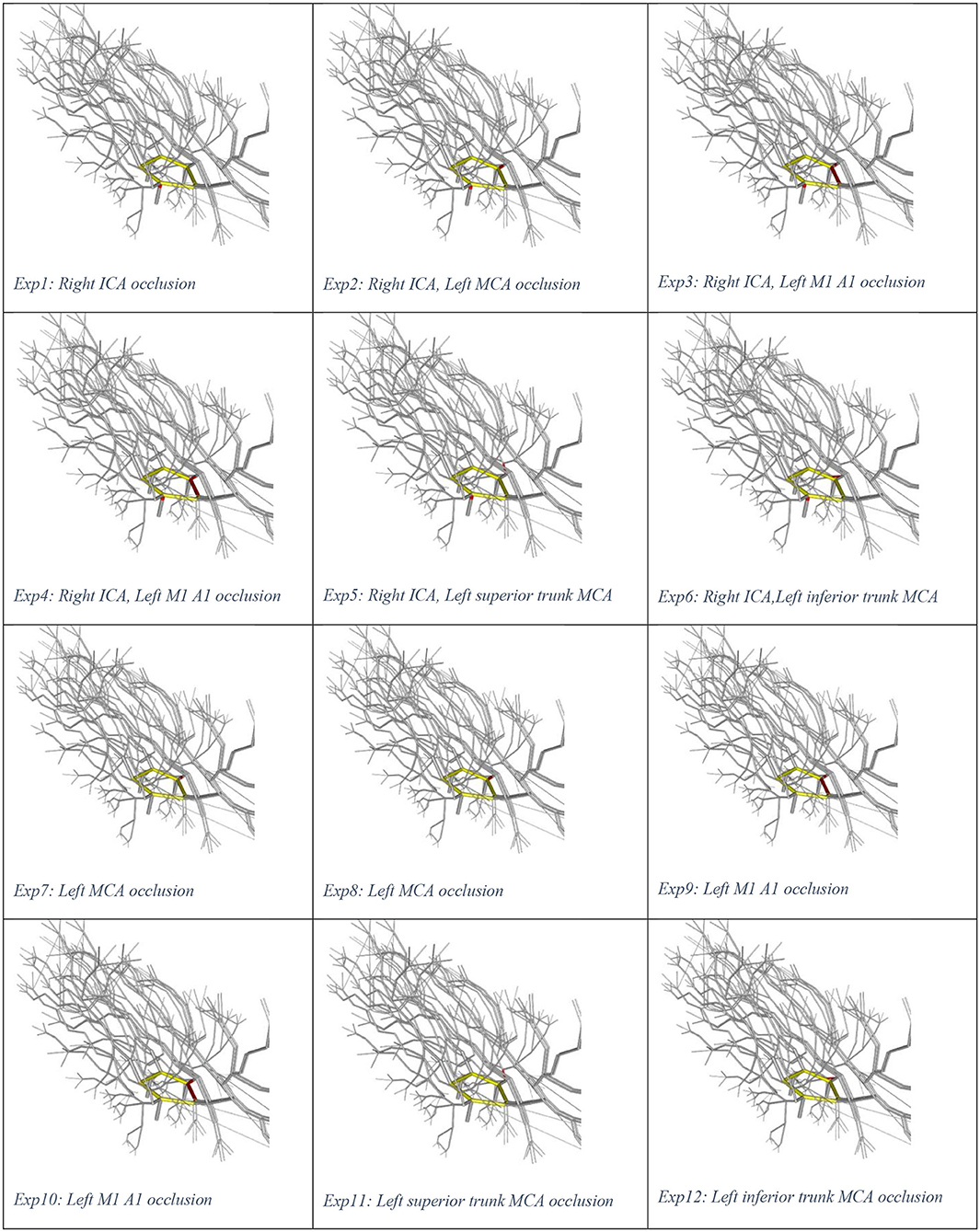
Illustrations of the experiments are presented in Figure 1. Experiment 0 is not displayed as there is no occlusion. We used the plotting library plotly in R to create a video play loop in which each frame of the video displays a single experiment. These experiments were combined so that the viewer can move through them with a “feel” of a movie. We have also collated the change in ICA stenosis into a movie, with each frame representing the degree of stenosis. These results are available at https://gntem3.shinyapps.io/ecrsim. The web-based app contains four tabs. The first tab, “Moving site of occlusion,” is for exploring CoW configurations, experiments, and LA diameter. The second tab, “Catheter causing progressive stenosis,” allows manipulation of the CoW configuration, degree of stenosis, and LA diameter. The third tab, “Artery model,” displays the three dimensional model of cerebral artery. The fourth tab, “Data table,” allows the viewers to peruse the results in a table format.
Results
Complete CoW
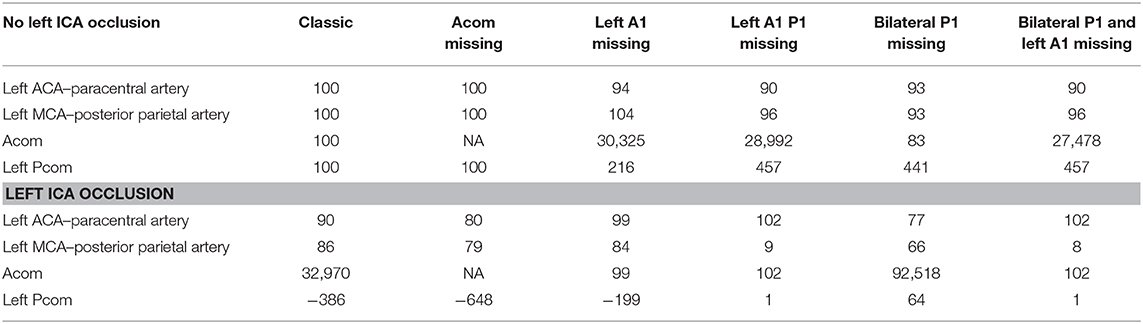
In the setting of left MCA occlusion, there was recruitment of the CoW vessels (Acom and left Pcom). This scenario resulted in compensatory cross flow as well as flow from the posterior circulation into the proximal left ACA and its branches, but not the MCA branches (see Table 1 and full results at https://gntem3.shinyapps.io/ecrsim). Augmentation of LA diameter improved flow in selected arteries, such as left posterior parietal, from 0% at LA = 0.5 mm to 13% at LA = 1 mm to 23% at LA = 1.5 mm. Even with increased LA diameters, there were no changes in flow in some arteries, such as the precentral (1%) and central (3%) arteries. These findings were also observed in the rare setting of concurrent left MCA occlusion and right ICA occlusion. Progressive stenosis of the ipsilateral ICA between 0 and 50% did not affect flow in the left ACA. At 75% ICA stenosis and above, flow in the left ACA branches dropped to 88%. At 100% stenosis, flow in the left ACA branches dropped to 86% (Figure 2).
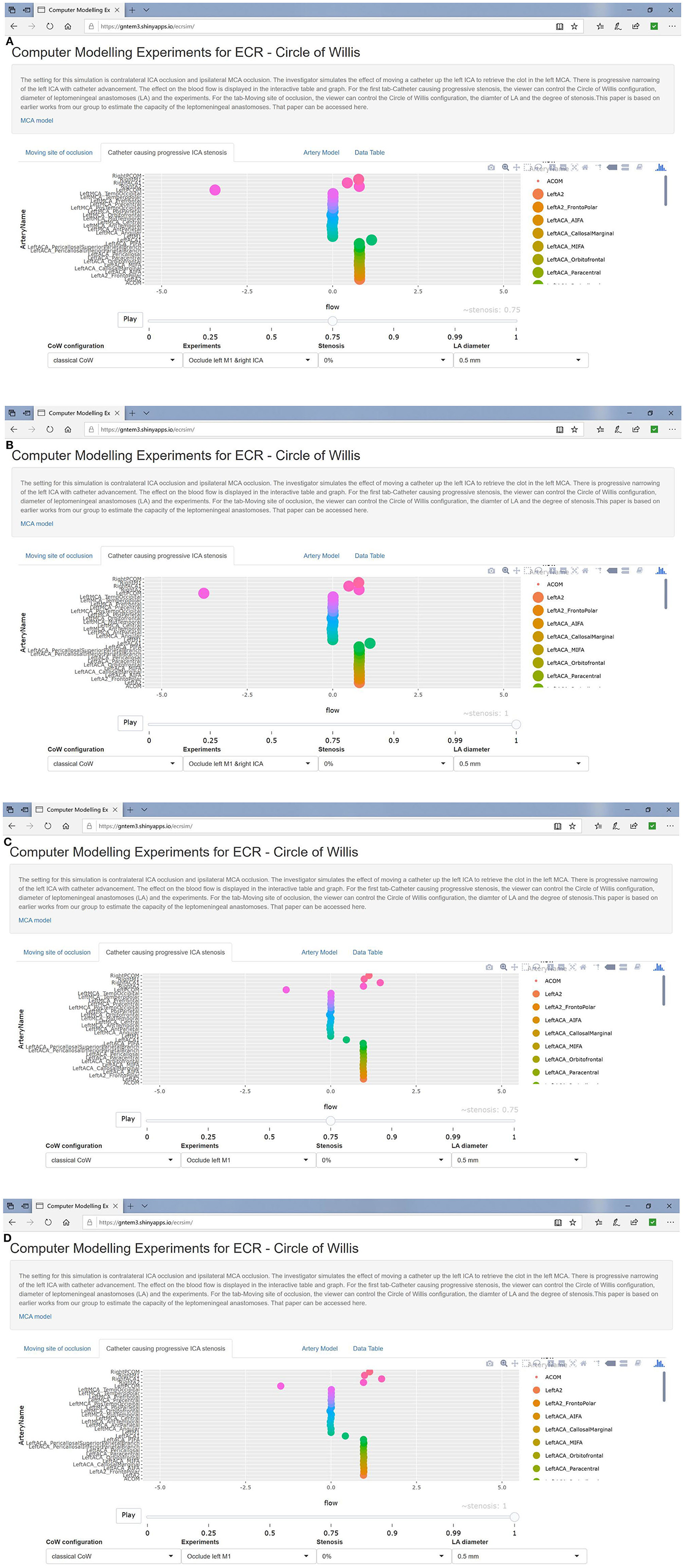
Figure 2. (A,B) Simulated catheter movement reduced flow to 86% of baseline in left ACA branches in setting of contralateral ICA occlusion. In (C,D), catheter movement has no effect even at 100% stenosis (absence of contralateral ICA occlusion).
Absent Acom
Flow was reduced in the left ACA (77%) and MCA (75%) in the presence of 100% left ICA occlusion. The additional left MCA (to left ICA) occlusion resulted in flow dropping below 12% in the left MCA branches and to 87% of baseline flow in the left ACA branches. We created T junction occlusion of the ICA by occluding the left MCA, ICA, and A1. In this experiment, flow in the left MCA branches dropped below ischemic threshold (12%), but flow in the left ACA branches was preserved. The same result occurred in the setting of complete CoW, as the left ACA has support from the right ICA via the Acom (Figure 3). In the setting of left MCA occlusion and contralateral right ICA occlusion, flow was reduced below ischemic threshold in the left MCA branches (<12%) and minimal changes in the left ACA branches (93%). In this scenario, stenosis of the ipsilateral (left) ICA between 0 and 100% did not further reduce flow in the left MCA branches, and there was only a small reduction in flow in the left ACA branches.
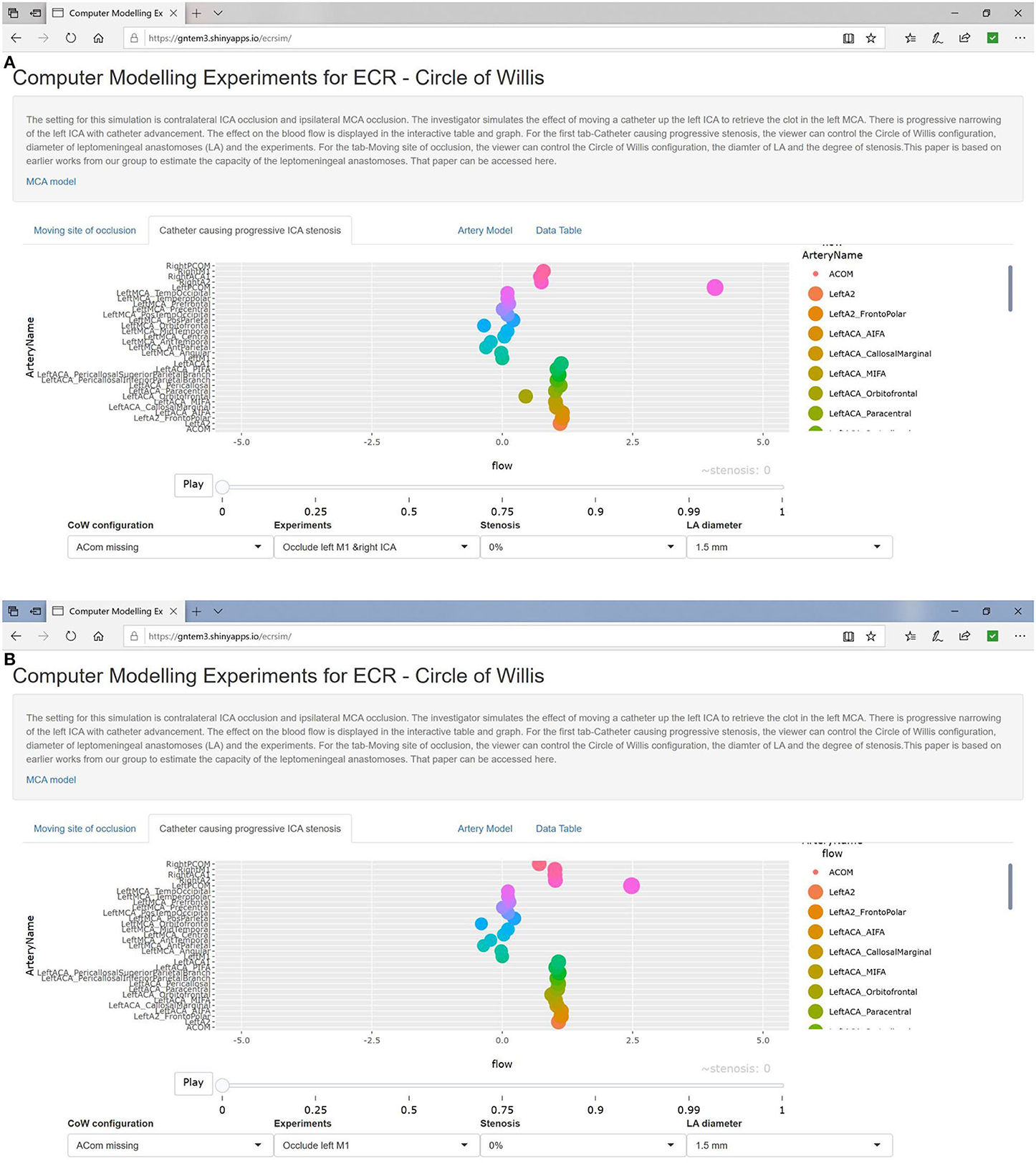
Figure 3. (A) Left MCA occlusion and contralateral ICA occlusion. (B) Left MCA occlusion only. Catheter movement has no effect even at 100% stenosis (absence of contralateral ICA occlusion).
Absent Left A1 Segment
In the setting of left MCA occlusion, there were reduced flow in the left MCA branches below ischemic threshold (<12%) and minimal changes in the left ACA branches (98%). In the setting of left MCA and right ICA occlusion, there was reduced flow in the left MCA branches below ischemic threshold (<12%) and a smaller reduction in flow in the left ACA branches (78%). Augmenting interterritory LA from 0.5 to 1.5 mm did not result in any change in flow.
Absent Left A1 and Left Fetal PCA
The findings were similar to the experiment with absent left A1 configuration of CoW. Augmentation of the LA from 0.5- to 1.5-mm diameter made no change to flow in the left ACA branches.
Bilateral Fetal PCA
In the setting of left MCA occlusion and contralateral (right) ICA occlusion, there was preserved flow in the ACA branches and recruitment of the CoW vessels (doubling of flow in the left Pcom) at low stenosis of the left ICA. Flow in Pcom completely collapsed as the stenosis in the ICA increased (≥50%) (Figure 4). Augmentation of the LA to 1.5-mm diameter made no change to flow in the left ACA branches.
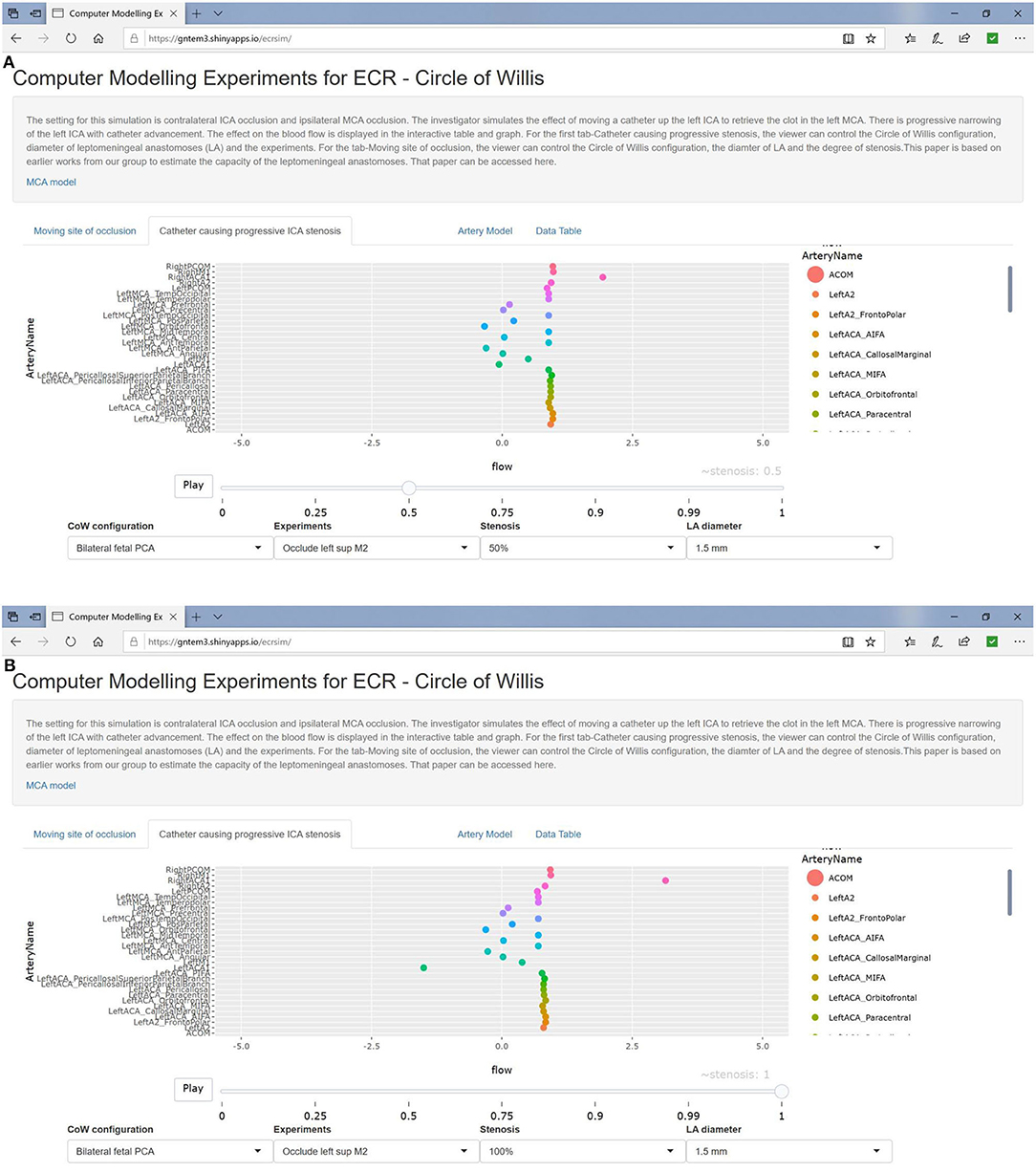
Figure 4. Simulated catheter movement on the left ICA show mild reduction in flow in the MCA branches at 50% stenosis (A) and at 100% stenosis (B).
Bilateral Fetal PCA and Absent Left A1
In the setting of left MCA and contralateral right ICA occlusion, there was no flow in the ACA branches even at no stenosis of the left ICA. Augmentation of the LA to 1.5-mm diameter made no change to flow in the left ACA branches.
Balloon Occlusion of Left ICA
Progressive stenosis (reduction of diameter) of left ICA did not result in any change in CoW flow until 50% occlusion (left MCA branches <91% and left ACA branches <95%). At 100% left ICA stenosis, flow was reduced in the left MCA branches <84% and left ACA branches <90%. In the setting of absent left A1, there was reduced flow in the left ACA branches (94%), mildly increased flow in the left MCA branches (104%), and markedly increased flow in the left Pcom (215%). In the setting of absent left A1 and P1, there was reduced flow in left MCA branches (9%). The changes were marked in the presence of CoW configuration with bilateral fetal PCA and absent left A1, where there was no flow in the left ACA branches.
In the presence of right ICA occlusion, progressive stenosis of the left ICA led to reduced flow in the left ACA (90%) and MCA branches (86%). With absent Acom, flows in the left ACA and MCA were reduced to 80 and 79%, respectively. With absent left A1, flows in the left ACA branches were reduced to 99%, and MCA branches to 84%. When both the left A1 and P1 were absent, flow in the left ACA was maintained at 102% and reduced in the left MCA to 9%. With bilateral fetal PCA and absent left A1, flow was maintained in the left ACA (102%) and reduced in the left MCA branches (8%).
Discussion
In this study, we have utilized the capability of a computer model of the cerebral arteries to explore the effect of advancing a catheter for clot retrieval under different hypothetical LVO scenarios. The simulations show that, under selected configurations of CoW (such as absent Acom or bilateral fetal PCA pattern), there is potential for a collapse of cerebral blood flow. We have animated the experiments to illustrate the dynamic process of catheter movement or balloon occlusion. Further, these experiments have been uploaded onto the web so that others are able to explore these hypothetical LVO scenarios interactively.
CoW Configuration
In this study, we corrected a limitation of the earlier model of the cerebral circulation, which only used a complete CoW configuration (9). The large range of CoW configurations used in this study reflected the different scenarios seen in clinical practice. Surprisingly, information on the individual configurations of the CoW in the cohorts of the randomized controlled ECR trials has not yet been fully published (3–8). The focus in secondary analyses of these pivotal trials has been on the leptomeningeal collateral system (15, 16). A secondary analysis of the data from the first positive multicentric randomized clinical trial of endovascular treatment for acute ischemic stroke (MRCLEAN) from the Netherlands observed smaller thrombi in patients with patent Pcom and ACA (16). In summary, these studies have not yet discussed the potential implications of different, individual CoW configurations on success or failure of ECR. There have also been studies using silicone-based phantoms of arterial trees (24, 25). These phantoms were built to test (endovascular) medical devices and for learning rather than to evaluate the circulation under different CoW configurations and LVO scenarios. One group performed physical simulation of the CoW with different degrees of ICA stenosis but, in contrast to our study, did not include LA collaterals in the model (26); they reported that configurations of the CoW with absent Pcom might be critical in terms of stroke outcome, based on their in vitro experiment. Furthermore, the collateral capacity of the Pcom became more important as ICA stenosis rose above 40%.
Simulation of Catheter Movement
There are sparse data in the literature on the effect of advancing a catheter along the ICA for clot retrieval under different CoW configurations. Investigators have discussed the impact of different types of ICA occlusions on collateral flow. The various ICA occlusions are as follows: I type (ICA occlusion), L type (ICA and MCA occlusion), and T type (ICA, MCA, and ACA occlusion) (27). However, there was no detailed discussion of the CoW in this article or in a more recent review on the topic of collateral circulation in the setting of thrombectomy (27, 28). One article described the importance of Acom and Pcom to the existence of LA collaterals (29). A similar scenario may occur during the performance of carotid endarterectomy in the setting of contralateral ICA occlusion. Carotid endarterectomy requires clamping of the ipsilateral ICA to achieve hemostasis and prevention of distal embolization. This procedure, but not angioplasty and stenting, has been associated with increased risk of stroke in the setting of contralateral carotid occlusion (30, 31). Unfortunately, there was no information on the status of the CoW in these studies. This is a subject that we hope would change in the future as investigators reevaluate the impact of CoW configurations on procedure feasibility and outcome.
To address the paucity of data on this topic, we imposed different degrees of ICA stenosis to simulate the effect of catheter advancement in the ICA or balloon inflation. These experiments were performed to evaluate downstream flow in the ACA and MCA branches. Our results suggested that under certain unfavorable CoW configurations, such as ipsilateral absent A1 and fetal PCA, there was collapse of the collateral systems. The results of these experiments are difficult to replicate in humans and would pose ethical issues given the findings here. Some support for our findings is provided by earlier studies in which there were descriptions of the use of manual techniques or balloon-guided compression of the ICA to evaluate safety of ICA occlusion for treatment of aneurysm (32–36). These studies surprisingly did not describe the CoW configuration before and after occlusion. A more recent article suggested the importance of cross-flow across the Acom and that its absence would result in a positive balloon occlusion test (37). Nikoubashman et al. have shown that a large-bore catheter can reduce, but not arrest, flow in the parent vessel in a porcine model (38). Complete flow arrest and flow reversal during clot retrieval, however, can be achieved by using a balloon-guided catheter and distal aspiration catheter (so-called dual aspiration).
Leptomeningeal Anastomoses
The size of the interterritorial LA in these experiments did not modify flow patterns in critical arteries when paired with unfavorable CoW configurations. As such, augmentation of LA may not improve flow when the proximal source of flow had been interrupted. Our original experiments were done with complete CoW configuration, where provision of flow to the left ACA branches and onto the selected MCA branches was possible (9).
Limitations
An issue with the earlier work on the computer model of cerebral circulation was that there was no method developed for depicting the results. We have attempted to rectify this issue by creating an app for exploring CoW configurations, ICA stenosis, arterial occlusion, and augmentation of LA. A potential drawback of this approach is that the reader needs to simultaneously read the article and explore the app. The “Artery model” tab can take several minutes to load on the web with Microsoft Edge. Loading this page is faster when using Google Chrome and fastest with Firefox on Linux operating system.
The experiments were created using a graph theory library (igraph) rather than the more computationally expensive physical model of the cerebral arteries with exact three-dimensional geometry. We are not aware of such a model at this stage and that would allow for this type of modeling or viewing. There are several groups who have described probabilistic map of the cerebral anatomy based on MR angiography performed on 3- and 7-T scanners (39–41). These atlases have labeling of the CoW, MCA, ACA, and PCA but not the major branches of these arteries and the LA. By contrast, this computer model of the cerebral arteries has labeling of the arterial branches down to the fifth order. A more rigorous method would have been to segment and label different segments of the entire cerebral circulation from whole-brain radiological images at a minimum on 7-T MR scanner; this is an enormous and currently infeasible task. Given the current technology, acquisition of micromillimeter high-resolution imaging of the LA and segmentation and labeling of these arteries are not yet possible. The challenge is less important in the context of our study because we did not intend to study fluid hemodynamics, but rather simply to examine the reserve capacity of the CoW and LA.
This simulation study showed that configurations of CoW play a major role in determining outcome. Augmentation of the leptomeningeal system is not sufficient in this setting to change blood flow to >30% of baseline. We hope that this study will lead to evaluation of the configurations of CoW in the ECR trials to better inform clinicians when faced with these clinical scenarios.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
TP: design, creation of app to display result, analysis, and writing of manuscript. RB: transfer original Matlab script into R, depiction of computer model analysis, creation of app to display result, and writing of manuscript. JH and MS: Matlab script for computer model. MG, VS, and HM: writing of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
TP has received honoraria as speaker for Bayer, Boehringer Ingelheim, Sanofi-Genzyme. He serves on the advisory board for Sanofi received-Genzyme for Fabry Disease.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACA, anterior cerebral artery; Acom, anterior communicating artery; BA, basilar artery; CoW, circle of Willis; ECR, endovascular clot retrieval; ICA, internal carotid artery; LA, leptomeningeal anastomoses; LVO, large vessel occlusion; MCA, middle cerebral artery; MR, magnetic resonance; MRCLEAN, multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands; PCA, posterior cerebral artery; Pcom, posterior communicating artery.
References
1. Rhoton AL Jr. The supratentorial arteries. Neurosurgery. (2002) 51:S53–120. doi: 10.1097/00006123-200210001-00003
2. Van der Eecken HM. The Anastomoses Between the Leptomeningeal Arteries of the Brain: Their Morphological, Pathological and Clinical Significance. Springfiled, IL: Charles C. Thomas (1959).
3. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
4. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
5. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
6. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
7. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
8. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
9. Phan TG, Hilton J, Beare R, Srikanth V, Sinnott M. Computer modeling of anterior circulation stroke: proof of concept in cerebrovascular occlusion. Front Neurol. (2014) 5:176. doi: 10.3389/fneur.2014.00176
10. Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. (2003) 34:2750–62. doi: 10.1161/01.STR.0000095791.85737.65
11. Cassot F, Vergeur V, Bossuet P, Hillen B, Zagzoule M, Marc-Vergnes JP. Effects of anterior communicating artery diameter on cerebral hemodynamics in internal carotid artery disease. A model study. Circulation. (1995) 92:3122–31. doi: 10.1161/01.CIR.92.10.3122
12. Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. (1994) 330:1565–70. doi: 10.1056/NEJM199406023302204
13. Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of willis collateral flow investigated by magnetic resonance angiography. Stroke. (1999) 30:2671–78. doi: 10.1161/01.STR.30.12.2671
14. Hendrikse J, Hartkamp MJ, Hillen B, Mali WP, van der Grond J. Collateral ability of the circle of willis in patients with unilateral internal carotid artery occlusion: Border zone infarcts and clinical symptoms. Stroke. (2001) 32:2768–73. doi: 10.1161/hs1201.099892
15. Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. (2016) 47:768–76. doi: 10.1161/STROKEAHA.115.011788
16. Alves HC, Treurniet KM, Dutra BG, Jansen IGH, Boers AMM, Santos EMM, et al. Associations between collateral status and thrombus characteristics and their impact in anterior circulation stroke. Stroke. (2018) 49:391–6. doi: 10.1161/STROKEAHA.117.019509
17. Phan TG, Donnan GA, Srikanth V, Chen J, Reutens DC. Heterogeneity in infarct patterns and clinical outcomes following internal carotid artery occlusion. Arch Neurol. (2009) 66:1523–28. doi: 10.1001/archneurol.2009.259
18. Kim DE, Park JH, Schellingerhout D, Ryu WS, Lee SK, Jang MU, et al. Mapping the supratentorial cerebral arterial territories using 1160 large artery infarcts. JAMA Neurol. (2018) 76:72–80. doi: 10.1001/jamaneurol.2018.2808
19. Csardi G, Nepusz T. The igraph software package for complex network research. Inter J. (2006) 1695:1–9.
20. Scheel P, Ruge C, Petruch UR, Schoning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. (2000) 31:147–50. doi: 10.1161/01.STR.31.1.147
21. Heywood J, Rannacher R, Turek S. Artificial boundaries and flux and pressure conditions for the incompressible navier-stokes equations. Int J Num Methods Fluids. (1996) 22:325–52. doi: 10.1002/(SICI)1097-0363(19960315)22:5<325::AID-FLD307>3.0.CO;2-Y
22. Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. (1994) 36:557–65. doi: 10.1002/ana.410360404
23. Campbell BCV, Majoie C, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. (2019) 18:46–55. doi: 10.1016/S1474-4422(18)30314-4
24. Chueh JY, Wakhloo AK, Gounis MJ. Neurovascular modeling: small-batch manufacturing of silicone vascular replicas. AJNR Am J Neuroradiol. (2009) 30:1159–64. doi: 10.3174/ajnr.A1543
25. Kaneko N, Mashiko T, Ohnishi T, Ohta M, Namba K, Watanabe E, et al. Manufacture of patient-specific vascular replicas for endovascular simulation using fast, low-cost method. Sci Rep. (2016) 6:39168. doi: 10.1038/srep39168
26. Zhu G, Yuan Q, Yang J, Yeo J. Experimental study of hemodynamics in the circle of Willis. Biomed Eng Online. (2015) 14:S10. doi: 10.1186/1475-925X-14-S1-S10
27. Liebeskind DS, Flint AC, Budzik RF, Xiang B, Smith WS, Duckwiler GR, et al. Carotid i's, l's and t's: collaterals shape the outcome of intracranial carotid occlusion in acute ischemic stroke. J Neurointerv Surg. (2015) 7:402–7. doi: 10.1136/neurintsurg-2014-011231
28. Piedade GS, Schirmer CM, Goren O, Zhang H, Aghajanian A, Faber JE, et al. Cerebral collateral circulation: A review in the context of ischemic stroke and mechanical thrombectomy. World Neurosurg. (2018) 122:33–42. doi: 10.1016/j.wneu.2018.10.066
29. Millesi K, Mutzenbach JS, Killer-Oberpfalzer M, Hecker C, Machegger L, Bubel N, et al. Influence of the circle of willis on leptomeningeal collateral flow in anterior circulation occlusive stroke: friend or foe? J Neurol Sci. (2019) 396:69–75. doi: 10.1016/j.jns.2018.11.002
30. Gasecki AP, Eliasziw M, Ferguson GG, Hachinski V, Barnett HJ. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. North American Symptomatic Carotid Endarterectomy Trial (NASCET) group. J Neurosurg. (1995) 83:778–82. doi: 10.3171/jns.1995.83.5.0778
31. Antoniou GA, Kuhan G, Sfyroeras GS, Georgiadis GS, Antoniou SA, Murray D, et al. Contralateral occlusion of the internal carotid artery increases the risk of patients undergoing carotid endarterectomy. J Vasc Surg. (2013) 57:1134–45. doi: 10.1016/j.jvs.2012.12.028
32. Linskey ME, Jungreis CA, Yonas H, Hirsch WL Jr, Sekhar LN, Horton JA, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced ct. AJNR Am J Neuroradiol. (1994) 15:829–43.
33. van Rooij WJ, Sluzewski M, Slob MJ, Rinkel GJ. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. (2005) 26:175–8.
34. Sato K, Shimizu H, Inoue T, Fujimura M, Matsumoto Y, Kondo R, et al. Angiographic circulation time and cerebral blood flow during balloon test occlusion of the internal carotid artery. J Cereb Blood Flow Metab. (2014) 34:136–43. doi: 10.1038/jcbfm.2013.176
35. Mathis JM, Barr JD, Jungreis CA, Yonas H, Sekhar LN, Vincent D, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. (1995) 16:749–54.
36. Imai K, Mori T, Izumoto H, Takabatake N, Kunieda T, Shimizu H, et al. Clot removal therapy by aspiration and extraction for acute embolic carotid occlusion. AJNR Am J Neuroradiol. (2006) 27:1521–27.
37. Kikuchi K, Yoshiura T, Hiwatashi A, Togao O, Yamashita K, Honda H. Balloon test occlusion of internal carotid artery: angiographic findings predictive of results. World J Radiol. (2014) 6:619–24. doi: 10.4329/wjr.v6.i8.619
38. Nikoubashman O, Wischer D, Hennemann HM, Sandmann J, Sichtermann T, Muschenich FS, et al. Balloon-guide catheters are needed for effective flow reversal during mechanical thrombectomy. AJNR Am J Neuroradiol. (2018) 39:2077–81. doi: 10.3174/ajnr.A5829
39. Wright SN, Kochunov P, Mut F, Bergamino M, Brown KM, Mazziotta JC, et al. Digital reconstruction and morphometric analysis of human brain arterial vasculature from magnetic resonance angiography. Neuroimage. (2013) 82:170–81. doi: 10.1016/j.neuroimage.2013.05.089
40. Dunas T, Wahlin A, Ambarki K, Zarrinkoob L, Malm J, Eklund A. A stereotactic probabilistic atlas for the major cerebral arteries. Neuroinformatics. (2017) 15:101–10. doi: 10.1007/s12021-016-9320-y
Keywords: circle of Willis, leptomeningeal anastomoses, thrombectomy, simulation, stroke, carotid endarterectomy, angioplasty, endovascular clot retrieval
Citation: Phan TG, Ma H, Goyal M, Hilton J, Sinnott M, Srikanth V and Beare R (2020) Computer Modeling of Clot Retrieval—Circle of Willis. Front. Neurol. 11:773. doi: 10.3389/fneur.2020.00773
Received: 07 April 2020; Accepted: 24 June 2020;
Published: 07 August 2020.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Woong Yoon, Chonnam National University Medical School, South KoreaAnastasios Mpotsaris, University Hospital RWTH Aachen, Germany
Copyright © 2020 Phan, Ma, Goyal, Hilton, Sinnott, Srikanth and Beare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thanh G. Phan, dGhhbmgucGhhbkBtb25hc2guZWR1
 Thanh G. Phan
Thanh G. Phan Henry Ma
Henry Ma Mayank Goyal3
Mayank Goyal3 James Hilton
James Hilton Velandai Srikanth
Velandai Srikanth Richard Beare
Richard Beare