- 1Swiss Distance Learning University, Brig, Switzerland
- 2Lyon Neuroscience Research Center, CNRS UMR 5292 - INSERM U1028 - Lyon 1 University, Lyon, France
- 3Clinical Health Psychology, University of Ulster, Ulster, United Kingdom
- 4Centre Hospitalier Annecy Genevois, Service Pneumologie, Épagny-Metz-Tessy, France
In order to ensure robust relationships between the dependent and independent variables in clinical dream/nightmare studies, the major factors which influence the frequency of reported dreams must be controlled. This article sets out methodological recommendations to both researchers seeking to ensure the equivalence of experimental groups of participants in group-matching designs, and to clinicians who wish to check that any change in frequency of reported nightmares over the course of a psychological or a pharmacological intervention is not caused by factors other than the experimental treatment itself. The main factors influencing the frequency of dream recall are presented: demographic variables, psychological characteristics, pathological dimensions, and substance consumption. A series of questionnaires is proposed for easily measuring these control variables.
Introduction
Dreaming is still a mysterious phenomenon and its definition remains controversial and the subject of epistemological debate (1). However, dreams can be studied using a scientific approach (2). The most renowned dream researchers, Hoffman (3) and Schredl (4), emphasized the necessity to increase and improve the use of quantitative methodological practices in dream research to improve results reliability. Quantitative dream studies should systematically control variables which are known to influence some aspects of dreaming, so as to verify that groups of various dreamers are matched in group-matching designs and also to ensure that the results obtained in pre-, post-, and follow-up design studies are due to experimental variables (e.g., patients treated for nightmares vs. patients on the waiting list) and not to confounding variables. For instance, studies testing treatments for nightmares usually compare dream/nightmare report frequency (DRF and NRF) measured at different time points (before and after exposure to the treatment, e.g., imagery rescripting therapy, prazosin) in one group of patients and possibly in one control group (e.g., patients from the waiting-list or exposed to a placebo). For the statistical comparison between groups to be valid, groups must not differ in DRF before the treatment, nor in factors which may influence the evolution of DRF. Consequently, as far as possible, factors influencing DRF should be measured and/or taken into consideration to match the groups to be compared [e.g., (5–12)] and to set the non-inclusion (i.e., psychoactive drugs consumption, and psychopathologies for experimental studies in healthy participants) and exclusion criteria. In pre-post treatment designs, for example, if a patient significantly changed the dose of one of their psychoactive medications (e.g., hypnotics, neuroleptic drugs, antidepressants) during the course of the study, it may have modified DRF & NRF, and this patient's data should be excluded from the analysis [e.g., (13)]. Similarly, if a participant has suffered a trauma during the course of the study, the change in DRF/NRF may be due to the trauma and not to the experimental condition, and their data should therefore be excluded. To improve the quality of future clinical research, outcome studies should also include relevant, and standardized measures known to covariate with some aspects of dreaming.
The objective of this paper is to set out a list of the main variables which should be controlled in dream studies, and more precisely those which influence dream report frequency (DRF), nightmare frequency and emotional valence of dreams. This list includes demographic data, sleep parameters, psychological and cognitive factors, and pathologies and drugs. The authors further recommend validated questionnaires to assess these factors. Note that we do not propose here an exhaustive review and discussion of all the parameters known to covary with some aspects of dreaming. Rather we intend to draw attention on the main variables well-known to influence dream report frequency (DRF), nightmare frequency and the emotional valence of dreams.
Demographic Variables
Gender
A meta-analysis comprising more than 40,000 participants showed that women present a slightly higher DRF than men (14). In a retrospective study including several thousand of participants, Nielsen showed that this difference occurs from early adolescence (14 years old) and disappears in middle age (44 years old) (15). This difference is also valid for nightmares except in the older population (older than 60 years old) (16).
Age
DRF covaries with age (17), it globally decreases with increasing age. An increase in DRF has been described during puberty (10–19 years old) and in early adulthood (19–29 years old) which is followed by a decline. This evolution is modulated by gender. For women, DRF increases in early adolescence, remains at a high level and decreases later in comparison to men (40–49 years old vs. 30–39 for men) (15). A similar profile applies for nightmare frequency, with a peak in childhood followed by a drastic decrease in adulthood. A nightmare rate of at least once a week [a frequency generally thought to reflect moderately severe pathology (18)], is observed in 18% of children and in 9% of adults (19, 20).
Sex ratio and age are important to match between groups in studies investigating dreams and nightmares.
Sleep Variables
Even if counter-intuitive, sleep duration is not a predictive factor of DRF (21). Sleep stages are. In average DRF is higher after awakenings in Rapid Eye Movement (REM) sleep (82%) than after awakenings in Non-REM (NREM) sleep (43–67%) (22, 23). Sleep is influenced by circadian rhythms which results in a higher DRF at the end of the night than at the beginning of the night irrespective of the sleep stages (24). Dream reports are in average longer, more vivid, story-like and bizarre in the second part of the night. DRF may be influenced by circadian-driven changes in cortical activity (according to increase in body temperature and reduction in melatonin) and by the ultradian rhythm of the NREM/REM cycle (17). Chronotype “morningness-eveningness” and nightmare frequency are correlated, especially among women (15).
Intrasleep wakefulness is a decisive parameter regarding DRF (5, 7, 17). The only sleep parameter differentiating high dream recallers (HR) from low dream recallers (LR) is the average duration of intra-sleep awakenings. While still in the normal range, HR show longer intra-sleep awakenings (about 2 min on average) than LR (about 0.95 min on average) (7). Long enough awakenings when the dream is still in short term memory appear to be necessary to transfer the short term dream memory into a long term memory (7, 25).
The time elapsed between awakening and dream report is also important for the precision and length of the report, the shorter, the better (26).
The sleeper's environment and sensory stimulation during sleep may also impact dream activity and notably its emotional content (2). It is for example the case for sounds (27, 28), odors (29, 30), and painful stimuli or thirst (31, 32) also for physical bodily position (33).
An assessment of wake/sleep rhythms, subjective quantity and quality of sleep using the Pittsburg Sleep Quality Index (34) is thus recommended in a dream study. The chronotype can be measured using the short version of the morningness/eveningness questionnaires (MEQ) (35).
Psychological Variables
Influence of Previous Waking Experiences
Some memories of wakefulness experiences are incorporated into dreams and are typically incorporated in a partial or indirect way (6, 10). The most recent memories from the day before (day residues) are typically more represented than other recent memories, as are emotional ones, however remote (6, 36, 37). According to some studies, positive and negative emotions occurring during a dream are strongly correlated with the emotional experiences of wakefulness (38). Stress can increase the rate of incorporation of waking memories into dreams (39), and it has also been associated with increased DRF (2, 40, 41) and emotional intensity of dream content (38). For studies investigating emotional dream content, it may thus be necessary to monitor/collect the emotional daily experiences of the participants during the period of dream collection. The Social Readjustment Rating Scale can be used to identify stressful life events (42).
Some researchers suggest that dream recall could be modified by certain conditions and suggestions before falling asleep. However, results are inconsistent. Thoughts or images repressed when falling asleep tend to come back in dreams (43–45). Thought suppression can be measured using the White Bear Suppression Inventory (46).
Attitudes toward dreams (ATD), which refers to the general interest in or attention to dreams, is consistently correlated with DRF and the effect of this parameter is large (17, 47). Doing a daily dream diary, which focuses attention on dreaming, is typically associated with a rapid and important increase in DRF. The simple fact of prospectively observing one's own dreams, has also been shown to decrease nightmare frequency (48). The Memory Experiences and Dreams Questionnaire can be used to measure ATD (49).
Memory
Several types of memory have been tested in HR and LR (short and long term, episodic and semantic, declarative and procedural memory…) but no reliable difference has been identified between the two groups (2). For control purposes, memory capacity can nevertheless be measured using the Mini Mental Score (10 min) (50).
Personality
Creativity, openness to experience and alexithymia are strongly correlated with DRF (2, 40, 51). A high score on an alexithymia scale is associated with difficulty in identifying and describing feelings, limited imaginative processes, and an externally oriented cognitive style (52). The 10-item short version of the Big Five Inventory can be used to measure openness to experience (53).
Subjects with a high DRF are also more likely to have a personality with thinner boundaries [Hartmann described people with thin boundaries as being open, trustworthy, vulnerable, and sensitive (52)], to be more anxious (54, 55), and to have a higher level of absorption [the absorption scale measures the capacity to become absorptively involved in imaginative and esthetic experiences (54, 55)]. The questionnaire assessing thin and thick personality boundaries has been developed by Hartmann (56). Absorption can be assessed with the absorption scale (34 items) (57). Neuroticism is correlated with nightmare frequency (58).
Methods for Studying Dreams
Several methods are classically used to assess some aspect of dreaming (retrospective or prospective DRF questionnaires, dream diaries with written or oral reports, and laboratory awakenings usually followed by an oral report), and these different methods result in different quantitative and qualitative measures (59–61). It is therefore important to use the same dream recording method in participants from different groups supposed to be compared. Retrospective measures are better correlated with prospective measurements using a dream logbook, compared to those using questionnaires for home measurements (62). The correlation between questionnaires and diaries measures are high. Nevertheless, recording a dream diary increases DRF in low and medium dream recallers, and decreases DRF in high dream recallers (63). It would therefore be relevant to measure dream recall frequency at baseline using a retrospective questionnaire.
Recording dreams using the method of provoked awakening in the laboratory–using a voice recording and the sudden awakening method (26)—is the best controlled method. The awakening method itself can influence DRF (abrupt awakenings favor dream recall compared to gradual awakening) (64). Oral reports using a voice recorder device are indeed longer (61). It is probably because oral report are less effortful and can be done right after awakening. When possible to control, the time lapse between awakening and the dream report should indeed be minimized in order to diminish memory loss and reconstruction bias.
Pathologies and Medications
Sleep Disorders
According to the existing literature, the main sleep disorders known to be associated with variations in DRF and/or negative dream emotions are insomnia, narcolepsy, nightmare disorders, and somnambulism or night terrors. There is less evidence for such variations in sleep-related breathing disorders (SBD) and restless legs syndrome (65, 66). The variations in dream frequency in patients suffering from idiopathic hypersomnia, REM sleep behavior disorder, and NREM parasomnia are less clear since fewer studies have been done in these pathologies (66, 67).
According to some studies, in primary insomnia, deterioration of sleep (decreased total sleep time, REM and NREM sleep duration, and sleep efficiency) is associated with a decreased frequency of dream and nightmare reports (68). However, several other studies have reported an increased DRF in insomnia patients (66, 69). In any case, insomnia is worth evaluating in dream/nightmare studies and its intensity can be measured using the Insomnia Severity Index (70).
Narcolepsy severity is correlated with an increase in DRF and higher occurrence of nightmares and lucid dreams (71, 72). Dream content in patients with narcolepsy is more bizarre, with a more negative emotional tone (73). The symptoms suggestive of this rare disease are uncontrollable sleep attacks, cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations.
Nightmare disorders are characterized by an increase in both dream and nightmare recall (66). And as DRF and nightmare frequency are usually positively correlated (20) both parameters are worth measuring in dream and nightmare studies.
In somnambulism and night terrors no variations in DRF has been observed but an increase in nightmare frequency or negative dream emotions has been reported (66). Night terrors are mainly observed in children, and sign of somnambulism can be provided by partners in adults.
Some studies have reported an increased DRF and duration of dreams in patients with SBD (74). Dreams are more negative in sleepers with an apnea-hypopnea index >15 (75). Continuous positive airway pressure therapy (Gold Standard treatment of SBD) decreases nightmare frequency in SBD patients suffering from traumatic disorders (76). In the absence of a polygraph or polysomnography, the Berlin questionnaire or Epworth Somnolence Scale (77) may be used as an indicator of SBD risk.
The severity of restless legs syndrome may be correlated with DRF, but a review of the literature shows no effect of this syndrome on dreaming (78). The Restless Legs Syndrome Rating Scale may be used to assess this disorder (79).
Preliminary screening should be carried out, especially for insomnia, narcolepsy and nightmare disorders.
Psychopathologies
Traumas are associated with nightmares and disruptive nocturnal behaviors (80). Nightmares are the most frequent symptom of post-traumatic stress disorder (PTSD) (81). Dreams are less frequent but significantly more threatening and hostile (82). Nightmare frequency is correlated with PTSD severity (83). Patients with PTSD experience significantly more replicative (i.e., reproducing the traumatic experience) nightmares (46%) than other patients (11%) (84). PTSD severity can be measured using the Post-traumatic Stress Disorder Checklist Scale (85) or the revised Impact of Event Scale (86).
Sleep related symptoms of depression include sleep fragmentation, early morning awakening, decreased rapid eye movement (REM) sleep latency and increased rapid eyes movements density in REM sleep (87). Decrease in DRF and intensity of negative emotions in dreams could be linked to the severity of symptoms (88, 89). Depression severity can be measured using the short form of the Beck Depression Inventory II (90).
Alteration of REM sleep in anxiety disorders is still the subject of debate (91). Elderly patients suffering from generalized anxiety disorder have more nightmares (91). The Spielberger State-Trait Anxiety scale measures the state and trait anxiety (92), a short version is also available (93).
Schizophrenic patients have stranger dreams, with stronger negative emotional valence compared to healthy subjects. However, DRF is not modified in these patients (94). Note that schizophrenia, as all other neurologic or psychiatric pathologies, is classically an exclusion criterion in dream studies targeting the healthy population.
In order to decrease heterogeneity among group participants in studies investigating dreams in patients, preliminary screening should be carried out, especially for post-traumatic stress disorders and depression.
Substances
Insomniac patients treated with benzodiazepine have lower DRF (69). Flunitrazepam leads to more aggressive, sexual and unpleasant dream content (95). Gradual reduction of doses of clonazepam increases nightmares in some patients (96). Z-drugs (e.g., zolpidem, zopiclone) can also modify both dream content and frequency. More generally, hypnotic drugs tend to decrease DRF (for a review on the effect of psychotropic drugs on dreaming, see Nicolas & Ruby in the same issue).
Most antidepressants increase sleep continuity and reduce intra-sleep awakenings, which may explain their reductive effect on DRF (see Nicolas and Ruby in the same issue).
Alcohol affects sleep quality and dreams. An acute pre-bedtime dose of alcohol in non-alcohol-dependent individuals deteriorates REM sleep (97, 98). There is debate about its effect: several studies have observed longer latency in REM sleep and decreased REM sleep only in the first half of the night, while other studies find this effect across the whole night (99–101). The link between alcohol at bedtime in non-alcoholic people and DRF does not seem to have been studied. Alcohol-dependent subjects report more negative dreams than those who are non-alcohol-dependent (102), with an increase in dream emotional negativity during the first weeks of abstinence (103). It may therefore be important to report and/or control alcohol consumption habits in dreams studies.
Cannabis increases the duration and latency of REM sleep (104). Intensification of vivid dreams is a cardinal symptom of cannabis withdrawal (104). Both non-clinical and clinical research has now characterized the profile of cannabis withdrawal, with sleep disturbances and vivid dreams representing its hallmark symptoms.
Given that the use of nicotine patches increases DRF (105), as does varenicline (used to help quit smoking) (106) one may expect that tobacco would influence DRF, however, this remains to be tested experimentally.
Prazosin is an alpha blocker recommended for the treatment of chronic nightmares in PTSD patients (107–109), and in this case, inhibition of the sympathetic nervous system may desensitize memories of fear which could explain the reduction of nightmare frequency induced by this treatment. To our knowledge, the impact of Prazosin on DRF is unknown. Other substances have been proposed for treating nightmares, but the current level of proof of their effectiveness is too low to be recommended (110).
Comparing groups in dream studies therefore requires consumption of the following substances to be controlled: benzodiazepine, antidepressant medication, prazosin, alcohol and cannabis. For longitudinal studies, the regularity of intake during the study should be monitored and any significant change in intake should be an exclusion criterion.
Conclusion
This article does not aim to review and discuss all the variables which are known to influence dream and nightmare recall frequency. Rather our objective is to provide a list of the main variables that are currently well-known to influence dream and nightmare recall frequency, and which, therefore, would be necessary to monitor or control in clinical studies investigating dreams. The authors therefore aim to set out methodological recommendations for conducting clinical studies on dreams and nightmares. It is essential for studies comparing different groups of dreamers to ensure that observed differences are due to experimental variables and not to uncontrolled differences between dreamers.
This methodological overview is nevertheless limited by the current literature on dream research i.e., the limited existing literature on the influence certain variables have on dreams. The influence of some parameters has been scarcely studied and the influence of some other parameters are debated in the dream research community. Table 1 summarizes the variables known to covary with DRF and dreams emotional valence/intensity and presents the reliability of the effects according to the current knowledge. The main recommendations resulting from this overview of the literature are that (1) in clinical studies, medical interviews should include a differential diagnosis for traumatic disorders, sleep disorders and mood disorders, and these disorders should be assessed in case of group comparison, (2) DRF should be measured in addition to NRF in longitudinal studies on nightmares, as well as psychoactive drug consumption (notably alcohol, antidepressant, cannabis, and prazosin), and significant variations in the consumption of psychoactive drugs during the course of the study should be considered as an exclusion criterion, (3) dreamers' groups to be compared should be matched for the maximum of the parameters covariating with DRF, notably: age, sex ratio, psychopathologies, and psychoactive drug consumption.
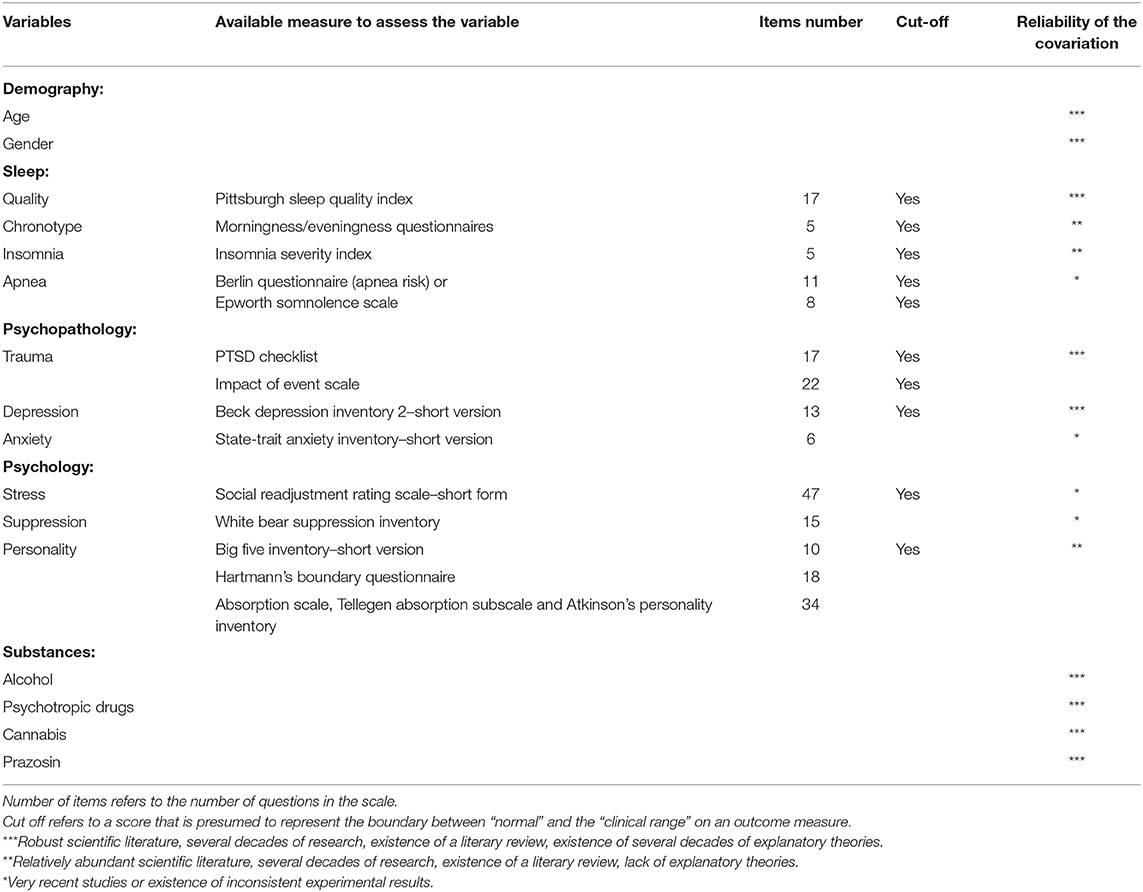
Table 1. Variables known to covary with dream recall frequency or dreams emotional valence/intensity.
With this methodological perspective we aim at increasing awareness of the need to control for several variables in clinical dream and nightmare studies and to stimulate discussion amongst experts in the field. A consortium could further develop this avenue of research to establish comprehensive guidelines and recommendations on the subject. As a first step, we propose here a list of variables currently known to influence DRF, nightmare frequency and the emotional valence of dreams—and which it may be necessary to monitor or control in clinical studies investigating dreams—and means to measure these variables. This article is intended to help improving methodological practices in clinical studies on dreams and nightmares, and is dedicated to clinician non-expert in sleep and dream science. This article may thus help to ensure that in future clinical studies, the observed differences between groups of dreamers are due to the experimental variables manipulated and not to confounding factors.
Author Contributions
BP developed the research subject presented herein and wrote the manuscript, with the support of CA, CS, SH, and WL under the supervision of PR. PR critically revised the manuscript and added important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Windt JM. Reporting dream experience : why (not) to be skeptical about dream reports. Front Hum Neurosci. (2013) 7:708. doi: 10.3389/fnhum.2013.00708
2. Ruby PM. Experimental research on dreaming: state of the art and neuropsychoanalytic perspectives. Front Psychol. (2011) 2:286. doi: 10.3389/fpsyg.2011.00286
3. Hoffman C. Research articles in dreaming: a review of the first 20 years. Dreaming. (2013) 23:216–31. doi: 10.1037/a0032905
4. Schredl M. Criteria for evaluating empirical research articles: a reply to curtiss Hoffman's “research articles in dreaming: a review of the first 20 years. Dreaming. (2013) 23:287–90. doi: 10.1037/a0035534
5. Eichenlaub JB, Bertrand O, Morlet D, Ruby P. Brain reactivity differentiates subjects with high and low dream recall frequencies during both sleep and wakefulness. Cereb Cortex. (2014) 24:1206–15. doi: 10.1093/cercor/bhs388
6. Vallat R, Chatard B, Blagrove M, Ruby P. Characteristics of the memory sources of dreams: a new version of the content-matching paradigm to take mundane and remote memories into account. PLoS ONE. (2017) 12:e0185262. doi: 10.1371/journal.pone.0185262
7. Vallat R, Lajnef T, Eichenlaub JB, Berthomier C, Jerbi K, Morlet D, et al. Increased evoked potentials to arousing auditory stimuli during sleep: implication for the understanding of dream recall. Front Hum Neurosci. (2017) 11:132. doi: 10.3389/fnhum.2017.00132
8. Vallat R, Nicolas A, Ruby P. Brain functional connectivity upon awakening from sleep predicts inter-individual differences in dream recall frequency. Sleep. (2020). doi: 10.1093/sleep/zsaa116. [Epub ahead of print].
9. Vallat R, Eichenlaub J-B, Nicolas A, Ruby P. Dream recall frequency is associated with medial prefrontal cortex white-matter density. Front Psychol. (2018) 9:1856. doi: 10.3389/fpsyg.2018.01856
10. Plailly J, Villalba M, Vallat R, Nicolas A, Ruby P. Incorporation of fragmented visuo-olfactory episodic memory into dreams and its association with memory performance. Sci. Rep. (2019) 9:1–14. doi: 10.1038/s41598-019-51497-y
11. Eichenlaub JB, Nicolas A, Daltrozzo J, Redouté J, Costes N, Ruby P. Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology. (2014) 39:1594–602. doi: 10.1038/npp.2014.6
12. Vallat R, Eskinazi M, Nicolas A, Ruby P. Sleep and dream habits in a sample of French college students who report no sleep disorders. J Sleep Res. (2018) 27:e12659. doi: 10.1111/jsr.1265
13. Putois B, Peter-Derex L, Leslie W, Braboszcz C, El-Hage W, Bastuji H. Internet-based intervention for posttraumatic stress disorder: using remote imagery rehearsal therapy to treat nightmares. Psychother Psychosom. (2019) 88:315–6. doi: 10.1159/000501105
14. Schredl M, Reinhard I. Gender differences in dream recall: a meta-analysis. J Sleep Res. (2008) 17:125–31. doi: 10.1111/j.1365-2869.2008.00626.x
15. Nielsen T. Variations in dream recall frequency and dream theme diversity by age and sex. Front Neurol. (2012) 3:106. doi: 10.3389/fneur.2012.00106
16. Nielsen T, Stenstrom P, Levin R. Nightmare frequency as a function of age, gender, and September 11, 2001: findings from an internet questionnaire. Dreaming. (2006) 16:145–58. doi: 10.1037/1053-0797.16.3.145
17. Schredl M, Wittmann L, Ciric P, Götz S. Factors of home dream recall: a structural equation model. J Sleep Res. (2003) 12:133–41. doi: 10.1046/j.1365-2869.2003.00344.x
18. American Sleep Disorders Association. ICSD-II. International Classification of Sleep Disorders: Diagnostic and Coding Manual. Chicago, IL: American Sleep Disorders Association (2005).
19. Levin R, Fireman G. Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep. (2002) 25:205–12. doi: 10.1093/sleep/25.2.205
20. Schredl M, Goeritz AS. Nightmare frequency and nightmare distress: socio-demographic and personality factors. Sleep Sci. (2019) 12:178–84. doi: 10.5935/1984-0063.20190080
21. Blagrove M, Pace-Schott EF. Trait and neurobiological correlates of individual differences in dream recall and dream content. Int Rev Neurobiol. (2010) 92:155–80. doi: 10.1016/S0074-7742(10)92008-4
22. Nielsen TA. A review of mentation in REM and NREM sleep: “Covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. (2000) 23:851–66. discussion 904–1121. doi: 10.1017/S0140525X0000399X
23. Montangero J. Dreaming and REM sleep: history of a scientific denial whose disappearance entailed a reconciliation of the neuroscience and the cognitive psychological approaches to dreaming. Int J Dream Res. (2018) 11:30–45. doi: 10.11588/ijodr.2018.1.42384
24. Chellappa SL, Cajochen C. Ultradian and circadian modulation of dream recall: EEG correlates and age effects. Int J Psychophysiol. (2013) 89:165–70. doi: 10.1016/j.ijpsycho.2013.03.006
25. Koulack D, Goodenough DR. Model for the recall of dreams upon waking, proposed to account for impairment of memory of dreams. Ann Med Psychol. (1977) 1:35–42.
26. Waterman D, Elton M, Kenemans L. Methodological issues affecting the collection of dreams. J Sleep Res. (1993) 2:8–12. doi: 10.1111/j.1365-2869.1993.tb00053.x
27. Hoelscher TJ, Klinger E, Barta SG. Incorporation of concern- and nonconcern-related verbal stimuli into dream content. J Abnormal Psychol. (1981) 90:88–91. doi: 10.1037/0021-843X.90.1.88
28. Berger RJ. Experimental modification of dream content by meanful verbal stimuli. Br J Psychiatry. (1963) 109:722–40. doi: 10.1192/bjp.109.463.722
29. Schredl M, Atanasova D, Hörmann K, Maurer JT, Hummel T, Stuck B. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. J Sleep Res. (2009) 18:285–90. doi: 10.1111/j.1365-2869.2009.00737.x
30. Schredl M, Hoffmann L, Sommer JU, Stuck BA. Olfactory stimulation during sleep can reactivate odor-associated images. Chemosens Percept. (2014) 7:140–6. doi: 10.1007/s12078-014-9173-4
31. Dement W, Wolpert EA. The relation of eye movements, body motility, and external stimuli to dream content. J Exp Psychol. (1958) 55:543–53. doi: 10.1037/h0040031
32. Bekrater-Bodmann R, Schredl M, Diers M, Reinhard I, Foell J, Trojan J, et al. Post-amputation pain is associated with the recall of an impaired body representation in dreams-results from a nation-wide survey on limb amputees. PLoS ONE. (2015) 10:e0119552. doi: 10.1371/journal.pone.0119552
33. Yu CK. The effect of sleep position on dream experiences. Dreaming. (2012) 22:212–21. doi: 10.1037/a0029255
34. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
35. Adan A, Almirall H. Horne et Ostberg morningness-eveningness questionnaire: a reduced scale. Pers Individ Diff. (1991) 12:241–53. doi: 10.1016/0191-8869(91)90110-W
36. Schredl M, Wittmann L. Dreaming: a psychological view. Dreaming. (2005) 156:484–92. doi: 10.4414/sanp.2005.01656
37. Schredl M, Hofmann F. Continuity between waking activities and dream activities. Conscious Cogn. (2003) 12:298–308. doi: 10.1016/S1053-8100(02)00072-7
38. Gilchrist S, Davidson J, Shakespeare-Finch J. Dream emotions, waking emotions, personality characteristics and well-being–A positive psychology approach. Dreaming. (2007) 17:172–85. doi: 10.1037/1053-0797.17.3.172
39. Picchioni D. Nightmares as a coping mechanism for stress. Dreaming. (2001) 12:155–69. doi: 10.1023/A:1020118425588
40. Brand S, Beck J, Kalak N, Gerber M, Kirov R, Pühse U, et al. Dream recall and its relationship to sleep, perceived stress, and creativity among adolescents. J Adolescent Health. (2011) 49:525–31. doi: 10.1016/j.jadohealth.2011.04.004
41. Armitage R. Gender differences and the effect of stress on dream recall: a 30-day diary report. Dreaming. (1992) 2:137–41. doi: 10.1037/h0094354
42. Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. (1967) 11:213–8. doi: 10.1016/0022-3999(67)90010-4
43. Wegner DM, Wenzlaff RM, Kozak M. Dream rebound: the return of suppressed thoughts in dreams. Psychol Sci. (2004) 15:232–6. doi: 10.1111/j.0963-7214.2004.00657.x
44. Schmidt RE, Gendolla GHE. Dreaming of white bears: the return of the suppressed at sleep onset. Conscious Cogn. (2008) 17:714–24. doi: 10.1016/j.concog.2007.09.002
45. Bryant RA, Wyzenbeek M, Weinstein J. Dream rebound of suppressed emotional thoughts: the influence of cognitive load. Conscious Cogn. (2011) 20:515–22. doi: 10.1016/j.concog.2010.11.004
46. Schmidt RE, Gay P, Courvoisier D, Jermann F, Ceschi G, David M, et al. Anatomy of the White Bear Suppression Inventory (WBSI): a review of previous findings and a new approach. J Pers Assess. (2009) 91:323–30. doi: 10.1080/00223890902935738
47. Schredl M, Ciric P, Götz S, Wittmann L. Dream recall frequency, attitude towards dreams and openness to experience. Dreaming. (2003) 13:145–53. doi: 10.1037/1053-0797.13.3.145
48. Neidhardt EJ, Krakow B, Kellner R, Pathak D. The beneficial effects of one treatment session and recording of nightmares on chronic nightmare sufferers. Sleep. (1992) 15:470–3.
49. Horton CL, Conway MA. The memory experiences and dreams questionnaire (MED-Q): a validated measure of dream remembering. Imag Cogn Pers. (2009) 29:3–29. doi: 10.2190/IC.29.1.b
50. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
51. Schredl M, Erlacher D. Self-reported effects of dreams on waking-life creativity: an empirical study. J Psychol. (2007) 141:35–46. doi: 10.3200/JRLP.141.1.35-46
52. De Gennaro L, Ferrara M, Cristiani R, Curcio G, Martiradonna V, Bertini M. Alexithymia and dream recall upon spontaneous morning awakening. Psychosom Med. (2003) 65:301–6. doi: 10.1097/01.PSY.0000058373.50240.71
53. Rammstedt B, John OP. Measuring personality in one minute or less: a 10-item short version of the big five inventory in English and German. J Res Pers. (2007) 41:203–12. doi: 10.1016/j.jrp.2006.02.001
54. Tart CT. Frequency of dream recall and some personality measures. J Consult Psychol. (1962) 26:467–70. doi: 10.1037/h0046056
55. Schonbar RA. Some manifest characteristics of recallers and nonrecallers of dreams. J Consult Psychol. (1959) 23:414–8. doi: 10.1037/h0047400
56. Hartmann E, Elkin R, Garg M. Personality and dreaming: the dreams of people with very thick or very thin boundaries. Dreaming. (1991) 1:311–24. doi: 10.1037/h0094342
57. Beaulieu-Prévost D, Zadra A. Absorption, psychological boundaries and attitude towards dreams as correlates of dream recall: two decades of research seen through a meta-analysis. J Sleep Res. (2007) 16:51–9. doi: 10.1111/j.1365-2869.2007.00572.x
58. Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. (2010) 33:774–80. doi: 10.1093/sleep/33.6.774
59. Zadra A, Robert G. Dream recall frequency: impact of prospective measures and motivational factors. Conscious Cogn. (2012) 21:1695–702. doi: 10.1016/j.concog.2012.08.011
60. Schredl M. Dream recall: research, clinical implications and future directions. Sleep Hypnosis. (1999) 1:72–81.
61. Schredl M, Schredl M, Dreer J, Mösle A, Rall M, Rauch L, et al. Voice-recorded vs written dream reports: a research note. Int J Dream Res. (2019) 12:138–40. doi: 10.11588/ijodr.2019.1.58801
62. Aspy DJ, Delfabbro P, Proeve M. Is dream recall underestimated by retrospective measures and enhanced by keeping a logbook? A review. Conscious Cogn. (2015) 33:364–74. doi: 10.1016/j.concog.2015.02.005
63. Schredl M. Questionnaires and diaries as research instruments in dream research: methodological issues. Dreaming. (2002) 12:17–26. doi: 10.1023/A:1013890421674
64. Shapiro A, Goodenough DR, Gryler RB. Dream recall as a function of method of awakening. Psychosom Med. (1963) 25:174–80. doi: 10.1097/00006842-196303000-00009
65. Schredl M. Dreams in patients with sleep disorders. Sleep Med Rev. (2009) 13:215–21. doi: 10.1016/j.smrv.2008.06.002
66. Schredl M. Do sleep disorders affect the dreaming process? Dream recall and dream content in patients with sleep disorders. Sleep Med Clin. (2010) 5:193–202. doi: 10.1016/j.jsmc.2010.01.008
67. Schredl M. Characteristics and contents of dreams. Int Rev Neurobiol. (2010) 92:135–54. doi: 10.1016/S0074-7742(10)92007-2
68. Pagel JF, Shocknesse S. Dreaming and insomnia: polysomnographic correlates of reported dream recall frequency. Dreaming. (2007) 17:140–51. doi: 10.1037/1053-0797.17.3.140
69. Schredl M, Schäfer G, Weber B, Heuser I. Dreaming and insomnia: dream recall and dream content of patients with insomnia. J Sleep Res. (1998) 7:191–8. doi: 10.1046/j.1365-2869.1998.00113.x
70. Smith M, Wegener S. Measures of sleep: the insomnia severity index, medical outcomes study (MOS) sleep scale, Pittsburgh Sleep Diary (PSD), and pittsburgh sleep quality index (PSQI). Arthritis Rheum. (2003) 49:S184–96. doi: 10.1002/art.11409
71. Rak M, Beitinger P, Steiger A, Schredl M, Dresler M. Increased lucid dreaming frequency in narcolepsy. Sleep. (2015) 38:787–92. doi: 10.5665/sleep.4676
72. Dodet P, Chavez M, Leu-Semenescu S, Golmard JL, Arnulf I. Lucid dreaming in narcolepsy. Sleep. (2015) 38:487–97. doi: 10.5665/sleep.4516
73. Schredl M. Dream content in patients with narcolepsy: preliminary findings. Dreaming. (1998) 8:103–7. doi: 10.1023/B:DREM.0000005900.63728.a7
74. Gross M, Lavie P. Dreams in sleep apnea patients. Dreaming. (1994) 4:195–204. doi: 10.1037/h0094412
75. Fisher S, Lewis KE, Bartle I, Ghosal R, Davies L, Blagrove M. Emotional content of dreams in obstructive sleep apnea hypopnea syndrome patients and sleepy snorers attending a sleep-disordered breathing clinic. J Clin Sleep Med. (2011) 7:69–74. doi: 10.5664/jcsm.28043
76. El-Solh AA, Vermont L, Homish GG, Kufel T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. (2017) 33:145–50. doi: 10.1016/j.sleep.2016.12.025
77. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
78. Schredl M. Dream recall frequency and sleep quality of patients with restless legs syndrome. Eur J Neurol. (2001) 8:185–9. doi: 10.1046/j.1468-1331.2001.00203.x
79. Walters A, LeBrocq C, Dhar A, Hening W, Rosen R, RP A, et al. Validation of the International restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. (2003) 4:121–32. doi: 10.1016/S1389-9457(02)00258-7
80. Mysliwiec V, Brock MS, Creamer JL, O'Reilly BM, Germain A, Roth BJ. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev. (2018) 37:94–104. doi: 10.1016/j.smrv.2017.01.004
81. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. (1989) 146:697–707. doi: 10.1176/ajp.146.6.697
82. Harvey A, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. (2003) 23:377–407. doi: 10.1016/S0272-7358(03)00032-1
83. Mellman TA, David D, Bustamante V, Torres J, Fins A. Dreams in the acute aftermath of trauma and their relationship to PTSD. J Traumatic Stress. (2001) 14:241–7. doi: 10.1023/A:1007812321136
84. Freese F, Wiese M, Knaust T, Schredl M, Schulz H, De Dassel T, et al. Comparison of dominant nightmare types in patients with different mental disorders. Int J Dream Res. (2018) 11:1–5. doi: 10.11588/ijodr.2018.1.38712
85. Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Traumatic Stress. (2015) 28:489–98. doi: 10.1002/jts.22059
86. Weiss DS. The impact of event scale: revised. In: Cross-Cultural Assessment of Psychological Trauma and PTSD. Boston, MA: Springer US (2007). p. 219–38. doi: 10.1007/978-0-387-70990-1_10
87. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. (2005) 66:1254–69. doi: 10.4088/JCP.v66n1008
88. Schredl M. Dream recall in depressed patients. Psychother Psychosom Med Psychol. (1995) 45:414–7.
89. Armitage R, Rochlen A, Fitch T, Trivedi M, Rush AJ. Dream recall and major depression: a preliminary report. Dreaming. (1995) 5:189–98. doi: 10.1037/h0094434
90. Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
91. Nadorff MR, Porter B, Rhoades HM, Greisinger AJ, Kunik ME, Stanley MA. Bad dream frequency in older adults with generalized anxiety disorder: prevalence, correlates, and effect of cognitive behavioral treatment for anxiety. Behav Sleep Med. (2014) 12:28–40. doi: 10.1080/15402002.2012.755125
92. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA:Consulting Psychologists Press. (1983).
93. Maretau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State–Trait Anxiety Inventory (STAI). Br J Clin Psychol. (1992) 31:301–6. doi: 10.1111/j.2044-8260.1992.tb00997.x
94. Schredl M. Dream research in schizophrenia: methodological issues and a dimensional approach. Conscious Cogn. (2011) 20:1036–41. doi: 10.1016/j.concog.2010.05.004
95. Gaillard JM, Phelippeau M. Benzodiazepine-induced modifications of dream content: the effect of flunitrazepam. Neuropsychobiology. (1976) 2:37–44. doi: 10.1159/000117527
96. Nardi AE, Freire RC, Valença AM, Amrein R, de Cerqueira ACR, Lopes FL, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. (2010) 30:290–3. doi: 10.1097/JCP.0b013e3181dcb2f3
97. Colrain IM, Nicholas CL, Baker FC. Alcohol and the sleeping brain. Handb Clin Neurol. (2014) 125:415–31. doi: 10.1016/B978-0-444-62619-6.00024-0
98. Gresham SC, Webb WB, Williams RL. Alcohol and caffeine: effect on inferred visual dreaming. Science. (1963) 140:1226–7. doi: 10.1126/science.140.3572.1226
99. Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. (2013) 37:539–49. doi: 10.1111/acer.12006
100. Ebrahim I, Fenwick P, Williams AJ, Shapiro C. Alcohol and sleep review: sound statistics and valid conclusions. Alcohol Clin Exp Res. (2015) 39:944–6. doi: 10.1111/acer.12708
101. Pressman MR, Grunstein RR, Mahowald MW, Schenck CH, Montplaisir JY, Bornemann MC, et al. Alcohol and sleep review: flawed design, methods, and statistics cannot support conclusions. Alcohol Clin Exp Res. (2015) 39:941–3. doi: 10.1111/acer.12712
102. Cernovsky ZZ. MMPI and nightmares in male alcoholics. Percept Mot Skills. (1985) 61:841–2. doi: 10.2466/pms.1985.61.3.841
103. Steinig J, Foraita R, Happe S, Heinze M. Perception of sleep and dreams in alcohol-dependent patients during detoxication and abstinence. Alcohol Alcohol. (2011) 46:143–7. doi: 10.1093/alcalc/agq087
104. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. (2017) 19:23. doi: 10.1007/s11920-017-0775-9
105. Page F, Coleman G, Conduit R. The effect of transdermal nicotine patches on sleep and dreams. Physiol Behav. (2006) 88:425–32. doi: 10.1016/j.physbeh.2006.04.009
106. Polini F, Principe R, Scarpelli S, Clementi F, de Gennaro L. Use of varenicline in smokeless tobacco cessation influences sleep quality and dream recall frequency but not dream affect. Sleep Med. (2017) 30:1–6. doi: 10.1016/j.sleep.2016.11.002
107. Waltman SH, Shearer D, Moore BA. Management of post-traumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. (2018) 20:108. doi: 10.1007/s11920-018-0971-2
108. George KC, Kebejian L, Ruth LJ, Miller CWT, Himelhoch S. Meta-analysis of the efficacy and safety of prazosin versus placebo for the treatment of nightmares and sleep disturbances in adults with posttraumatic stress disorder. J Trauma Dissoc. (2016) 17:494–510. doi: 10.1080/15299732.2016.1141150
109. Yücel DE, van Emmerik AAP, Souama C, Lancee J. Comparative efficacy of imagery rehearsal therapy and prazosin in the treatment of trauma-related nightmares in adults: a meta-analysis of randomized controlled trials. Sleep Med Rev. (2020) 50:101248. doi: 10.1016/j.smrv.2019.101248
Keywords: dream, nightmare, methodology, dream report frequency, experimental control
Citation: Putois B, Leslie W, Asfeld C, Sierro C, Higgins S and Ruby P (2020) Methodological Recommendations to Control for Factors Influencing Dream and Nightmare Recall in Clinical and Experimental Studies of Dreaming. Front. Neurol. 11:724. doi: 10.3389/fneur.2020.00724
Received: 25 October 2019; Accepted: 15 June 2020;
Published: 15 September 2020.
Edited by:
S. R. Pandi-Perumal, Somnogen Canada Inc., CanadaReviewed by:
Bjoern Rasch, Université de Fribourg, SwitzerlandJoseph De Koninck, University of Ottawa, Canada
Copyright © 2020 Putois, Leslie, Asfeld, Sierro, Higgins and Ruby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Putois, YmVuamFtaW4ucHV0b2lzQHVuaWRpc3RhbmNlLmNo
 Benjamin Putois
Benjamin Putois Wendy Leslie3
Wendy Leslie3 Claire Asfeld
Claire Asfeld Caroline Sierro
Caroline Sierro Susan Higgins
Susan Higgins Perrine Ruby
Perrine Ruby