
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 17 July 2020
Sec. Movement Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00547
This article is part of the Research Topic Orofacial Functions: From Neural Mechanisms to Rehabilitation View all 50 articles
The effect of deep brain stimulation (DBS) on swallowing function in movement disorders is unclear. Here, we systematically reviewed this topic by searching keywords following PICOS strategy of problem (swallowing or swallow or dysphagia or aspiration) and intervention (deep brain stimulation, or DBS) in the PubMed and Web of Science in English in April 2020, with comparators [subthalamic nucleus (STN), globus pallidus interna (GPi), ventralis intermedius, (ViM), post-subthalamic area, or caudal zona incerta (PSA/cZi); ON/OFF DBS state/settings, ON/OFF medication state, Parkinson's disease (PD), dystonia, tremor], outcomes (swallowing function measures, subjective/objective) and study types (good quality original studies) in mind. We found that STN DBS at usual high-frequency stimulation could have beneficial effect (more so on subjective measures and/or OFF medication), no effect, or detrimental effect (more so on objective measures and/or ON medication) on swallowing function in patients with PD, while low-frequency stimulation (LFS) could have beneficial effect on swallowing function in patients with freezing of gait. GPi DBS could have a beneficial effect (regardless of medication state and outcome measures) or no effect, but no detrimental effect, on swallowing function in PD. GPi DBS also has beneficial effects on swallowing function in majority of the studies on Meige syndrome but not in other diseases with dystonia. PSA/cZi DBS rarely has detrimental effect on swallowing functions in patients with PD or tremor. There is limited information on ViM to assess. Information on swallowing function by DBS remains limited. Well-designed studies and direct comparison of targets are further needed.
Dysphagia, or impaired swallow function, is one of the two major causes of mortalities in Parkinson's disease (PD) (along with falls related to the loss of balance). Dysphagia usually does not respond well to dopaminergic medication treatment (1, 2). Although deep brain stimulation (DBS) has significant beneficial effects in PD patients with motor fluctuation, dyskinesia, or medication refractory tremor (3–7), it has less benefits in axial symptoms of balance, speech, and swallowing function. Some studies even raise concerns about worsening of the axial symptom after DBS, particularly with long-term DBS at the usual high-frequency stimulation (HFS) (8–13), while axial symptoms have been found to predict the mortality of PD patients with STN DBS (14). Low-frequency stimulation (LFS) has been reported to have beneficial effect on axial symptoms in patients with freezing of gait (FOG) at usual HFS (15–18). Most common DBS targets to treat PD are STN (subthalamic nucleus) or GPi (globus pallidus interna) (3–7). They both have a similar effect on motor function of PD, but different effects in non-motor symptoms, such as cognitive function and depression, with different extents in medication reduction after the surgery as well (5, 19). GPi also seems to have a better outcome on axial symptoms, particularly after more than 2-year stimulation compared to STN (12).
The effect of DBS on swallowing function has not been well-studied across various movement disorders and targets. There was a retrospective study on the effect of unilateral STN vs. unilateral GPi on swallowing function in PD patients, which demonstrated a better swallowing function in penetration–aspiration (PA) scores on the videofluoroscopic swallow study (VFSS) in GPi compared to STN at medication OFF status, although there was a difference in baseline swallowing function between these two groups (20). LFS of STN was found to have beneficial effect on dysphagia compared to HFS in patients with FOG refractory to usual HFS of STN (16, 17). DBS targeting the post-subthalamic area and caudal zona incerta (PSA/cZi) was thought to be associated with fewer side effects compared to ventralis intermedius (ViM) or STN (21), including the swallowing function (22–24). GPi DBS has also been used to treat various dystonia (25–28), including Meige syndrome (29–32), and its effect on the swallowing function is also of interest to review compared to that in PD.
Besides diseases and targets, ON/OFF DBS state and stimulation frequencies, ON/OFF medication state, outcome measures for swallowing function (subjective questionnaires or scales vs. objective assessments, such as VFSS), and study designs (randomized double blind vs. open label retrospective or prospective) could also affect the swallowing function.
There was only one review article specifically focusing on the effect of DBS on swallowing function comparing different targets in the literature, mainly on unilateral GPi to STN DBS in patients with PD (33), which was published about 7 years ago. Therefore, it is necessary to have a comprehensive review with updated information on the effect of DBS on swallowing function covering various targets and movement disorders to reflect recent advances in the field, which will help guide our clinical practice in applying DBS for movement disorders.
We systematically searched the PubMed and the Web of Science in April 2020 for all available publications in English by keywords following PICOS concepts: problem = (dysphagia or swallowing or swallow or aspiration) and intervention = (DBS or deep brain stimulation) to include all pertinent articles, with comparators [subthalamic nucleus (STN), globus pallidus interna (GPi), ventralis intermedius (ViM), post-subthalamic area or caudal zona incerta (PSA/cZi), ON/OFF DBS state/settings (ON/OFF) medication state; Parkinson's disease (PD), dystonia, tremor], outcomes (swallowing function measures, subjective/objective) and study types (good quality original studies) in mind during the search. We followed PRISMA guideline for systematic review, and the flow chart of the literature search and selection process of the review is depicted in Figure 1 (34, 35). A total of 145 publications were found from PubMed and 169 from Web of Science. After removing the duplicate entries, screening was performed to narrow down to 177 articles by excluding reviews, comments, viewpoints, author responses, letters, book chapters, single case reports with insufficient information, and meeting abstracts. Then the full texts were assessed, and we removed studies without clear outcome measures on swallowing function by DBS. We finally identified 32 unique articles. We included DBS studies targeting STN, GPi, ViM, or PSA/cZi on patients with PD, various dystonia (including Meige syndrome), and essential tremor (ET), and compared swallowing function measures (subjective vs. objective) at ON/OFF DBS state under different settings (including stimulation frequencies), or post-operative to pre-operative baseline, at ON/OFF medication state. Basic demographics and types of study designs (retrospective vs. prospective, open vs. blind) were also taken into consideration in assessments.
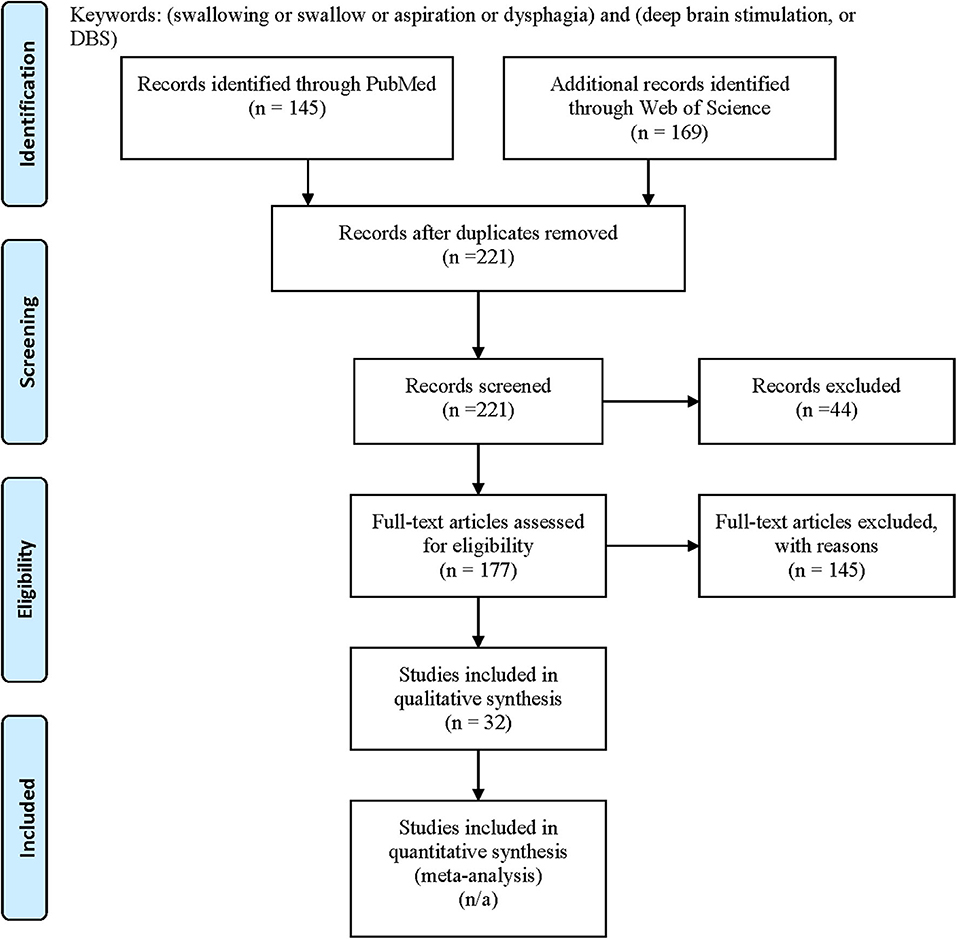
Figure 1. PRISMA flow diagram: literature search and selection with numbers of articles at each stage.
Each pertinent publication is listed in detail in Table 1, with information on references, diseases, DBS targets, basic demographics, study designs (randomized double blind vs. open label retrospective or prospective), outcome measures (subjective vs. objective measures) on swallowing functions at ON/OFF DBS or post-operational vs. pre-operational state under different DBS settings (if available) and ON/OFF medication state, and major conclusions. Among the 32 articles identified, 22 articles were on PD patients, with 19 targeting STN, 3 targeting GPi, and 3 targeting PSA/cZi, as some studies were targeting more than one target. There were six articles on Meige syndrome and five on non-Meige dystonia or dyskinesia (including primary generalized dystonia, segmental dystonia, and cerebral palsy), all targeting GPi. There was only one article on ET targeting PSA and none on ET targeting ViM on swallowing function. The majority of the studies used HFS of 125–210 Hz, but two studies used LFS of 60 Hz (16, 17). The assessments included subjective measures, such as swallowing questionnaires or scales, and objective measures, such as VFSS and fiberoptic endoscopic evaluation of swallowing (FEES).
We summarized the result as below, based on the diseases and targets.
STN DBS in patients with PD can have no effects (36–38). Kitashima et al. reported no improvement in swallowing function in 18 PD patients assessed by VFSS at ON medication state 6 months after the bilateral STN DBS (37). Olchik et al. found no change in swallowing function 6 months after bilateral STN DBS in 10 PD patients assessed by anamnesis, functional oral intake scale, and clinical swallowing function (38).
STN DBS in patients with PD can also have detrimental effects on the swallowing function. STN DBS impaired the jaw opening and closing velocities by scales 6 months after DBS compared to baseline regardless of ON/OFF medication state in a randomized double blind study in 14 patients with bilateral STN DBS (39). Xu et al. did not find any improvement on swallowing function based on the item on Unified Parkinson's Disease Rating Scale (UPDRS) Part II in 85 PD patients assessed on an average of 4.9 years after STN DBS (mixed unilateral and bilateral STN DBS) at ON/OFF medication state (and the swallowing function was even worse ON DBS) (40). Troche et al. reported significantly worse in the PA score of VFSS in 14 PD 6 months after unilateral STN DBS at ON medication state (20). Kraus reported that at least three patients developed worsening dysphagia or new dysphagia after bilateral STN DBS in a group of 27 PD patients during a mean of 30 months follow-up, based on the assessment for adverse effect, with unclear medication state though (8). Add-on stimulation of substantia nigra reticular (SNr) to STN did not have beneficial effect (41). Worsening of the dysphagia could be related to the suboptimal placement of the DBS electrodes or suboptimal programming in some cases, as turning off or reprogramming of the DBS made the swallowing symptoms better or go away in these cases (42, 43).
Some studies even reported beneficial effects on the swallowing function but mostly at OFF medication status, on subjective measures, or at LFS. Ciucci et al. reported significantly improved pharyngeal composite score and transit time by VFSS in ON DBS compared to OFF DBS at OFF medication status in 14 PD patients assessed at least 3 months after STN DBS (44). Kulnef et al. reported a subjective improvement in a self-assessment of swallowing function, but not on objective FEES, at ON DBS compared to OFF DBS at ON medication state in 11 PD patients 6 and 12 months after STB DBS (a mixed bilateral and unilateral DBS) (36). A similar result was also reported by Silbergleit et al. in 14 PD patients 3 and 12 months after bilateral STN DBS assessed by VFSS who found subjective but not objective improvement in swallowing function at ON/OFF medication state (45). Zibetti et al. found improved salivation and swallowing function in 36 patients with PD and bilateral STN DBS at 12 and 24 months after DBS at OFF medication state but no difference at ON medication compared to the pre-operational state (although the levodopa dosage was also reduced then) (46). Lengerer et al. reported no clinically relevant influence of DBS on swallowing function using qualitative parameters in 18 PD patients with bilateral STN DBS, but quantitative parameters found improved pharyngeal parameters with ON DBS compared to preoperative condition or OFF DBS, mostly with fluid consistency (47). Krygowska-Wajs et al. reported a 50% improvement on dysphagia on the gastrointestinal dysfunction questionnaire in 20 PD patients, assessed 3 months after bilateral STN DBS at ON DBS but OFF medication state (48). Wolz et al. studied 34 PD patients at a median of 13 months after the bilateral STN DBS and found improved dysphagia in subjective visual analog (VA) scale at ON DBS compared to OFF DBS and OFF medication state (49). Xie et al. reported acute and short-term improvement of objective and subjective swallowing function on PD patients with bilateral STN DBS in randomized double blind crossover studies under LFS (60 Hz) compared to those under HFS (130 Hz) in patients with HFS and medication refractory FOG at ON medication state (16, 17). However, the long-term (more than a year) benefit of LFS on the swallowing function was not demonstrated (17).
Troche et al. performed a retrospective chart review in 33 PD patients, with unilateral GPi DBS in 19 and unilateral STN DBS in 14 patients, looking at PA score of VFSS and patient-reported swallowing-related quality of life (SWAL-QOL) before and 6 months after DBS (20). PA scores significantly worsened in STN but not in GPi DBS assessed at ON medication state. No change in SWAL-QOL score was found before and after the DBS in either group of patients. The GPi group patients had worse swallowing function than the STN group at baseline. Robertson et al. randomized the PD patients to STN or GPi in double-blind study in 14 PD with bilateral STN and 13 PD with bilateral GPi, assessed before (OFF medication vs. ON) and 6 months after DBS (OFF medication vs. ON medication and OFF DBS vs. ON DBS) on self-scaled and externally scaled jaw peak velocity (39). At OFF medication state, DBS in STN worsened, while GPi improved the jaw velocities after DBS compared to baseline. At ON medication state, the velocities in STN were worse than the baseline, but no difference in GPi. The authors concluded that there was no benefit of STN or GPi on jaw velocity in PD compared to the best medication therapy, and that STN could even be harmful.
The swallowing function of eight PD patients with bilateral cZi DBS was assessed before and after DBS by FEES and questionnaire (22). There was no clear-cut effect of DBS at 6 and 12 months on any of the swallowing parameters except for the pre-swallow spillage, which was slightly worse in the ON stimulation state 12 months after DBS, although the medication was cut down by one-third post-operatively. Sundstedt et al. found no significant difference in SWAL-QOL score and VA scale score 12 months after the DBS at ON medication state in nine PD patients with bilateral cZi (23). Sundstedt et al. also did a prospective longitudinal study on 14 PD patients with bilateral cZi, extending their previous report on swallowing function, before and after DBS at ON medications and ON DBS vs. OFF DBS state by FEES (24). They found that cZi DBS did not have a negative impact on swallowing function, with no changes on PA scores, pharyngeal residual or premature spillage, although the medication was cut down by one-third post-operatively.
Bilateral GPi DBS has been shown to improve the swallowing function in majority of the studies in patients with Meige syndrome, as demonstrated by improved Burke–Fahn–Masden Dystonia Rating Scale (BFMDRS) speech and swallowing scores in 12 patients who followed up to 38 months on average (29), in 11 patients who followed up for 23 months on average (30), in 6 patients who followed up to 60 months (31), and in 40 patients who followed up at 6, 12, and 24 months after surgery (32). There was one study by Limotai et al. in six patients with Meige syndrome, with one unilateral and five bilateral GPi, evaluated 6 and 12 months after DSB for Unified Dystonia Rating Scale (UDRS) and BFMDR speech and swallowing function, but they did not find improvement in speech and swallowing function in this cohort (50). Bilateral GPi also has been used in patients with 11 non-Meige dystonia patients and 9 Meige syndrome patients (51), with significantly improved swallowing and speech scores in BFMDR up to 36 months after the DBS. Bilateral GPi also has been used in primary generalized dystonia and segmental dystonia patients (25–27), and dyskinetic cerebral palsy patients (28), but no changes or just slightly worsening in speech and swallowing function after DBS compared to baseline were reported.
There is no specifically designed study on the evaluation of dysphagia in ET by ViM or PSA/cZi DBS, although transient mild dysphagia after the DBS implantation surgery was reported, which usually resolved within several weeks (21, 52).
The majority of the studies were open label, retrospective or prospective, small-size studies, with subjective and/or objective assessments of swallowing function, at ON DBS compared to OFF DBS and ON/OFF dopaminergic medication state. There were only a few prospective randomized double blind studies (16, 17, 39), a few on comparing different targets (20, 39), and a few on comparing different frequency stimulations (16, 17). Most studies used bilateral targets although some were unilateral or mixed targets, as bilateral DBS is more likely to affect the axial symptoms, including dysphagia. Some of them were not fairly compared, as there were reduced dopaminergic medications post-operatively. Although the medications probably would not have a major impact on the objective swallowing functions (1, 2), beneficial effect of dopaminergic medication was also reported in a small proportion of patients (53). Taking dopaminergic medications could also affect the subjective measure with overall improvement of the parkinsonism. Therefore, it probably could explain why some studies showed improved swallowing function at subjective measures but not objective measures at ON medication state, and why the beneficial effect of DBS is more appreciated at OFF medication state or less appreciated at ON medication state.
We found that STN DBS at usual HFS could have beneficial effect (more so on subjective measures of scales, questionnaires, or swallowing item in UPDRS-II, and/or OFF medication state), no effect, or detrimental effect (more so on objective measures of VFSS or FEES, and/or ON medication state) on swallowing function in patients with PD. The effect of LFS stimulation on FOG has been consistently reported positively by many studies, as summarized in a review article (18). However, there have been only a few studies addressing its effect on dysphagia. Two studies of randomized double blinded crossover prospective studies in the short- and long- term effects did find significant benefit of LFS on acute and short-term studies (16, 17), but not the long-term benefits (17), although the long-term effect remains unclear given the small sample size and sub-clinical dysphagia in participants, which could limit the power to detect the potential difference. These studies were conducted at ON medication state in bilateral STN DBS patients with refractory FOG to HFS; hence, the beneficial effect should not necessarily be generalized to the whole PD population.
GPi DBS seems more likely to improve the swallowing function or process compared to the STN DBS, more so at OFF medication state (20, 39). In contrast to STN DBS, GPi DBS does not have detrimental effect on swallowing function or process at ON medication state (20, 39). Even though the non-matched baseline swallowing function in the two groups, and the retrospective and non-randomized design in assigning the targets could all affect the interpretation of the favorable PA scores in unilateral GPi compared to STN DBS (20), similar results were also obtained in a randomized, double-blind study comparing the effect of bilateral GPi to bilateral STN DBS on jaw velocity (39), suggesting that GPi DBS is probably more favorable than STN DBS in overall swallowing function for PD patients, particularly at OFF medication state. Although there is no benefit of STN or GPi DBS on swallowing function in PD compared to the best medication therapy (at ON medication state), STN DBS could even be harmful at ON medication state, based on limited studies available so far.
Targeting GPi seemed to have positive results on Meige syndrome in the majority of the studies (29–32). One of the possibilities behind the benefit is the direct effect on the pharyngeal and laryngeal dystonia by GPi, which could help to improve dysphagia symptoms. There was no study on using STN in Meige syndrome and other dystonia on dysphagia. Hence, it is not certain if targeting STN would have similar benefit, as STN has also been found to be beneficial to dystonia in PD (54). There is no beneficial effect of GPi DBS on dysphagia in patients with primary generalized dystonia, segmental dystonia, and dyskinesic cerebral palsy patients, and there rarely is worsening effect either (25–28).
The PSA and cZi are relatively new targets. They have the potential to provide more efficient stimulations but fewer side effects due to their anatomic characteristics, with the fibers from both the basal ganglia and cerebellar merging together at the PSA/cZi area, and studies so far found that PSA/cZi DBS rarely has a detrimental effect on swallowing functions in patients with PD or tremor (21, 55). There has been limited information on the effect of ViM DBS on swallowing function to assess so far.
In summary, we found that STN DBS at usual HFS could have beneficial effect (more so on subjective measures and/or OFF medication state), no effect, or detrimental effect (more so on objective measures and/or ON medication state) on swallowing function in patients with PD, while LFS of STN could have beneficial effect on swallowing functions in PD patients with FOG refractory to HFS. GPi DBS could have a beneficial effect (regardless of medication state, and subjective or objective measures), or no effect (more so at ON medication state), but no detrimental effect (in contrast to STN DBS, even at ON medication state) on swallowing function in PD, suggesting that GPi DBS could be probably more favorable than STN DBS in overall swallowing function for PD patients, particularly at OFF medication state. GPi DBS also has beneficial effects on swallowing function in the majority of the studies on Meige syndrome but no beneficial effect on swallowing function in other dystonia. Stimulation of PSA/cZi rarely has detrimental effect on swallowing functions. The effect of ViM on swallowing function in ET patients is too limited to assess. Overall, most of them are retrospective, open label, small-size studies, with medication reduction post-operatively. There are only a few randomized, double blind studies, a few on direct comparisons among targets or between stimulation frequencies. The overall evidence levels of these studies are low, ranging from IV to III. Information on swallowing function by DBS remains limited. Well-designed studies and direct comparison of targets and stimulating parameters are further needed to gain more insights on the effect of DBS on swallowing function in movement disorders.
All datasets analyzed for this study are included in the article and the Table 1.
KT and TX initiated the study and made the study PRISMA compatible systematic review. HY, SQ, LB, and TX performed search and initial writeup. All authors edited and worked on the submitted manuscript.
KT was partially supported by NIH R03DE028395. TX was partially supported by NIH U01 project and National Parkinson's Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Fuh J-L, Lee R-C, Wang S-J, Lin C-H, Wang P-N, Chiang J-H, et al. Swallowing difficulty in Parkinson's disease. Clin Neurol Neurosurg. (1997) 99:106–12. doi: 10.1016/S0303-8467(97)80006-6
2. Hunter PC, Crameri J, Austin S, Woodward MC, Hughes AJ. Response of Parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry. (1997) 63:579–83. doi: 10.1136/jnnp.63.5.579
3. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. (2006) 355:896–908. doi: 10.1056/NEJMoa060281
4. Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. (2009) 301:63–73. doi: 10.1001/jama.2008.929
5. Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. (2010) 362:2077–91. doi: 10.1056/NEJMoa0907083
6. Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. (2010) 9:581–91. doi: 10.1016/S1474-4422(10)70093-4
7. Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. (2013) 368:2038. doi: 10.1056/NEJMc1303485
8. Krause M, Fogel W, Mayer P, Kloss M, Tronnier V. Chronic inhibition of the subthalamic nucleus in Parkinson's disease. J Neurol Sci. (2004) 219:119–24. doi: 10.1016/j.jns.2004.01.004
9. Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord. (2010) 25:578–86. doi: 10.1002/mds.22735
10. Østergaard K, Aa Sunde N. Evolution of Parkinson's disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord. (2006) 21:624–31. doi: 10.1002/mds.20776
11. Romito LM, Contarino MF, Vanacore N, Bentivoglio AR, Scerrati M, Albanese A, et al. Replacement of dopaminergic medication with subthalamic nucleus stimulation in Parkinson's disease: long-term observation. Mov Disord. (2009) 24:555–61. doi: 10.1002/mds.22390
12. St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. (2010) 75:1292–9. doi: 10.1212/WNL.0b013e3181f61329
13. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto J-L, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. (2005) 128:2240–9. doi: 10.1093/brain/awh571
14. Lau B, Meier N, Serra G, Czernecki V, Schuepbach M, Navarro S, et al. Axial symptoms predict mortality in patients with Parkinson disease and subthalamic stimulation. Neurology. (2019) 92:e2559–70. doi: 10.1212/WNL.0000000000007562
15. Moreau C, Defebvre L, Destée A, Bleuse S, Clement F, Blatt JL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. (2008) 71:80–4. doi: 10.1212/01.wnl.0000303972.16279.46
16. Xie T, Vigil J, MacCracken E, Gasparaitis A, Young J, Kang W, et al. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology. (2015) 84:415–20. doi: 10.1212/WNL.0000000000001184
17. Xie T, Bloom L, Padmanaban M, Bertacchi B, Kang W, MacCracken E, et al. Long-term effect of low frequency stimulation of STN on dysphagia, freezing of gait and other motor symptoms in PD. J Neurol Neurosurg Psychiatry. (2018) 89:989–94. doi: 10.1136/jnnp-2018-318060
18. Xie T, Padmanaban M, Bloom L, MacCracken E, Bertacchi B, Dachman A, et al. Effect of low versus high frequency stimulation on freezing of gait and other axial symptoms in Parkinson patients with bilateral STN DBS: a mini-review. Transl Neurodegener. (2017) 6:13. doi: 10.1186/s40035-017-0083-7
19. Ramirez-Zamora A, Hess CW, Nelson DR. Is interferon therapy for hepatitis c infection a treatable risk factor for parkinson disease? JAMA Neurol. (2019) 76:1006–7. doi: 10.1001/jamaneurol.2019.1377
20. Troche MS, Brandimore AE, Foote KD, Morishita T, Chen D, Hegland KW, et al. Swallowing outcomes following unilateral STN vs GPi surgery: a retrospective analysis. Dysphagia. (2014) 29:425–31. doi: 10.1007/s00455-014-9522-0
21. Xie T, Bernard J, Warnke P. Post subthalamic area deep brain stimulation for tremors: a mini-review. Transl Neurodegener. (2012) 1:20. doi: 10.1186/2047-9158-1-20
22. Sundstedt S, Olofsson K, van Doorn J, Linder J, Nordh E, Blomstedt P, et al. Swallowing function in Parkinson's patients following Zona Incerta deep brain stimulation. Acta Neurol Scand. (2012) 126:350–6. doi: 10.1111/j.1600-0404.2012.01658.x
23. Sundstedt S, Nordh E, Linder J, Hedström J, Finizia C, Olofsson K, et al. Swallowing quality of life after zona incerta deep brain stimulation. Ann Otol Rhinol Laryngol. (2017) 126:110–6. doi: 10.1177/0003489416675874
24. Sundstedt S, Holmén L, Rova E, Linder J, Nordh E, Olofsson K, et al. Swallowing safety in Parkinson's disease after zona incerta deep brain stimulation. Brain Behav. (2017) 7:e00709. doi: 10.1002/brb3.709
25. Danielsson A, Carecchio M, Cif L, Koy A, Lin J-P, Solders G, et al. Pallidal deep brain stimulation in DYT6 dystonia: clinical outcome and predictive factors for motor improvement. J Clin Med. (2019) 8:2163. doi: 10.3390/jcm8122163
26. Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider G-H, Poewe W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. (2006) 355:1978–90. doi: 10.1056/NEJMoa063618
27. Krause P, Völzmann S, Ewert S, Kupsch A, Schneider GH, Kühn AA, et al. Long-term effects of bilateral pallidal deep brain stimulation in dystonia: a follow-up between 8 and 16 years. J Neurol. (2020) 267:1622–31. doi: 10.1007/s00415-020-09745-z
28. Koy A, Pauls KAM, Flossdorf P, Becker J, Schönau E, Maarouf M, et al. Young adults with dyskinetic cerebral palsy improve subjectively on pallidal stimulation, but not in formal dystonia, gait, speech and swallowing testing. Eur Neurol. (2014) 72:340–8. doi: 10.1159/000360984
29. Reese R, Gruber D, Schoenecker T, Bäzner H, Blahak C, Capelle HH, et al. Long-term clinical outcome in meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. (2011) 26:691–8. doi: 10.1002/mds.23549
30. Ghang JY, Lee MK, Jun SM, Ghang CG. Outcome of pallidal deep brain stimulation in meige syndrome. J Korean Neurosurg Soc. (2010) 48:134–8. doi: 10.3340/jkns.2010.48.2.134
31. Sobstyl M, Brzuszkiewicz-Kuzmicka G, Zaczynski A, Pasterski T, Aleksandrowicz M, Zabek M, et al. Long-term clinical outcome of bilateral pallidal stimulation for intractable craniocervical dystonia (Meige syndrome) Report of 6 patients. J Neurol Sci. (2017) 383:153–7. doi: 10.1016/j.jns.2017.10.017
32. Tian H, Yu Y, Zhen X, Zhang L, Yuan Y, Zhang B, et al. Long-term efficacy of deep brain stimulation of bilateral globus pallidus internus in primary meige syndrome. Stereotact Funct Neurosurg. (2019) 97:356–61. doi: 10.1159/000504861
33. Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson's disease: a systematic review. Parkinsonism Relat Disord. (2013) 19:783–8. doi: 10.1016/j.parkreldis.2013.05.001
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
35. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
36. Kulneff L, Sundstedt S, Olofsson K, van Doorn J, Linder J, Nordh E, et al. Deep brain stimulation – effects on swallowing function in Parkinson's disease. Acta Neurol Scand. (2013) 127:329–36. doi: 10.1111/ane.12019
37. Kitashima A, Umemoto G, Tsuboi Y, Higuchi M, Baba Y, Kikuta T, et al. Effects of subthalamic nucleus deep brain stimulation on the swallowing function of patients with Parkinson's disease. Parkinsonism Relat Disord. (2013) 19:480–2. doi: 10.1016/j.parkreldis.2012.10.023
38. Olchik MR, Ghisi M, Ayres A, Schuh AFS, Oppitz PP, Rieder CR de M, et al. The impact of deep brain stimulation on the quality of life and swallowing in individuals with Parkinson's Disease. Int Arch Otorhinolaryngol. (2018) 22:125–9. doi: 10.1055/s-0037-1603466
39. Robertson LT, St George RJ, Carlson-Kuhta P, Hogarth P, Burchiel KJ, Horak FB, et al. Site of deep brain stimulation and jaw velocity in Parkinson disease. J Neurosurg. (2011) 115:985–94. doi: 10.3171/2011.7.JNS102173
40. Xu C, Zhuang P, Hallett M, Zhang Y, Li J, Li Y, et al. Parkinson's disease motor subtypes show different responses to long-term subthalamic nucleus stimulation. Front Hum Neurosci. (2018) 12:365. doi: 10.3389/fnhum.2018.00365
41. Pflug C, Nienstedt JC, Gulberti A, Müller F, Vettorazzi E, Koseki J-C, et al. Impact of simultaneous subthalamic and nigral stimulation on dysphagia in Parkinson's disease. Ann Clin Transl Neuron. (2020) 7:628–38. doi: 10.1002/acn3.51027
42. Fagbami OY, Donato AA. Stridor and dysphagia associated with subthalamic nucleus stimulation in Parkinson disease: case report. J Neurosurg. (2011) 115:1005–6. doi: 10.3171/2011.7.JNS11602
43. Troche MS, Brandimore AE, Hegland KW, Zeilman PR, Foote KD, Okun MS, et al. Tailored deep brain stimulation optimization for improved airway protective outcomes in Parkinson's disease. Interdiscip Neurosurg. (2016) 5:3–5. doi: 10.1016/j.inat.2016.03.003
44. Ciucci MR, Barkmeier-Kraemer JM, Sherman SJ. Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson's disease. Mov Disord. (2008) 23:676–83. doi: 10.1002/mds.21891
45. Silbergleit AK, LeWitt P, Junn F, Schultz LR, Collins D, Beardsley T, et al. Comparison of dysphagia before and after deep brain stimulation in Parkinson's disease. Mov Disord. (2012) 27:1763–8. doi: 10.1002/mds.25259
46. Zibetti M, Torre E, Cinquepalmi A, Rosso M, Ducati A, Bergamasco B, et al. Motor and nonmotor symptom follow-up in Parkinsonian patients after deep brain stimulation of the subthalamic nucleus. Eur Neurol. (2007) 58:218–23. doi: 10.1159/000107943
47. Lengerer S, Kipping J, Rommel N, Weiss D, Breit S, Gasser T, et al. Deep-brain-stimulation does not impair deglutition in Parkinson's disease. Parkinsonism Relat Disord. (2012) 18:847–53. doi: 10.1016/j.parkreldis.2012.04.014
48. Krygowska-Wajs A, Furgala A, Gorecka-Mazur A, Pietraszko W, Thor P, Potasz-Kulikowska K, et al. The effect of subthalamic deep brain stimulation on gastric motility in Parkinson's disease. Parkinsonism Relat Disord. (2016) 26:35–40. doi: 10.1016/j.parkreldis.2016.02.010
49. Wolz M, Hauschild J, Fauser M, Klingelhöfer L, Reichmann H, Storch A, et al. Immediate effects of deep brain stimulation of the subthalamic nucleus on nonmotor symptoms in Parkinson's disease. Parkinsonism Relat Disord. (2012) 18:994–7. doi: 10.1016/j.parkreldis.2012.05.011
50. Limotai N, Go C, Oyama G, Hwynn N, Zesiewicz T, Foote K, et al. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. J Neurol. (2011) 258:2069–74. doi: 10.1007/s00415-011-6075-0
51. Sensi M, Cavallo MA, Quatrale R, Sarubbo S, Biguzzi S, Lettieri C, et al. Pallidal stimulation for segmental dystonia: long term follow up of 11 consecutive patients. Mov Disord. (2009) 24:1829–35. doi: 10.1002/mds.22686
52. Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord. (2010) 25:1350–6. doi: 10.1002/mds.22758
53. Warnecke T, Oelenberg S, Teismann I, Hamacher C, Lohmann H, Ringelstein EB, et al. Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Mov Disord. (2010) 25:1239–45. doi: 10.1002/mds.23060
54. Ostrem JL, San Luciano M, Dodenhoff KA, Ziman N, Markun LC, Racine CA, et al. Subthalamic nucleus deep brain stimulation in isolated dystonia. Neurology. (2017) 88:25–35. doi: 10.1212/WNL.0000000000003451
Keywords: deep brain stimulation (DBS), dysphagia, swallowing function, subthalamic nucleus (STN), globus pallidus interna (GPi), post-subthalamic area (PSA), Parkinson's disease, movement disorders
Citation: Yu H, Takahashi K, Bloom L, Quaynor SD and Xie T (2020) Effect of Deep Brain Stimulation on Swallowing Function: A Systematic Review. Front. Neurol. 11:547. doi: 10.3389/fneur.2020.00547
Received: 10 February 2020; Accepted: 14 May 2020;
Published: 17 July 2020.
Edited by:
Steven Frucht, Mount Sinai Hospital, United StatesReviewed by:
Adolfo Ramirez-Zamora, University of Florida Health, United StatesCopyright © 2020 Yu, Takahashi, Bloom, Quaynor and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Xie, dHhpZUBuZXVyb2xvZ3kuYnNkLnVjaGljYWdvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.