- 1Department of Medicine and Pediatrics, Kilimanjaro Christian Medical Centre, Moshi, Tanzania
- 2Service de Neurologie, Centre Hospitalier Universitaire du Point “G”, Bamako, Mali
- 3Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 4Department of Neuroscience, Mayo Clinic, Jacksonville, FL, United States
- 5Department of Clinical Genomics, Mayo Clinic, Jacksonville, FL, United States
- 6Division of Neurology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 7Department of Medicine, College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria
The burden of Parkinson's disease (PD) is becoming increasingly important in the context of an aging African population. Although PD has been extensively investigated with respect to its environmental and genetic etiology in various populations across the globe, studies on the African continent remain limited. In this Perspective article, we review some of the obstacles that are limiting research and creating barriers for future studies. We summarize what research is being done in four sub-Saharan countries and what the key elements are that are needed to take research to the next level. We note that there is large variation in neurological and genetic research capacity across the continent, and many opportunities for unexplored areas in African PD research. Only a handful of countries possess appropriate infrastructure and personnel, whereas the majority have yet to develop such capacity. Resource-constrained environments strongly determines the possibilities of performing research locally, and unidirectional export of biological samples and genetic data remains a concern. Local-regional partnerships, in collaboration with global PD consortia, should form an ethically appropriate solution, which will lead to a reduction in inequality and promote capacity building on the African continent.
Introduction
Mirroring global trends, life expectancy on the African continent has greatly increased in recent decades, paralleling economic growth, and related to a decline in a number of infectious diseases. The World Health Organization reports that overall life expectancy at birth in Africa is currently 61.2 years (1). As a result of improved control of the HIV epidemic, malaria and diarrhoeal diseases, non-communicable disorders (NCD) have become increasingly important as a public health concern for Africa (2), which is a global pattern observed initially in higher income countries. The common movement disorder, Parkinson's disease (PD), is one example of an important neurological NCD in the aging African population.
Exploring the epidemiology and genetic etiology of NCDs is essential in order to dissect out patterns of disease susceptibility, environmental clustering, and medication responses at a population level, as well as on an individual basis. In this Perspective article, we provide an overview of the main difficulties that we consider to be hindering the progress of PD epidemiological and genetic research on the African continent, and the solutions needed. We also provide summaries of the healthcare infrastructure in four African countries [represented by the four neurologists listed as authors; Tanzania (MD), Nigeria (MK), Mali (TC), and South Africa (JC)], and of the research done in these countries to illustrate the local obstacles but also the potential global opportunities for the field.
Obstacles to PD Research on the African Continent
Limited Number of Neurologists
Epidemiological patterns are heavily dependent on the power of detection of disease, which is an interplay of diagnostic factors as well as the accuracy of determining the correct diagnosis in the general population. In the case of PD, the first and foremost factor in this is the availability of neurologists. On the African continent, there is a wide discrepancy in the number of neurologists and medical facilities between different countries.
Some urban centers may have comprehensive neurological and auxiliary services available, consisting of neurologists, neurosurgeons, neurophysiologists, and related equipment (such as electro-encephalography, electromyography, and nerve conduction studies). However, even in these centers, accessibility to such services by the general population is limited due to financial restrictions and barriers related to cultural perception of disease. Reviewing the situation in Africa as a whole, neurological services are either scarce or not available, as illustrated by a survey conducted in 2005 that included 11 countries that were entirely without neurological services (3). Currently, in most countries in Africa, there is still a dearth of neurologists, nurses, physiotherapists, and other allied professions due to limited training facilities for neurologists within Africa, as well as emigration of skilled personnel to more economically developed countries. Although, some research on the clinical and epidemiological aspect of PD has been conducted, genetics research of PD is limited in Africa, as a result of poor awareness and lack of facilities. The low number of scientific publications on PD mirrors the low density of neurological professionals (Figure 1). As can be seen in the figure, the Northern African Arabic countries bordering the Mediterranean Sea and South Africa at the tip of the continent are the two regions with better access to neurological surveillance and care than the remainder of Africa.
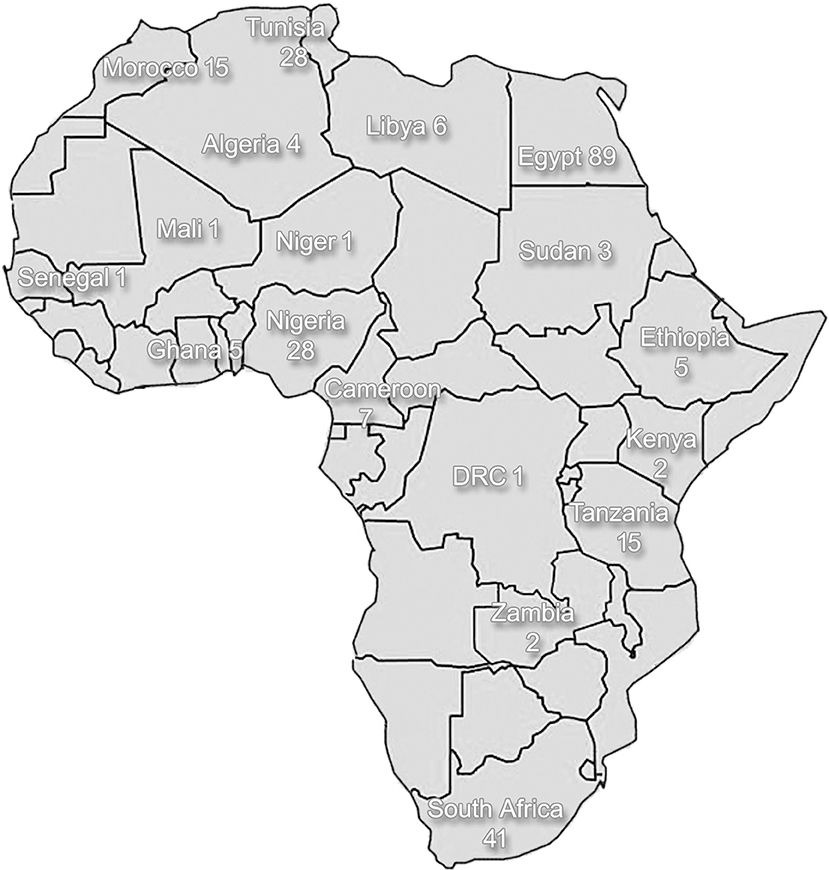
Figure 1. A schematic diagram of African countries indicating the number of published articles on Parkinson's disease per country (source: https://www.ncbi.nlm.nih.gov/pubmed/). The search was conducted in December 2019 and the term “Parkinson” was used in addition to the country name for all countries located on the African continent (Algeria-Zimbabwe); the ensuing results were then reviewed for being appropriate as a publication related to Parkinson's disease. Duration of search extended from 2019 until 1952.
Public Health Education and PD
A potential obstacle for access to neurological care may be preconceived beliefs that exist among the community about neurological conditions. Erroneous beliefs may arise from the absence of knowledge and education regarding a particular disorder, as well as culturally determined perceptions. Public health education is therefore of considerable importance. Absence of such education, or the cultural inappropriateness of educational content (for instance by direct translation of information leaflets and videos from other global regions) might lead not only to ongoing lack of recognition of medical disorders but also to missing out on the benefits of effective treatments. If one is not attuned to the specific culturally appropriate requirements of a region, the impression might arise that there is a resistance toward receiving educational information. However, with an approach adjusted to the specific ethnic, geographical, or religious needs of a target population, the same information may be better understood and therefore accepted.
Stigma Associated With PD
In a Northern Tanzanian door-to-door survey in a semi urban setting, it appeared that many people suffering from PD met with various misconceptions about the disorder (4). Similarly, in a study conducted in South Africa, there was lack of knowledge about PD, with half of the members of the community believing that patients with PD should not live within the community (5). Ideas about guilt, witchcraft, and presumed mental disease all attribute to stigmatization. Such factors delay or prevent correct diagnosis or access to appropriate treatment for PD. The Tanzanian setting does have access to basic neurological services close to the survey area (6), which highlights the fact that targeting a community's perception of disease is potentially as important as is the improvement of structural facilities such as neurology clinics, laboratory diagnostics, and brain imaging. When educational material for patients and their caregivers is made available by a direct translation of quality material available from websites such as the International Parkinson and Movement Disorder Society (MDS; www.movementdisorders.org), it is expected to correctly reflect currently available evidence-based information. However, whether its contents will actually appeal to groups other than those in high-income regions, is less clear. In addition to appropriate translation, stigma due to superstitious beliefs and misconception (4, 5) also needs to be addressed in the educational material in order address target populations respectfully and effectively.
Consequently, we believe that some of the main challenges for PD research faced on the African continent are:
• Stigma of a visible impairment and the perception that the disease may be caused by a curse or is a bad omen.
• Delay in diagnosis and treatment due to traditional medicine being used as a first step for the majority of patients outside urbanized regions.
• Low rate of healthcare insurance coverage preventing affordability of long-term treatment in chronic disorders such as PD.
• Denial of a positive family history of a possibly genetic condition so as to prevent discredit to individuals or their relatives.
PD Research in Four African Countries
In this section, we highlight the situation regarding PD research in four countries, Mali, Nigeria, South Africa, and Tanzania to illustrate the obstacles and the opportunities. A summary of the resources and infrastructure currently available for PD studies in each country is provided in Table 1. This table clearly shows the severe shortages of suitable resources, infrastructure and facilities in comparison to developed countries. However, despite this, high quality research has been done in these countries, as indicated below.
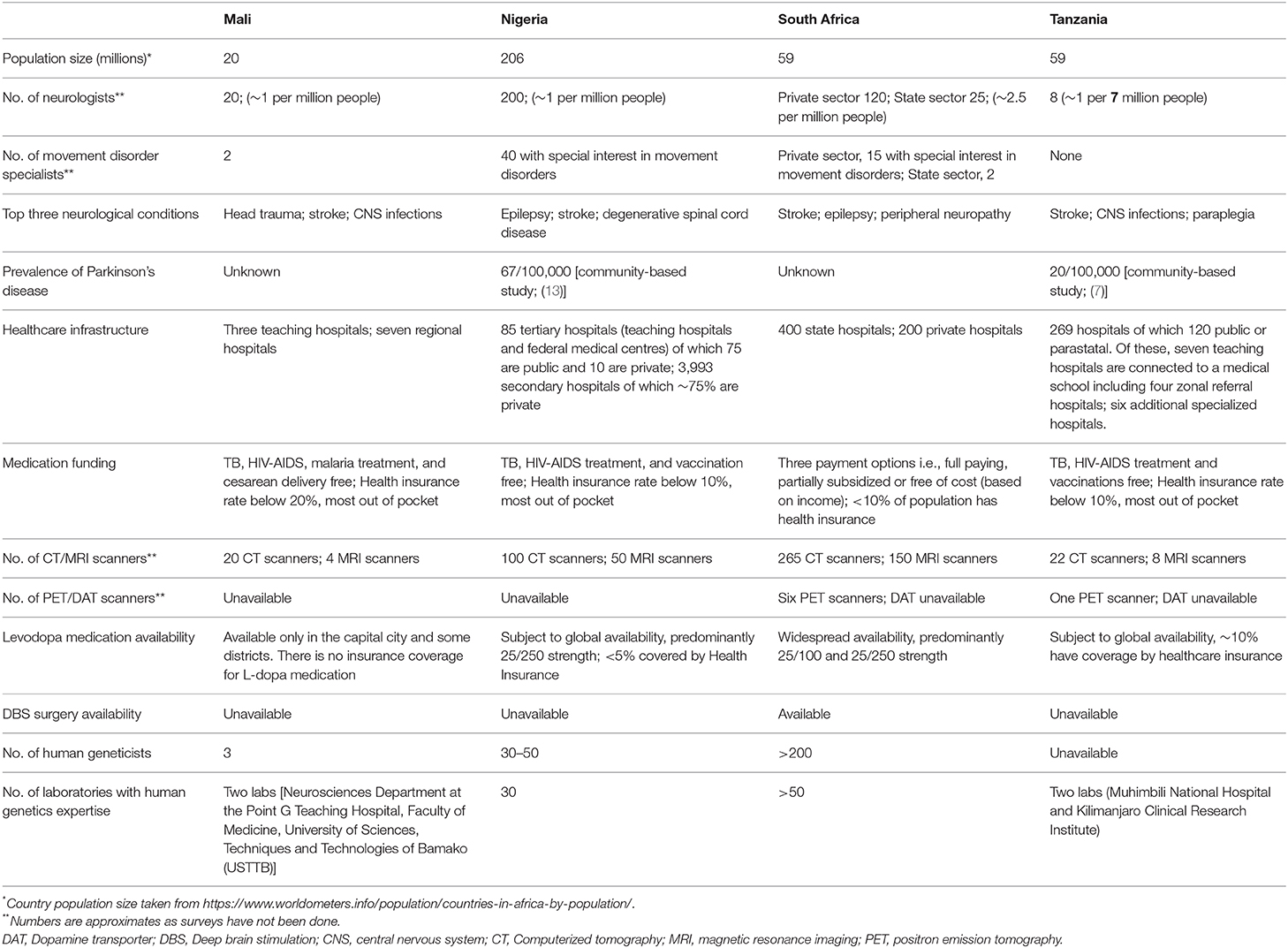
Table 1. Summary of healthcare and clinical resources available for the clinical management of Parkinson's disease in four sub-Saharan African countries.
Table 1 also shows the problems with treatment strategies for PD in Africa. In this setting, after clinically diagnosing parkinsonism, drug treatment usually starts with a levodopa/carbidopa trial. Dopamine agonists are unavailable in the majority of African countries. Treatment can be called unsuccessful when about one gram of levodopa/carbidopa daily for a number of weeks does not elicit a significant treatment response. Practically however, the high cost of the treatment may necessitate patients to terminate this titration prematurely, or to reduce dosage frequency to once daily or very low dosages. There will be a proportion of patients who would have responded better had there been no financial limitations. Physiotherapy is also a useful treatment modality, but is best given in limited sessions due to long travel distances and low resources. Physiotherapy in lower income regions is aimed at education and low frequency follow up: patients and relatives may attend for a few days consecutively, perform home exercises and return a number of months later.
Tanzania
Tanzania, situated in East Africa, is one of the few countries in Sub-Saharan Africa where door-to-door prevalence data on PD are available from a survey of a semi urban and rural area (7). This survey also examined perception of disease, including that PD is considered to be an age-related phenomenon, which does not require treatment, or that it may be a punishment for having done something wrong (4, 7). The research group who conducted the survey has been funding levodopa therapy for newly diagnosed patients identified from the survey, in addition to following up the patients (8), and also studying physiotherapy interventions (9). A nearby tertiary referral center in Moshi, at the foot of Mount Kilimanjaro, also has neurologists available for this patient population (6). However, a limited number of patients follow up to obtain levodopa maintenance therapy, illustrating that there are additional, obstacles to care in an African rural population over and above the availability of a neurologist.
A levodopa containing crop, Mucuna Pruriens, is presently being studied for its medicinal properties in Moshi. Its use as monotherapy or add-on medication in PD has proven to be successful in Bolivia and Ghana (10, 11). The crop is being grown, and will be roasted and ground at the hospital premises using readily available facilities since Moshi is known for its coffee industry, which uses the same procedures. The availability of locally sourced medication of this nature may also allow patients with chronic illness to grow their own medicine at home, and titrate it themselves for daily use. In the framework of the above study, an assessment on candidate and pharmacologically relevant genes (e.g., Catechol-O-methyltransferase; COMT) will be performed. To date, only one genetically confirmed PD kindred is known from the East African region, which was identified in North Tanzania and is due to a homozygous PRKN deletion (12).
Nigeria
Nigeria is the most populous and diverse nation in Africa with a growing population estimated to be over 200 million people, and home to many different ethnic groups speaking three major languages and over 250 other languages. Community based studies on the prevalence of PD obtained an age adjusted rate of 67 per 100,000 which is low compared to the frequency observed in African Americans (13). The clinical profile, etiology of Parkinsonism and PD and their complications have been described and are similar to the clinical profile in other regions of the world (14–16). Similarly, there have been studies on the non–motor features of neuropsychiatric impairment (17), cognitive impairment (18, 19), depression (20), gait instability (21), autonomic (22), gastrointestinal (23), and respiratory (24) involvement in PD. Studies to dissect the risk factors and etiology of PD among Nigerians include biochemical and pathological studies. Some authors observed the occurrence of Lewy bodies (25, 26), xenobiotics (27), and risk factors such as manganese among blacksmiths (28) and increased levels of trace metals (29). A few genetic studies have also been conducted and did not detect pathogenic mutations in PRKN (parkin), LRRK2, and ATXN3 (30–32).
The challenges to care include low numbers of health care personnel, poor access to care, late presentation, as well as lack of medicine availability (33). However, new technologies, particularly telemedicine, have been identified as a promising area to improve access to care, especially for patients in rural communities (33). Educational campaigns and awareness efforts to tackle misconceptions as well as a multidisciplinary team care approach at the community level are anticipated to improve access and quality of care (34, 35).
Mali
Mali is situated in the midst of the Sahara Desert between North African and sub-Saharan African countries. The demography is diverse and consists of Sub-Saharan ethnic groups living in the southern part of the country (black African origins) and nomadic racial groups (Arabic-Berber origins) living in the northern part of the country (36). The two ethnic groups share similar historic, cultural and religious traditions with each other, and there are high rates of consanguinity. These features are also shared with neighboring countries, namely the North African countries across the Sahara Desert and the sub-Saharan countries in the South. Almost all facilities and health care personnel in Mali are located in a geographic area representing <10% of the country, where only 14% of the population live (37). PD is not regarded as an urgent health priority when compared to the disease burden of infectious diseases and other NCDs. Long-term availability of medication, follow up and patient education are also lacking.
Due to a lack of trained movement disorders specialists and severe constraints in health care infrastructure, only two hospital-based studies of PD have been conducted in Mali. From January 2012 to November 2013, all cases of PD were collected using in-patient and out-patient visit data at Point G Hospital in Bamako, which is the main teaching hospital in Mali. Among the 8,372 patients seen at the Neurology Department, 60 patients (0.7%) had PD (38). Mostly, individuals aged 61–80 years were affected, the frequency of young onset cases was 12.2%, and a positive family history of PD was present in 7.3%. Another study done in 2016 revealed non-motor signs in 90% of all patients with PD (39). To date, there are no published genetic studies on PD patients from Mali.
South Africa
South Africa has been described as a “melting pot,” since the country is ethnically diverse due to its history, comprising people from a range of different ancestral backgrounds. South Africa has reasonably well-established healthcare and facilities for clinical management of PD but there are wide discrepancies in facilities between different provinces (largely as a legacy of the apartheid era) and between the urban and rural areas. The PD research group is based in Cape Town and was initiated in 2006. As the country has some of the best resources and infrastructure for human genetics studies on the continent, the focus of the PD research group is to study the genetic etiology of the different ethnic groups by establishing a DNA bank of clinically well-characterized PD patients. Initially, the group concentrated on familial and early onset PD, of all ethnic origins, but more recently, a focus has been on recruitment of South African patients of Black African ancestry. The group has identified pathogenic mutations, albeit at low frequencies, in all of the commonly associated PD genes, as elaborated on in the next section. Recently, a PTRHD1 mutation was identified in a Xhosa family with Parkinsonism and intellectual disability (40).
Genetics Of PD in African Populations
As has been highlighted by many previous reports, genetic studies on African populations have been very limited (35, 41–43). All of the published studies and their findings are summarized in Supplementary Table 1. On the African continent, most of the work has been done on patients from North African Arabic countries where the frequency of the LRRK2 G2019S mutation was reported to be as high as 41% of patients (44) due to the presence of genetic founder effects. A number of studies have been conducted in South Africa but the mutation detection rate has been low (32, 40, 45–55). The other studies have been done in Nigeria (30–32), Tanzania (12), Zambia (56), and Ghana (57) but for the vast majority of the countries in Africa, no genetic studies have been reported. This is a striking omission since African populations have the oldest genomes and the greatest genetic diversity in the world, and are therefore likely to reveal novel insights into disease mechanisms and pathways underlying PD (58).
Notably, findings conducted on LRRK2 and in particular, the G2019S mutation, have revealed interesting findings (Supplementary Table 1). Although common in North African Arabic populations, this mutation has not been identified in a single individual of Black African ancestry (30, 31, 49, 56, 57). A recent study conducted in South Africa found that 8 out of 647 patients screened were G2019S-carriers but all are of Ashkenazi Jewish origin except one (whose grandfather was German) (49). In a study on African Arabic patients in Tunisia, G2019S-carriers had similar PD symptoms to non-G2019S idiopathic PD cases but had a younger age at onset (AAO), a more benign phenotype and less cognitive impairment (59). In the South African study, the average AAO of the eight G2019S carriers was 56.6 years (SD 10.9), they had typical PD symptoms, and the homozygous mutation carrier did not exhibit a more severe disease to the others, although two patients had severe lower limb dystonia (49). It is plausible that patients of Black ancestry harbor other mutations in LRRK2 but this would require comprehensive screening of all 51 exons of this gene.
In summary, genetic studies in African populations have the potential to be of great benefit for PD research globally but have largely been unexplored.
Solutions Needed
In order to tackle the major challenges and obstacles to care of PD patients and to facilitate more research on this disorder, we believe the following issues need to be urgently addressed: lack of awareness and wrong perceptions, lack of trained personnel and the unavailability of drugs. To tackle the lack of awareness, awareness campaigns, and culture specific educational materials need to be developed in the local languages. Governments should improve awareness and reduce stigma through the use of radio and television jingles, adverts, and drama. Celebrities in each country who have the disease could be encouraged to talk about PD. This will improve awareness in the populations and may encourage patients to seek care earlier than they do currently. Observance of World Parkinson's Day (on 11 April annually) in healthcare institutions as well as obtaining sponsorship for other events such as quiz competitions and arts and cultural activities amongst school learners would be important. It has been observed that school learners can help to raise awareness of neurological disorders among the older members of the family (60).
In addition, in the short term, training of multidisciplinary teams comprising primary care physicians, and geriatricians as well as training of neurology nurses has been established in some parts of Africa and should be encouraged. The training of other team members such physiotherapists, occupational and speech therapists, and dieticians, should be promoted through local neurological and international societies such as the International Parkinson and Movement Disorder Society. A previous review suggested that tele-neurology can be deployed for training of health care workers through local, regional, and intercontinental networks (61).
To tackle the problem of the non-availability and un-affordability of drugs, a multisectorial strategy involving governments, pharmaceutical organizations, and other key stakeholders is necessary. It will be important for governments across Africa to include drugs for PD in the National Drug Formulary and to enroll patients in the health insurance programme. Incorporating PD care into health insurance systems will also enable patients to have access to neuroimaging (62). Neuroimaging facilities are becoming more widely available in Africa, but the cost of investigation is not affordable for most patients.
Finally, a holistic approach to care could be developed and implemented. The organization of PD support groups and clubs as well as organization of community-based rehabilitation will help in the care of patients living in rural communities.
Conclusions
The genetic and environmental diversity across the African continent provides a wealth of information and opportunities for research into the epidemiological patterns of PD occurrence, its clinical phenotypes and the genetic and environmental causal factors. However, there is large variation in neurological and genetic research capacity across the African continent, and many unexplored areas in African PD research. Some countries are relatively well-equipped, but most are severely resource-constrained. A low resource environment strongly limits the possibilities of performing research locally, and therefore unidirectional export of genetic material to scientifically more developed countries remains a major concern. Local and regional partnerships can form an ethically appropriate solution, reducing inequalities, and promoting capacity building. There is also the possibility for collaboration of these partnerships with global consortia studying the genetic etiology of PD [Genetic Epidemiology of Parkinson's Disease (GEoPD; www.geopd.net) and The International Parkinson Disease Genomics Consortium (IPDGC; www.pdgenetics.org)], to provide training in genomics and bioinformatics to African scientists.
Furthermore, a major concern for the adequate treatment of PD patients is the availability of affordable levodopa-containing medication. Various African countries have difficulties in obtaining levodopa for their patients largely due to manufacturing capacity and supply chain constraints, which has prompted the need for development of alternative therapies. The levodopa-containing plant Mucuna Pruriens thrives in the subtropics and can be grown by patients for their own use. Options such as these and others should be considered to provide African-based solutions to uniquely African problems when dealing with the emerging PD pandemic (63).
Author Contributions
MD contributed to design, conception, writing of the first draft, and editing of the manuscript. TC contributed to writing sections of the manuscript. SB and OR contributed to conception and editing. JC contributed to editing and compiled the figure. MK contributed to writing sections and editing. All authors reviewed and approved the final version of the manuscript.
Funding
The authors are financially supported by a grant (R21NS098862) from the National Institute of Neurological Disorders and Stroke, and Fogarty International Center, the National Institutes of Health, USA; the National Research Foundation of South Africa (Grant Number: 106052); the South African Medical Research Council (Self-Initiated Research Grant); and Stellenbosch University, South Africa. OR was funded by grants from the NIH, Michael J. Fox Foundation; the American Parkinson Disease Association (APDA) Mayo Clinic Information and Referral Center; Mayo Clinic APDA Center for Advanced Research and the Mayo Clinic Lewy Body Dementia Association (LBDA) Research Center of Excellence and Mayo Clinic LBD Functional Genomics Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors OR.
Acknowledgments
We also acknowledge the support of the NRF-DST Centre of Excellence for Biomedical Tuberculosis Research; South African Medical Research Council Centre for Tuberculosis Research; Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00512/full#supplementary-material
References
1. World, Health Statistics 2019 Monitoring Health for the SDG's 2019. Available online at: https://apps.who.int/iris/bitstream/handle/10665/311696/WHO-DAD-2019.1-eng.pdf (accessed April 11, 2020).
2. Howlett WP. Neurology in Africa : Clinical Skills and Neurological Disorders. Cambridge, UK: Cambridge University Press (2015).
3. Bower JH, Zenebe G. Neurologic services in the nations of Africa. Neurology. (2005) 64:412–5. doi: 10.1212/01.WNL.0000150894.53961.E2
4. Mshana G, Dotchin CL, Walker RW. We call it the shaking illness: perceptions and experiences of Parkinson's disease in rural northern Tanzania. BMC Public Health. (2011) 11:219. doi: 10.1186/1471-2458-11-219
5. Mokaya J, Gray WK, Carr J. Beliefs, knowledge and attitudes towards Parkinson's disease among a xhosa speaking black population in South Africa: a cross-sectional study. Park Relat Disord. (2017) 41:51–7. doi: 10.1016/j.parkreldis.2017.05.009
6. Dekker MCJ, Urasa SJ, Howlett WP. Neurological letter from Kilimanjaro. Pract Neurol. (2017) 17:412–6. doi: 10.1136/practneurol-2017-001693
7. Dotchin C, Msuya O, Kissima J, Massawe J, Mhina A, Moshy A, et al. The prevalence of Parkinson's disease in rural Tanzania. Mov Disord. (2008) 23:1567–672. doi: 10.1002/mds.21898
8. Dotchin C, Jusabani A, Walker R. Three year follow up of levodopa plus carbidopa treatment in a prevalent cohort of patients with Parkinson's disease in Hai, Tanzania. J Neurol. (2011) 258:1649–56. doi: 10.1007/s00415-011-5988-y
9. Rochester L, Rafferty D, Dotchin C, Msuya O, Minde V, Walker RW. The effect of cueing therapy on single and dual-task gait in a drug naïve population of people with Parkinson's disease in Northern Tanzania. Mov Disord. (2010) 25:906–11. doi: 10.1002/mds.22978
10. Cilia R, Laguna J, Cassani E, Cereda E, Pozzi NG, Isaias IU, et al. Mucuna pruriens in Parkinson disease: a double-blind, randomized, controlled, crossover study. Neurology. (2017) 89:432–8. doi: 10.1212/WNL.0000000000004175
11. Cilia R, Laguna J, Cassani E, Cereda E, Raspini B, Barichella M, et al. Daily intake of Mucuna pruriens in advanced Parkinson's disease: a 16-week, noninferiority, randomized, crossover, pilot study. Park Relat Disord. (2018) 49:60–6. doi: 10.1016/j.parkreldis.2018.01.014
12. Dekker MCJ, Suleiman JM, Bhwana D, Howlett WP, Rashid SM, van Minkelen R, et al. PRKN-related familial Parkinson's disease: first molecular confirmation from East Africa. Park Relat Disord. (2020) 73:14–5. doi: 10.1016/j.parkreldis.2020.02.014
13. Schoenberg BS, Osuntokun BO, Adeuja AOG, Bademosi O, Nottidge V, Anderson DW, et al. Comparison of the prevalence of Parkinson's disease in black populations in the rural United States and in rural Nigeria: door-to-door community studies. Neurology. (1988) 38:645–6. doi: 10.1212/WNL.38.4.645
14. Okubadejo NU, Ojo OO, Oshinaike OO. Clinical profile of parkinsonism and Parkinson's disease in Lagos, Southwestern Nigeria. BMC Neurol. (2010) 10:1. doi: 10.1186/1471-2377-10-1
15. Akinyemi RO. Epidemiology of parkinsonism and parkinson's disease in Sub-Saharan Africa: Nigerian profile. J Neurosci Rural Pract. (2012) 3:233–4. doi: 10.4103/0976-3147.102586
16. Ugoya SO, Agaba EI, Daniyam CA. Parkinsonism caused by adverse drug reactions: a case series. J Med Case Rep. (2011) 5:105. doi: 10.1186/1752-1947-5-105
17. Adebayo PB, Ajani AA, Adeniji OA, Akinyemi RO. Neuropsychiatric and parkinsonian manifestations of dementia: a case report in a Nigerian woman. Ann Afr Med. (2013) 12:46–8. doi: 10.4103/1596-3519.108252
18. Ojagbemi A. Relationship between cognitive dysfunction and behavioural symptoms in Nigerian patients with Parkinson's disease no dementia. J Parkinsons Dis. (2013) 3:293–300. doi: 10.3233/JPD-130210
19. Ojo O, Okubadejo N, Danesi M, Ojini F. Frequency of cognitive impairment and depression in Parkinson′s disease: a preliminary case-control study. Niger Med J. (2012) 53:65–70. doi: 10.4103/0300-1652.103544
20. Okunoye O, Asekomeh G. Depression among patients with Parkinson's disease in a Nigerian tertiary hospital. Niger Heal J. (2013) 13:96–103. Available online at: http://www.tnhjph.com/index.php/tnhj/article/view/98/92
21. Farombi TH, Owolabi MO, Ogunniyi A. Falls and their associated risks in Parkinson's disease patients in Nigeria. J Mov Disord. (2016) 9:160–5. doi: 10.14802/jmd.16011
22. Okubadejo NU, Danesi MA. Frequency and predictors of autonomic dysfunction in Parkinson's disease: a study of African patients in Lagos, Nigeria. Niger Postgrad Med J. (2004) 11:45–9.
23. Owolabi LF, Samaila AA, Sunmonu T. Gastrointestinal complications in newly diagnosed Parkinson's disease: a case-control study. Trop Gastroenterol. (2014) 35:227–31. doi: 10.7869/tg.221
24. Owolabi LF, Nagoda M, Babashani M. Pulmonary function tests in patients with Parkinson's disease: a case-control study. Niger J Clin Pract. (2016) 19:66–70. doi: 10.4103/1119-3077.173714
25. Muthane UB, Chickabasaviah YT, Henderson J, Kingsbury AE, Kilford L, Shankar SK, et al. Melanized nigral neuronal numbers in Nigerian and British individuals. Mov Disord. (2006) 21:1239–41. doi: 10.1002/mds.20917
26. Jendroska K, Olasode BJ, Daniel SE, Elliott L, Ogunniyi AO, Aghadiuno PU, et al. Incidental lewy body disease in black Africans. Lancet. (1994) 344:882–3. doi: 10.1016/S0140-6736(94)92854-1
27. Igbokwe E, Ogunniyi AO, Osuntokun BO. Xenobiotic metabolism in idiopathic Parkinson's disease in Nigerian Africans. East Afr Med J. (1993) 70:807–9.
28. Falope ZF, Osuntokun BO, Ogunniyi A. Risk factors for Parkinson's disease in Nigerian Africans: a case-control study. J Trop Geogr Neurol. (1992). 2:177–80. Available online at: https://eurekamag.com/research/002/484/002484190.php
29. Ogunrin O, Komolafe MA, Sanya OE, Osubor CC, Ajose OA Akande AA, et al. Trace metals in patients with Parkinson's disease: a multi-center case-control study of Nigerian patients. J Neurol Epidemiol. (2013) 1:31–8. doi: 10.12974/2309-6179.2013.01.01.4
30. Okubadejo NU, Rizig M, Ojo OO, Jonvik H, Oshinaike O, Brown E, et al. Leucine rich repeat kinase 2 (LRRK2) GLY2019SER mutation is absent in a second cohort of Nigerian Africans with Parkinson disease. PLoS ONE. (2018) 13:e0207984. doi: 10.1371/journal.pone.0207984
31. Okubadejo N, Britton A, Crews C, Akinyemi R, Hardy J, Singleton A, et al. Analysis of Nigerians with apparently sporadic Parkinson disease for mutations in LRRK2, PRKN and ATXN3. PLoS ONE. (2008) 3:e3421. doi: 10.1371/journal.pone.0003421
32. Oluwole OG, Kuivaniemi H, Abrahams S, Haylett WL, Vorster AA, van Heerden CJ, et al. Targeted next-generation sequencing identifies novel variants in candidate genes for Parkinson's disease in Black South African and Nigerian patients. BMC Med Genet. (2020) 21:23. doi: 10.1186/s12881-020-0953-1
33. Ben-Pazi H, Browne P, Chan P, Cubo E, Guttman M, Hassan A, et al. The promise of telemedicine for movement disorders: an interdisciplinary approach. Curr Neurol Neurosci Rep. (2018) 18:26. doi: 10.1007/s11910-018-0834-6
34. Oluwole OG, Kuivaniemi H, Carr JA, Ross OA, Olaogun MOB, Bardien S, et al. Parkinson's disease in Nigeria: a review of published studies and recommendations for future research. Park Relat Disord. (2019) 62:36–43. doi: 10.1016/j.parkreldis.2018.12.004
35. Williams U, Bandmann O, Walker R. Parkinson's disease in Sub-Saharan Africa: a review of epidemiology, genetics and access to care. J Mov Disord. (2018) 11:53–64. doi: 10.14802/jmd.17028
36. The World Population Prospects: 2015 Revision. (2015). Available online at: https://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html (accessed April 11, 2020).
37. Maiga Y, Albakaye M, Diango D, Kanikomo D, Seybou H, Minta I, et al. Modalites de prise en charge des accidents vasculaires cerebraux au Mali (Afrique de l'Ouest): une enquete de pratiques. Mali Médical. (2013) 28:30–5. Available online at: https://www.bibliosante.ml/handle/123456789/3065
38. Coulibaly T, Sissoko AS, Coulibaly T, Guida L, Mamadou K, Oumar GC. Clinical profile of Parkinson's disease at the neurology department of point G teaching hospital. Mov Disord. (2016) 31(Suppl 2). Available online at: https://www.mdsabstracts.org/abstract/clinical-profile-of-parkinsons-disease-at-the-neurology-department-ofpoint-g-teaching-hospital/ (accessed May 5, 2020).
39. Maïga B, Koné A, Landouré G, Coulibaly T, Sangaré M, Dembélé K, et al. Non-motor signs in patients with Parkinson's disease at the university hospital of point “G”, Mali. eNeurologicalSci. (2016) 3:35–6. doi: 10.1016/j.ensci.2016.02.001
40. Kuipers DJS, Carr J, Bardien S, Thomas P, Sebate B, Breedveld GJ, et al. PTRHD1 loss-of-function mutation in an african family with juvenile-onset parkinsonism and intellectual disability. Mov Disord. (2018) 33:1814–9. doi: 10.1002/mds.27501
41. Akinyemi RO, Owolabi MO, Oyeniyi T, Ovbiagele B, Arnett DK, Tiwari HK, et al. Neurogenomics in Africa: perspectives, progress, possibilities and priorities. J Neurol Sci. (2016) 366:213–23. doi: 10.1016/j.jns.2016.05.006
42. Blanckenberg J, Bardien S, Glanzmann B, Okubadejo NU, Carr JA. The prevalence and genetics of Parkinson's disease in sub-Saharan Africans. J Neurol Sci. (2013) 335:22–5. doi: 10.1016/j.jns.2013.09.010
43. Lekoubou A, Echouffo-Tcheugui JB, Kengne AP. Epidemiology of neurodegenerative diseases in sub-Saharan Africa: a systematic review. BMC Public Health. (2014) 14:653. doi: 10.1186/1471-2458-14-653
44. Lesage S, Dürr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, et al. LRRK2 G2019S as a cause of Parkinson's disease in North African arabs. N Engl J Med. (2006) 354:422–3. doi: 10.1056/NEJMc055540
45. Bardien S, Keyser R, Yako Y, Lombard DCJ. Molecular analysis of the parkin gene in South African patients diagnosed with Parkinson's disease. Park Relat Disord. (2009) 15:116–21. doi: 10.1016/j.parkreldis.2008.04.005
46. Bardien S, Marsberg A, Keyser R, Lombard D, Lesage S, Brice A, et al. LRRK2 G2019S mutation: frequency and haplotype data in South African Parkinson's disease patients. J Neural Transm. (2010) 117:847–53. doi: 10.1007/s00702-010-0423-6
47. Blanckenberg J, Ntsapi C, Carr JA, Bardien S. EIF4G1 R1205H and VPS35 D620N mutations are rare in Parkinson's disease from South Africa. Neurobiol Aging. (2014) 35:445.e1–3. doi: 10.1016/j.neurobiolaging.2013.08.023
48. Carr J, Guella I, Szu-Tu C, Boolay S, Glanzmann B, Farrer MJ, et al. Double homozygous mutations (R275W and M432V) in the ParkinGene associated with late-onset Parkinson's disease. Mov Disord. (2016) 31:423–5. doi: 10.1002/mds.26524
49. du Toit N, van Coller R, Anderson DG, Carr J, Bardien S. Frequency of the LRRK2 G2019S mutation in South African patients with Parkinson's disease. Neurogenetics. (2019) 20:215–8. doi: 10.1007/s10048-019-00588-z
50. Haylett WL, Keyser RJ, du Plessis MC, van der Merwe C, Blanckenberg J, Lombard D, et al. Mutations in the parkin gene are a minor cause of Parkinson's disease in the South African population. Park Relat Disord. (2012) 18:89–92. doi: 10.1016/j.parkreldis.2011.09.022
51. Keyser RJ, Lesage S, Brice A, Carr J, Bardien S. Assessing the prevalence of PINK1 genetic variants in South African patients diagnosed with early- and late-onset Parkinson's disease. Biochem Biophys Res Commun. (2010) 398:125–9. doi: 10.1016/j.bbrc.2010.06.049
52. Keyser RJ, Lombard D, Veikondis R, Carr J, Bardien S. Analysis of exon dosage using MLPA in South African Parkinson's disease patients. Neurogenetics. (2010) 11:305–12. doi: 10.1007/s10048-009-0229-6
53. Mahne AC, Carr JA, Bardien S, Schutte CM. Clinical findings and genetic screening for copy number variation mutations in a cohort of South African patients with Parkinson's disease. S Afr Med J. (2016) 106:623. doi: 10.7196/SAMJ.2016.v106i6.10340
54. Mahungu AC, Anderson DG, Rossouw AC, van Coller R, Carr JA, Ross OA, et al. Screening of the glucocerebrosidase (GBA) gene in South Africans of African ancestry with Parkinson's disease. Neurobiol Aging. (2020) 88:156.e11–4. doi: 10.1016/j.neurobiolaging.2019.12.011
55. van der Merwe C, Carr J, Glanzmann B, Bardien S. Exonic rearrangements in the known Parkinson's disease-causing genes are a rare cause of the disease in South African patients. Neurosci Lett. (2016) 619:168–71. doi: 10.1016/j.neulet.2016.03.028
56. Yonova-Doing E, Atadzhanov M, Quadri M, Kelly P, Shawa N, Musonda STS, et al. Analysis of LRRK2, SNCA, Parkin, PINK1, and DJ-1 in Zambian patients with Parkinson's disease. Park Relat Disord. (2012) 18:567–71. doi: 10.1016/j.parkreldis.2012.02.018
57. Cilia R, Sironi F, Akpalu A, Cham M, Sarfo FS, Brambilla T, et al. Screening LRRK2 gene mutations in patients with Parkinson's disease in Ghana. J Neurol. (2012) 259:569–70. doi: 10.1007/s00415-011-6210-y
58. Rotimi CN, Bentley AR, Doumatey AP, Chen G, Shriner D, Adeyemo A. The genomic landscape of African populations in health and disease. Hum Mol Genet. (2017) 26:225–36. doi: 10.1093/hmg/ddx253
59. Ben Romdhan S, Farhat N, Nasri A, Lesage S, Hdiji O, Ben Djebara M, et al. LRRK2 G2019S Parkinson's disease with more benign phenotype than idiopathic. Acta Neurol Scand. (2018) 138:425–31. doi: 10.1111/ane.12996
60. Komolafe MA, Olorunmoteni OE, Fehintola FO. Effect of health education on level of awareness and knowledge of Nigerian in-school adolescents on stroke and its risk factors. J Stroke Cerebrovasc Dis. (2020) 29:104757. doi: 10.1016/j.jstrokecerebrovasdis.2020.104757
61. Sarfo FS, Adamu S, Awuah D, Ovbiagele B. Tele-neurology in sub-Saharan Africa: a systematic review of the literature. J Neurol Sci. (2017) 380:196–9. doi: 10.1016/j.jns.2017.07.037
62. Okubadejo NU, Ojo OO, Wahab KW, Abubakar SA, Obiabo OY, Salawu FK, et al. A nationwide survey of Parkinson's disease medicines availability and affordability in Nigeria. Mov Disord Clin Pract. (2019) 16:27–33. doi: 10.1002/mdc3.12682
Keywords: Parkinson's disease, Africa, public health, awareness, epidemiology, genetics
Citation: Dekker MCJ, Coulibaly T, Bardien S, Ross OA, Carr J and Komolafe M (2020) Parkinson's Disease Research on the African Continent: Obstacles and Opportunities. Front. Neurol. 11:512. doi: 10.3389/fneur.2020.00512
Received: 31 January 2020; Accepted: 08 May 2020;
Published: 19 June 2020.
Edited by:
Chin-Hsien Lin, National Taiwan University Hospital, TaiwanReviewed by:
Taku Hatano, Juntendo University, JapanEng-King Tan, National Neuroscience Institute (NNI), Singapore
Copyright © 2020 Dekker, Coulibaly, Bardien, Ross, Carr and Komolafe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marieke C. J. Dekker, bWFyaWVrZUB6d2V0cy5jb20=
 Marieke C. J. Dekker
Marieke C. J. Dekker Toumany Coulibaly
Toumany Coulibaly Soraya Bardien
Soraya Bardien Owen A. Ross
Owen A. Ross Jonathan Carr
Jonathan Carr Morenikeji Komolafe
Morenikeji Komolafe