
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 19 May 2020
Sec. Neuroinfectious Diseases
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00498
This article is part of the Research TopicCOVID-19 in CNS and PNS: Basic and Clinical Focus on the Mechanisms of Infection and New Tools for the Therapeutic ApproachView all 26 articles
Objective: Review and integrate the neurologic manifestations of the Coronavirus Disease 2019 (COVID-19) pandemic, to aid medical practitioners who are combating the newly derived infectious disease.
Methods: We reviewed the clinical research, consisting of mainly case series, on reported neurologic manifestations of COVID-19. We also reviewed basic studies to understand the mechanism of these neurologic symptoms and signs.
Results: We included 79 studies for qualitative synthesis and 63 studies for meta-analysis. The reported neurologic manifestations were olfactory/taste disorders (35.6%), myalgia (18.5%), headache (10.7%), acute cerebral vascular disease (8.1%), dizziness (7.9%), altered mental status (7.8%), seizure (1.5%), encephalitis, neuralgia, ataxia, Guillain-Barre syndrome, Miller Fisher syndrome, intracerebral hemorrhage, polyneuritis cranialis, and dystonic posture.
Conclusions: Neurologic manifestations in COVID-19 may alert physicians and medical practitioners to rule in high-risk patients. The increasing incidence of olfactory/taste disorders, myalgia, headache, and acute cerebral vascular disease renders a possibility that COVID-19 could attack the nervous system. The cytokine secretion and bloodstream circulation (viremia) are among the most possible routes into the nervous system.
COVID-19 first occurred in late 2019 in Wuhan, China (1). As of May 01, 2020, the COVID-19 pandemic had infected 3,291,008 worldwide and caused 232,478 deaths (data from the World Health Organization). The most common clinical symptoms are cough, sputum production, fatigue, shortness of breath, and mainly respiratory tract symptoms. However, an increasing number of cases have presented with neurologic manifestations, such as olfactory and taste disorders (2), and the phenomenon requires further attention.
COVID-19 is a new RNA virus strain from the family Coronaviridae (including the Middle East respiratory syndrome CoV [MERS-CoV] and severe acute respiratory syndrome CoV [SARS-CoV]). Phylogenetic analysis of the complete viral genome revealed that the virus was most closely related (89.1% nucleotide similarity) to a group of SARS-like coronaviruses (3). As such, it was previously termed SARS-CoV-2. In the review article published in 2018 (4), researchers found that the human coronavirus can enter the central nervous system through the olfactory bulb, causing demyelination and inflammation (cultured glial cells have been described to secrete cytokines including IL-6, IL-12p40, IL-15, TNF-a, CXCL9, and CXCL10 upon viral infection). The authors of a recent article (5) investigated the mechanism of COVID-19 nervous system involvement, and they stated that similar to SARS-CoV, the COVID-19 virus exploits the angiotensin-converting enzyme 2 (ACE2) receptor to gain entry inside the cells. The brain has been reported to express ACE2 receptors that have been detected over glial cells and neurons, which makes them a potential target of COVID-19. Recently, the research team in Harvard Medical School identified three main cells co-expressing ACE2 and TMPRSS2 (Type II transmembrane serine protease): lung type II pneumocytes, ileal absorptive enterocytes, and nasal goblet secretory cells (6). And the other research team used single-cell RNA-Seq datasets to suggest possible mechanisms through which CoV-2 infection could lead to anosmia or other forms of olfactory dysfunction (7).
We searched the MEDLINE, CENTRAL, and EMBASE databases for eligible publications from December 2019 to April 30, 2020 written in English, using the following keywords: COVID-19, SARS-CoV-2, neuro, clinical, characteristics, manifestations. We also checked the reference lists of relevant studies to identify any missing publications. We reviewed the clinical researches, including case series and case reports, for neurologic manifestations of COVID-19 and organized them into tables. A confirmed case of COVID-19 (SARS-CoV-2) was defined and mostly diagnosed using the triple algorithm (epidemiological history, clinical symptoms, and laboratory or radiological findings) as a standard procedure proposed by the World Health Organization. We also reported data from the Taiwan Centers for Disease Control until May 01, 2020. Then we did the meta-analysis of all the case series to pool the data together and make it easier to understand. We used the software of Comprehensive Meta-Analysis Software (CMA), version 3, and chose the model of one group event rate, random effect, to draw the Forest Plot (Supplementary Figures 2–8).
We used Preferred Reporting Items for Systematic reviews and Meta-Analyzes (PRISMA) guidelines for searching and listed our flowchart (Supplementary Figure 1). Then we made a list of the neurologic manifestations in the current COVID-19 pandemic (Table 1). We included 9 case series and 4 case reports of olfactory or taste disorders. We pooled the case series together and found around 35.6% of patients got these symptoms (Supplementary Figure 2). We included 43 studies of myalgia, about 18.5% of patients had this symptom (Supplementary Table 1 and Supplementary Figure 3). And 45 studies of headache, the percentage was 10.7% (Supplementary Table 2 and Supplementary Figure 4); 2 studies of acute cerebral vascular disease, the percentage was 8.1% (Supplementary Figure 5); 7 studies of dizziness, the percentage was 7.9% (Supplementary Figure 6); 4 case series and 2 case reports of altered mental status, the percentage was 7.8% (Supplementary Figure 7); and 2 studies of seizure, the percentage was 1.5% (Supplementary Figure 8). And still other case reports of encephalitis, neuralgia, ataxia, Guillain-Barre syndrome, Miller Fisher syndrome, intracerebral hemorrhage, polyneuritis cranialis, and dystonic posture.
In addition, we reviewed some basic studies (4, 5, 44, 45) to determine the mechanism of these neurologic symptoms and signs. The cartoon figure summarized the possible mechanism (Figure 1).
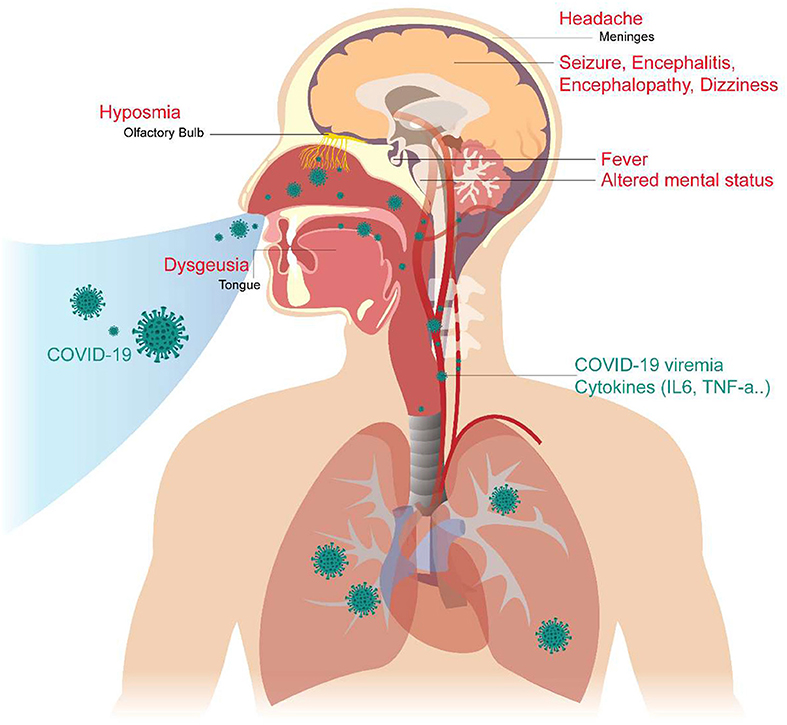
Figure 1. Neurological Manifestations of COVID-19 and the proposed mechanism. The COVID-19 virus may cause neurologic manifestations by cytokines secretion, general circulation (viremia), or direct invasion via the numerous ACE2 receptors in the olfactory epithelium. The olfactory disorder may cause by the olfactory epithelium damage. Fever was believed to be caused by the effect of cytokines or hypothalamus functional pertubation. The seizure may cause by cytokines storm, severely illed condition, or the brain parenchyma involvement, especially the mesial temporal lobe. Altered mental status may be a consequence of multiple organ failure, severe infection, or brainstem involvement. Headache is caused by meningeal irritation.
The COVID-19 pandemic is currently progressing, and neurologists and medical practitioners worldwide will face additional challenges from the neurologic complications of the disease (46). An updated review focusing on the neurologic features may help clinicians early identify potential patients.
Interestingly, previous coronavirus infections, including MERS and SARS, did not have a large proportion of patients with olfactory and taste disorders (47). However, patients with COVID-19 frequently complain of abnormalities in smell and taste. In our analysis of data from Taiwan (9), we found that between January 21 and March 24, 2020, a total of 216 patients were confirmed to have COVID-19 infection, and 5 of them (2.3%) had olfactory or taste disorders. Between March 25 and May 01, 48 cases in 213 patients (22.5%) had olfactory or taste disorders. In the beginning, most COVID-19 patients had a contact history related to Wuhan. But after the government of China locked down many big cities, Taiwan's COVID-19 cases mostly originated from travelers from Europe, the Middle East, or the United States. Besides, according to 88 cases series (see Supplementary Tables 1, 2) in China (from December 2019 to April 25, 2020), only one study (2) conducted by neurologists in Wuhan reported olfactory or taste disorder.
On the other hand, an Italian researcher reported that 33.9% of COVID-19 patients in Italy experienced this problem (8). In the Middle East, researchers in Iran found a surge in the outbreak of olfactory dysfunction during the COVID-19 epidemic (based on an online checklist of 10,069 voluntary cases between March 12 and 17, 2020) (48). The different incidence of the olfactory and/or taste dysfunction by the timing and geographic distribution might reveal important information that the virus may carry the potential to alter its affinity to the central nervous system (49–51). However, the possibilities of a higher detection rate of olfactory dysfunction in patients diagnosed by certain sub-specialists, such as neurologists (2) or otolaryngologists, cannot be completely excluded. For example, the study conducted by otolaryngologists (17) found olfactory/taste disorders in more than 80% of the patients.
Fever is generally known as an elevation in body temperature caused by a cytokine-induced upward displacement of the set point of the hypothalamic thermoregulatory center. Small elevations in body temperature appear to enhance immune function and inhibit pathogen growth (52). In 2005, pathologists in Beijing performed autopsies of SARS patients and found signals of the SARS viral genome detected in numerous neurons in the hypothalamus (53). As a result, it is conceivable that fever may be caused mainly by the effect of cytokines or possible direct viral invasion to the hypothalamus.
Concerning seizure in viral infection, generally the paroxysmal spell may be a consequence of multiple complications of systemic disease, such as metabolic disturbances, hypoxia, etc. Considering the viral encephalitis, it frequently manifests with seizures in its acute phase (54). The most widely reported virus was HSV-1 (herpes simplex virus), which involves the highly epileptogenic mesial temporal lobe structures, including the hippocampus (54). In the two case reports (37, 39), both had mesial temporal lobe involvement (one by acute inflammation, one by previous ischemic stroke). Since the case number is limited, we can only speculate that seizures may be caused by the generalized poor condition, cytokine storm (55), or mesial temporal lobe involvement in severe COVID-19 patients.
Several countries are currently encountering a crisis of ventilator shortage. The respiratory failure of COVID-19 infected patients may be partly related to brainstem failure. The COVID-19 virus passes into the cell via the ACE2 receptor (5). ACE2 is expressed in the brain and is mainly found in the brainstem, specifically in the nuclei associated with cardio-respiratory control (56, 57). In the previous research on SARS-CoV-1 and MERS-COV, the brainstem was severely infected, which possibly contributes to the degradation and failure of respiratory centers (45). Besides, the ascending reticular activating system (ARAS), which is responsible for human consciousness, also originates from the brainstem (and then advances into the thalamus and cortex) (58). This may partly explain the altered mental status of COVID-19 patients. However, the maintenance of consciousness is complex. Considering many COVID-19 patients were severely ill with multi-organ failure, both the cytokine effect and systemic impact of organ dysfunction can also lead to the consciousness disturbance.
Both dizziness and headache are considered to be general non-specific symptoms. Etiologies attributed to infectious causes are important secondary causes of headache (59). It is known that cytokines induced by viral infection increase the permeability of vessels. This causes cerebral swelling and meningeal irritation. The meningeal irritation stimulates the trigeminal nerve terminals and triggers pain sensation (60).
Ischemic stroke also occurs in COVID-19 patients because the infection may cause D-dimer elevation, thrombocytopenia, and hypercoagulable state (61–66). Besides, the exaggerated systemic inflammation or a “cytokine storm” (55), cardioembolism from virus-related cardiac injury (67) could further increase the risk of stroke (68).
Most cases of Guillain-Barre syndrome appeared with a lag time from the primary infection of COVID-19 (33, 34); the pathogenesis is therefore likely to be postinfectious immune-mediated.
This review is obviously constrained by the current information and limited reports. And there was considerable heterogeneity in the data. In addition, the researches of the novel pandemic emerge fastly. We could only review the results up to April 30, 2020 in this regard. The cause of neurologic manifestation may be a cytokine storm, multiple organ failure, or direct viral infection. However, the detailed pathophysiology of causing COVID-19 nervous system involvement remains to be elucidated. We sincerely hope the review can help the first line clinicians identify the emerging neurologic manifestations when combating the viral pandemic.
S-TT and M-KL did the literature search and drafted this manuscript. C-HT initiated this review and integrated the clinical and basic research. SS did the meta-analysis and made all the Forest Plot figures.
The review was supported in part by grants from the Ministry of Science and Technology (MOST 105-2314-B-039-004-MY2, MOST 106-2410-H-008-054-, MOST 107-2314-B-039−017 -MY3, MOST 107-2221-E-008-072-MY2, and MOST 105-2410-H-039-003-) and China Medical University Hospital (DMR-108-206, DMR-109-069, DMR-109-229), Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Ms. Hsiu-Chen Lu for the help in graphing, the Enago (www.enago.tw) for the English language review, Dr. I-Chen Tsai, MD, Ph.D. for the PRISMA template.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00498/full#supplementary-material
Supplementary Figure 1. Preferred Reporting Items for Systematic reviews and Meta-Analyzes (PRISMA), our searching strategy.
Supplementary Figure 1. Forest plot for olfactory/taste disorder.
Supplementary Figure 3. Forest plot for myalgia.
Supplementary Figure 4. Forest plot for headache.
Supplementary Figure 5. Forest plot for acute cerebral vascular disease.
Supplementary Figure 6. Forest plot for dizziness.
Supplementary Figure 7. Forest plot for altered mental status.
Supplementary Figure 8. Forest plot for seizure.
Supplementary Table 1. Patients presented with myalgia.
Supplementary Table 2. Patients presented with headache (ordered by cases recruitment date).
1. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. (2020) 323:1341–2. doi: 10.1001/jama.2020.3151
2. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) e201127. doi: 10.1001/jamaneurol.2020.1127
3. Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, et al. A new coronavirus associated with human respiratory disease in China. Nature. (2020) 579:265–9. doi: 10.1038/s41586-020-2008-3
4. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci. (2018) 12:386. doi: 10.3389/fncel.2018.00386
5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
6. Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. (2020). doi: 10.2139/ssrn.3555145
7. Brann DH, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv. (2020) doi: 10.1101/2020.03.25.009084
8. Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. (2020) ciaa330. doi: 10.1093/cid/ciaa330
9. Taiwan Centers for Disease Control (Chinese Version Website). Taipei. Available online at: https://data.cdc.gov.tw.
10. Yan CH, Faraji F, Prajapati DP, Ostrander BT, Deconde AS. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol. (2020) doi: 10.1002/alr.22592
11. Popescu CP, Marin A, Melinte V, Gherlan GS, Banicioiu FC, Dogaru A, et al. COVID-19 in a tertiary hospital from Romania: epidemiology, preparedness and clinical challenges. Travel Med Infect Dis. (2020) 101662. doi: 10.1016/j.tmaid.2020.101662
12. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. (2020) doi: 10.1002/lary.28692
13. Beltran-Corbellini A, Chico-Garcia JL, Martinez-Poles J, Rodriguez-Jorge F, Natera-Villalba E, Gomez-Corral J, et al. Acute-onset smell and taste disorders in the context of Covid-19: a pilot multicenter PCR-based case-control study. Eur J Neurol. (2020) doi: 10.1111/ene.14273
14. Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. (2020) doi: 10.1001/jama.2020.6771
15. Villalba NL, Maouche Y, Ortiz MBA, Sosa ZC, Chahbazian JB, Syrovatkova A, et al. Anosmia and dysgeusia in the absence of other respiratory diseases: should COVID-19 infection be considered? Eur J Case Rep Intern Med 7:001641. doi: 10.12890/2020_001641
16. Gutierrez-Ortiz C, Mendez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Manas R, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. (2020) doi: 10.1212/WNL.0000000000009619
17. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. (2020) 1–11. doi: 10.1007/s00405-020-05965-1
18. Qian G, Yang N, Ma AHY, Wang L, Li G, Chen X, et al. A COVID-19 Transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis. (2020) ciaa316. doi: 10.1093/cid/ciaa316
19. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
20. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. (2020) ciaa248. doi: 10.1093/cid/ciaa248
21. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
22. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) ciaa272. doi: 10.1093/cid/ciaa272
23. Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. (2020) 63:706–11. doi: 10.1007/s11427-020-1661-4
24. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. (2020) 20:425–34. doi: 10.1016/S1473-3099(20)30086-4
25. Godaert L, Proye E, Demoustier-Tampere D, Coulibaly PS, Hequet F, Drame M. Clinical characteristics of older patients: The experience of a geriatric short-stay unit dedicated to patients with COVID-19 in France. J Infect. (2020) doi: 10.1016/j.jinf.2020.04.009
26. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
27. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. (2020) 92:441–7. doi: 10.1002/jmv.25689
28. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI Features. Radiology. (2020) 201187. doi: 10.1148/radiol.2020201187
29. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by Severe Acute Respiratory Syndrome Coronavirus−2 (SARS-CoV-2). J Med Virol. (2020) doi: 10.1002/jmv.25915
30. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. (2020) 323:1612–14. doi: 10.1001/jama.2020.4326
31. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. (2020) NEJMc2008597. doi: 10.1056/NEJMc2008597
32. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. (2020) e60. doi: 10.1056/NEJMc2009787
33. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. (2020) NEJMc2009191. doi: 10.1056/NEJMc2009191
34. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
35. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. (2020) doi: 10.1016/j.jocn.2020.04.062
36. Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, et al. Guillain-Barré syndrome related to COVID-19 infection. J Clin Neurosci. (2020) 7:e741. doi: 10.1212/NXI.0000000000000741
37. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
38. Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis. (2020) 101642. doi: 10.1016/j.tmaid.2020.101642
39. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of Coronavirus Disease (COVID-19): encephalopathy. Cureus. (2020) 12:e7352. doi: 10.7759/cureus.7352
40. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. (2020) doi: 10.1016/j.bbi.2020.04.017
41. Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2010. Brain Behav Immun. (2020) doi: 10.1016/j.bbi.2020.04.024
42. Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. (2020) 35:100669. doi: 10.1016/j.nmni.2020.100669
43. Dugue R, Cay-Martinez KC, Thakur K, Garcia JA, Chauhan LV, Williams SH, et al. Neurologic manifestations in an infant with COVID-19. Neurology. (2020) doi: 10.1212/WNL.0000000000009653
44. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. (2020) doi: 10.1002/jmv.25728
45. Steardo L, Steardo L Jr, Zorec R, Verkhratsky A. Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. (2020) 29:e13473. doi: 10.1111/apha.13473
46. Bersano A, Pantoni L. On being a neurologist in Italy at the time of the COVID-19 outbreak. Neurology. (2020) doi: 10.1212/WNL.0000000000009508
47. Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol Taiwan. (2006) 15:26–8.
48. Bagheri SHR, Asghari AM, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. medRxiv. (2020) doi: 10.1101/2020.03.23.20041889
49. Delanghe JR, Speeckaert MM, De Buyzere ML. The host's angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. (2020) 505:192–3. doi: 10.1016/j.cca.2020.03.031
50. Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci USA. (2020) 117:9241–3. doi: 10.1073/pnas.2004999117
51. Rodriguez-Morales AJ, Rodriguez-Morales AG, Mendez CA, Hernandez-Botero S. Tracing new clinical manifestations in patients with covid-19 in chile and its potential relationship with the SARS-CoV-2 divergence. Curr Trop Med Rep. (2020) 18:1–4. doi: 10.1007/s40475-020-00205-2
52. Elizabeth AA, Dottie B, Carlton GB. Understanding the pathophysiology of fever. Nursing. (2008) 38:56cc51–2. doi: 10.1097/01.NURSE.0000327497.08688.47
53. Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. (2005) 202:415–24. doi: 10.1084/jem.20050828
54. Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. (2008) 49(Suppl. 6):13–8. doi: 10.1111/j.1528-1167.2008.01751.x
55. Mehta P, Mcauley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
56. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. (2010) 12:170–5. doi: 10.1007/s11906-010-0105-7
57. Gowrisankar YV, Clark MA. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J Neurochem. (2016) 138:74–85. doi: 10.1111/jnc.13641
58. Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci. (2020) 7:416. doi: 10.3389/fnhum.2013.00416
59. Prakash S, Patel N, Golwala P, Patell R. Post-infectious headache: a reactive headache? J Headache Pain. (2011) 12:467–73. doi: 10.1007/s10194-011-0346-0
60. Ogunlaja OI, Cowan R. Subarachnoid Hemorrhage and Headache. Curr Pain Headache Rep. (2019) 23:44. doi: 10.1007/s11916-019-0785-x
61. Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): A pooled analysis of published literature. Int J Stroke. (2020) doi: 10.1177/1747493020921664
62. Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. (2020) doi: 10.1111/ijlh.13230
63. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. (2020) 127:104362. doi: 10.1016/j.jcv.2020.104362
64. Klok FA, Kruip MJHA, Van Der Meer NJM, Arbous MS, Gommers DAMPJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) doi: 10.1016/j.thromres.2020.04.013
65. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. (2020) doi: 10.1111/jth.14850
66. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. (2020) doi: 10.1016/j.jacc.2020.04.031
67. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
Keywords: COVID-19, pandemic, neurologic, headache, taste, olfactory, ACE2, cytokine
Citation: Tsai S-T, Lu M-K, San S and Tsai C-H (2020) The Neurologic Manifestations of Coronavirus Disease 2019 Pandemic: A Systemic Review. Front. Neurol. 11:498. doi: 10.3389/fneur.2020.00498
Received: 18 April 2020; Accepted: 06 May 2020;
Published: 19 May 2020.
Edited by:
Jordi A. Matias-Guiu, Hospital Clínico San Carlos, SpainReviewed by:
Diogo Goulart Corrêa, Federal University of Rio de Janeiro, BrazilCopyright © 2020 Tsai, Lu, San and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chon-Haw Tsai, d2luZHltb3ZlbWVudEBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.