- 1Dementia Research Centre, Department of Neurodegenerative Diseases, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom
- 2UK Dementia Research Institute at University College London, UCL, London, United Kingdom
- 3Department of Psychology, The Hebrew University of Jerusalem, Jerusalem, Israel
Introduction
Alzheimer's disease (AD) is the most common form of neurodegenerative dementia, accounting for 2/3 of all dementia cases and currently recognized as a global public health challenge. In 2015, 46.8 million people were estimated to have dementia, and this number is expected to almost double every 20 years reaching 75 million in 2030 and 131.5 million in 2050 (1). Early detection may offer the best chance of therapeutic success amid these raising numbers.
It is now recognized that a preclinical period may precede the symptomatic phase up to 25 years (2). The development of suitable behavioral markers to detect and track this stage is important, before more expensive and invasive biomarkers are used. One important line of AD research in the last decade has provided evidence that the ability to bind object features together in short-term memory (STM) is affected in AD even at asymptomatic stages (3, 4). In cognition, binding is the function that supports the integration of multiple elements together (5–7).
Popular in clinical psychology is the Memory Binding Test (MBT), which assesses the binding of a category cue (e.g., flower) to a word target [e.g., tulip; see (8) for detail on test] (9–11). However, its verbal nature causes susceptibility to semantic interference and cognitive reserve [CR; the ability to find alternative ways of performing a task, bypassing any deficits (12, 13)]. Instead, visual short-term memory (VSTM) binding relies on the integration of visual features and is less susceptible to semantic and verbal strategies. The focus of this article will be on binding of visual information across short time scales. Yet, before we tackle this in more depth, it is relevant to define a series of terms.
In clinical practice, “prodromal” is usually the period immediately preceding the onset of dementia, when patients might meet criteria for mild cognitive impairment (MCI) (14) and “preclinical” generally refers to the stage preceding this, before the onset of the clinical phenotype. Here, the term “preclinical AD” will be restricted to asymptomatic familial Alzheimer's disease (FAD)—a rare autosomal dominantly inherited variant of Alzheimer's and clinically healthy individuals (at time of testing) who, over time, developed AD dementia. We will provide brief theoretical reasoning for assessing VSTM binding in AD and a summary of the research lines in the field. In the context of clinical practice, we will also reflect on its use for the differential diagnosis of AD and as a tool for preclinical AD.
Theoretical Reasoning
The “feature integration theory of attention” (6) suggests that when attention is focused on an object, all its attributes (e.g., shape, color, motion, and texture) (15) are rapidly bound into a unified representation that is then used by higher-level cognitive processes (16, 17). The different attributes on objects have to be bound together also after the perceptual stage, even when maintained in STM. STM binding is a cognitive function known to support the integration of features necessary to maintain a coherent representation of an object in immediate memory. Two types of VSTM binding deficits have been reported in AD: relational and conjunctive binding.
Some conceptual differences are mentioned below:
° Conjunctive binding: is the integration of features within an object, ultimately forming a single representations of the item with multiple elements (e.g., color and shape) (18). It does not seem to depend on the hippocampus (19–21) and appears supported by a network involving the entorhinal and perirhinal cortex as well as occipital–parietal regions (22–24).
° Relational binding: is the association of an object identity's to other “independent” features such as its location, context, or source [see (25)]. Successful performance relies on the integrity of the hippocampus (3) and appears to engage a network in which it plays a fundamental role (19, 20).
Memory Binding in Relation to the Detection of Preclinical AD
Parra et al. were the first to suggest conjunctive binding as a preclinical marker of AD (26, 27). They used a change-detection task in which individuals detected a change between two consecutive displays. While a shift in one feature (e.g., color) was easily detected, when only the binding between features was changed (green circle and blue square green square and blue circle), asymptomatic presenilin 1 (PSEN1) carriers carrying an E280A mutation (causing FAD) were significantly impaired. Such tasks showed greater sensitivity than standard neuropsychological tests, which revealed no differences in this asymptomatic group compared to healthy controls.
More recently, Liang and colleagues (3) assessed relational binding by asking individuals to remember the identity and location of several objects. After a delay of a few seconds, subjects were required to report which one of two objects was presented and move the selected object to its correct location on a touch screen (28, 29). Asymptomatic FAD carriers, who performed similarly to matched controls on identifying the correct object, and in localization precision, exhibited more binding errors in which the correct object was localized precisely near the location of one of the other objects from the memory array (30).
Memory Binding in Relation to the Differential Diagnosis Of Sporadic Ad
Binding tasks have also proven useful in the differential diagnosis of sporadic AD. In 2009, Parra and colleagues showed that AD patients had deficits in conjunctive binding (5). These were later revealed specific to AD relative to other neurological conditions [i.e., major depression (4) and non-AD dementias (30, 31)].
Implications of Differences Between Relational and Conjunctive Binding for Clinical Use
Arguably, if relational and conjunctive binding rely on different brain mechanisms and regions, they may have distinct sensitivity to different disease stages. This is a debated topic in the field, as some believe that atrophy in the entorhinal and perirhinal cortex (conjunctive) precedes hippocampal changes (relational) (32) in AD. Others sustain both types of binding decline at a preclinical phase (33). There is insufficient evidence to determine whether one or both may act as effective predictive clinical markers. Convincing evidence would require longitudinal studies that follow individuals at risk of sporadic or familial AD from an asymptomatic stage through MCI (33).
Memory Binding And Aging
Conjunctive binding appears preserved across lifespan in healthy aging (34, 35), whereas relational binding seems to decline as the hippocampus degenerates with age regardless of risk for AD (36, 37). Importantly, the basis of this differential impact is not fully understood and susceptibility to reduction of attentional resources in working memory needs further exploration (38). Whether suitable preclinical tests for AD should not show effects of healthy aging (39) is still debated among the scientific community. Arguably the highest predictive power of such tools could also be reached when comparing individuals who will develop AD to age-matched controls who do not share the same risk factors (33).
A summary of the findings showing sensitivity to preclinical and symptomatic AD is presented in Table 1.
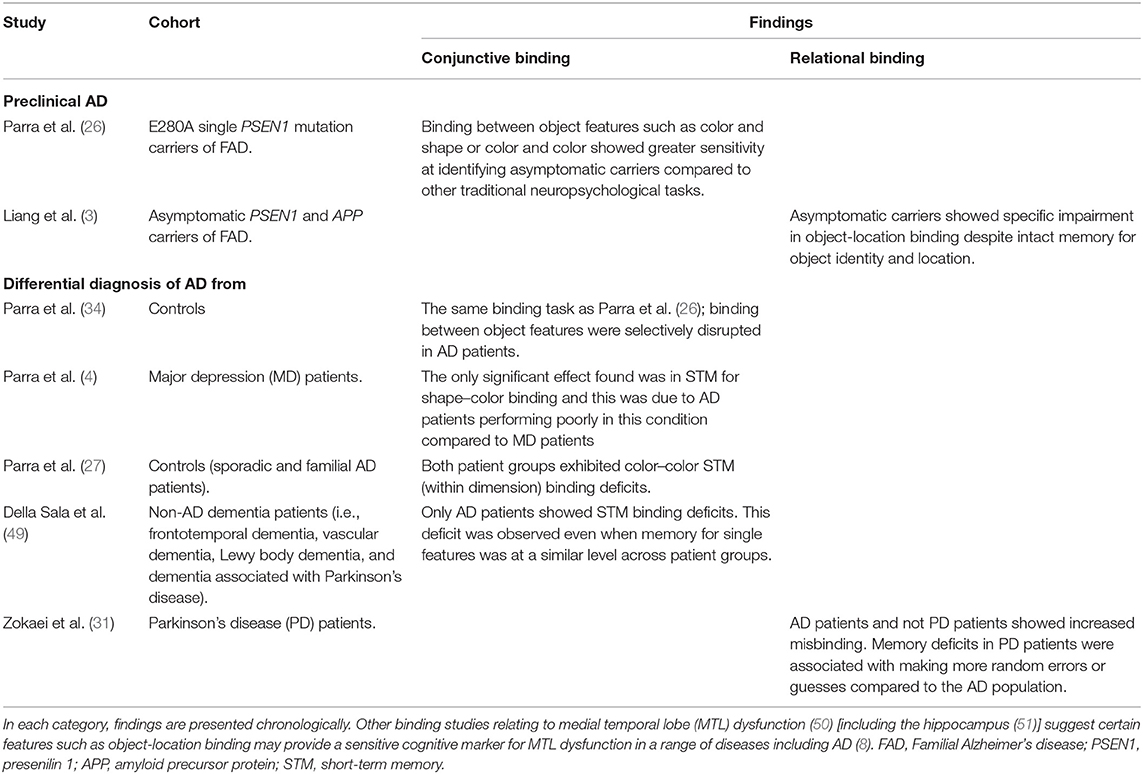
Table 1. Summary of binding studies suggesting utility for the detection of preclinical AD and differential diagnosis of AD.
Translational Potential of VSTM Binding
The effort to build on basic scientific research and develop therapies, screening, and diagnostics for individuals is central in the field of dementia research. In 2015, the European Society for Translational Medicine stated its goal was to “combine disciplines, resources, expertise, and techniques within benchside, bedside and community to promote enhancements in prevention, diagnosis, and therapies” (40). Crucial to clinical practice is the transition from (a) the initial confirmation of association with the outcome of interest (e.g., VSTM binding impairment is associated with a diagnosis of AD) to (b) acquiring sensitivity to a treatment or an intervention (e.g., VSTM binding deficits decline in response to a therapy) and (c) showing a “meaningful” change in patient behavior (e.g., change in VSTM binding score results in a different treatment strategy) (41).
Below, we reflect on the translational potential of VSTM binding in a clinical setting (before any regulatory approval is attempted) and outline the pros and cons in this context.
Pros
• Diagnostic potential for asymptomatic and MCI stages of AD (3, 10, 26, 27).
• Potential as a behavioral marker for preclinical AD outperforms traditional memory measures that lack the same sensitivity (3, 26).
• Sensitivity in predicting cognitive decline and conversion from aMCI to AD (11, 41).
• Such computerized tasks are non-invasive, easy to administer, inexpensive, and easily portable.
• Usually do not require verbal reports and thus are not language-constrained.
• Impervious to education and intercultural differences (27).
• Reduced susceptibility to subjective interpretation of results compared to other traditional neuropsychology tasks (9).
• Reduced susceptibility to practice or learning effects as the repeated presentation of semantically meaningless stimuli such as polygon–color or fractal–location combinations is quickly overwritten (42, 43).
• The use of shape and color or abstract figures limits the variability in the way information is rehearsed, organized, and encoded. Controlled learning minimizes the use of individualized strategies increasing the probability that retrieval is based on direct access to what was learned in the first place (9).
Cons
• There is greater need for validation of test–retest reliability of such tasks for the purpose of detecting and monitoring AD-related populations. This should ideally involve different research groups with ethnically diverse populations.
• Although some conjunctive binding tasks have proven impervious to differences in cognitive reserve (26), further validation and additional exploration on relational binding in healthy aging is necessary.
• Longitudinal studies are needed to establish the suitability for the detection of preclinical AD (in non-genetic forms) (33). This will provide greater validation for its use in preclinical sporadic AD.
• At present, the detection of preclinical AD or differential diagnosis of MCI or AD relies on group differences. Future studies should focus on its use at an individual screening level and evaluate the best threshold for determining “impaired performance”.
• Large-scale testing of non-clinical populations are lacking, and this is necessary for acquiring appropriate norms.
• There is no current evidence for a relationship between task performances and prediction of treatment outcomes (i.e., are patients with high VSTM binding scores more likely to benefit from specific medications or interventions?)
• There is a need for validation of task performances against AD biomarkers like abnormal amyloid beta–positron emission tomography (Aβ-PET), or abnormal Aβ in cerebrospinal fluid (CSF), or abnormal tau-PET, or abnormal tau in CSF—before considering using these approaches on their own.
Discussion
The recent Food and Drug Administration guidelines suggest that biomarkers alone will not be sufficient as surrogate outcomes to show effectiveness of treatment (44). There is increasing recognition that therapies should be associated with changes in clinically meaningful endpoints (whether cognitive or functional) (45). It is therefore paramount to identify cognitive behavioral measures that are sensitive to detecting early disease states and ideally converge with biological markers of AD pathology. These become particularly important for identifying individuals at risk, monitoring disease progression, and ascertaining treatment efficacy (9, 46). If such tests were developed to identify cognitive deficits resulting from the earliest identifiable brain pathology in AD, such as the deposition of Aβ or abnormal phosphorylated tau (47, 48), measures could then serve as both highly powerful cognitive markers and, in turn, clinically significant end points.
In our opinion, there is currently insufficient evidence to determine the translational potential of VSTM binding tasks to clinical settings. However, the development of novel tests that are cognitively challenging, minimize variability in learning strategies, decrease the subjectivity to interpretation, and exploit vulnerabilities caused by AD is needed (9). VSTM binding tasks are indeed headed in this direction.
This leaves a number of questions unanswered: What are the most important characteristics of tests administered in a clinical setting? Do these characteristics vary depending on purpose (e.g., diagnosis, dementia incidence, and prognosis)? Is it far too early for the use of VSTM binding tasks in clinical practice?
Considering the current evidence, we propose that the greatest translational potential of VSTM binding tasks might lie on the preclinical stages of AD. Nonetheless, large-population-based studies, longitudinal designs, and correlations to biomarkers are paramount to validate this further. Lastly and crucial to clinical practice, it is yet to be determined if such tests are actionable, i.e., whether their prognostic and predictive value gives grounds for improving patient management.
Author Contributions
IP: conceived a first version of the manuscript. IP, AS-G, and YP: conception and interpretation of the work, final approval of the manuscript, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor CB declared a past co-authorship with one of the authors, AS-G.
Acknowledgments
This report was undertaken at the Dementia Research Centre at University College London, in collaboration with The Hebrew University of Jerusalem. The Dementia Research Centre was supported by Alzheimer's Research UK, the Brain Research Trust, and The Wolfson Foundation. This work was also supported by the UK Dementia Research Institute which receives its funding from DRI Ltd., funded by the UK Medical Research Council, Alzheimer's Society and Alzheimer's Research UK. YP was supported by the Israel Science Foundation (ISF) grant (1747/14).
Abbreviations
STM, short-term memory; FAD, familial Alzheimer's disease.
References
1. Dementia statistics. Alzheimer's Disease International. (2019). Available online at: https://www.alz.co.uk/research/statistics
2. Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. (2012) 367:795–804. doi: 10.1056/NEJMoa1202753
3. Liang Y, Pertzov Y, Nicholas JM, Henley SMD, Crutch S, Woodward F, et al. Visual short-term memory binding deficit in familial Alzheimer's disease. Cortex J Devoted Study Nerv Syst Behav. (2016) 78:150–64. doi: 10.1016/j.cortex.2016.01.015
4. Parra MA, Abrahams S, Logie RH, Della Sala S. Visual short-term memory binding in Alzheimer's disease and depression. J Neurol. (2010) 257:1160–9. doi: 10.1007/s00415-010-5484-9
5. Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Della Sala S. Short-term memory binding deficits in Alzheimer's disease. Brain J Neurol. (2009) 32:1057–66. doi: 10.1093/brain/awp036
6. Treisman AM, Gelade G. A feature-integration theory of attention. Cognit Psychol. (1980) 12:97–136. doi: 10.1016/0010-0285(80)90005-5
7. Zimmer H, Mecklinger A, Lindenberger U. Handbook of Binding and Memory: Perspectives From Cognitive Neuroscience. Oxford; New York, NY: Oxford University Press (2006). p. 752. doi: 10.1093/acprof:oso/9780198529675.001.0001
8. Buschke H. Rationale of the memory binding test. Dement Mem. (2013) 2013:55–71. doi: 10.1037/t56415-000
9. Loewenstein DA, Curiel RE, Duara R, Buschke H. Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer's disease. Assessment. (2018) 25:348–59. doi: 10.1177/1073191117691608
10. Buschke H, Mowrey WB, Ramratan WS, Zimmerman ME, Loewenstein DA, Katz MJ, et al. Memory binding test distinguishes amnestic mild cognitive impairment and dementia from cognitively normal elderly. Arch Clin Neuropsychol. (2017) 32:29–39. doi: 10.1093/arclin/acw083
11. Mowrey WB, Lipton RB, Katz MJ, Ramratan WS, Loewenstein DA, Zimmerman ME, et al. Memory binding test predicts incident dementia: results from the einstein aging study. J Alzheimers Dis JAD. (2018) 62:293–304. doi: 10.3233/JAD-170714
12. Stern Y. Cognitive reserve. Neuropsychologia. (2009) 47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004
13. Cognitive Reserve. (2020). Available online at: https://www.ageuk.org.uk/information-advice/health-wellbeing/mind-body/staying-sharp/thinking-skills-change-with-age/cognitive-reserve/
14. Visser PJ, Vos S, van Rossum I, Scheltens P. Comparison of international working group criteria and national institute on aging-alzheimer's association criteria for alzheimer's disease. Alzheimers Dement. (2012) 8:560–3. doi: 10.1016/j.jalz.2011.10.008
15. Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci Off J Soc Neurosci. (1987) 7:3416–68. doi: 10.1523/JNEUROSCI.07-11-03416.1987
16. Kahneman D, Treisman A, Gibbs BJ. The reviewing of object files: object-specific integration of information. Cognit Psychol. (1992) 24:175–219. doi: 10.1016/0010-0285(92)90007-O
17. Treisman A. The binding problem. Curr Opin Neurobiol. (1996) 6:171–8. doi: 10.1016/S0959-4388(96)80070-5
18. Moses SN, Ryan JD. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. (2006) 16:43–65. doi: 10.1002/hipo.20131
19. Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. (2007) 11:126–35. doi: 10.1016/j.tics.2006.12.003
20. Parra MA, Fabi K, Luzzi S, Cubelli R, Hernandez Valdez M, Della Sala S. Relational and conjunctive binding functions dissociate in short-term memory. Neurocase. (2015) 21:56–66. doi: 10.1080/13554794.2013.860177
22. Shafritz KM, Gore JC, Marois R. The role of the parietal cortex in visual feature binding. Proc Natl Acad Sci U S A. (2002) 99:10917–22. doi: 10.1073/pnas.152694799
23. Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. (2006) 440:91–5. doi: 10.1038/nature04262
24. Parra MA. Cognitive assessment in Alzheimer's disease. Adv Alzheimers Dis. (2013) 2:123–5. doi: 10.4236/aad.2013.24016
25. Hannula DE, Tranel D, Allen JS, Kirchhoff BA, Nickel AE, Cohen NJ. Memory for items and relationships among items embedded in realistic scenes: disproportionate relational memory impairments in amnesia. Neuropsychology. (2015) 29:126–38. doi: 10.1037/neu0000119
26. Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain J Neurol. (2010) 133:2702–13. doi: 10.1093/brain/awq148
27. Parra MA, Sala SD, Abrahams S, Logie RH, Méndez LG, Lopera F. Specific deficit of colour-colour short-term memory binding in sporadic and familial Alzheimer's disease. Neuropsychologia. (2011) 49:1943–52. doi: 10.1016/j.neuropsychologia.2011.03.022
28. Bays PM, Catalao RFG, Husain M. The precision of visual working memory is set by allocation of a shared resource. J Vis. (2009) 9:1–11. doi: 10.1167/9.10.7
29. Gorgoraptis N, Catalao RFG, Bays PM, Husain M. Dynamic updating of working memory resources for visual objects. J Neurosci Off J Soc Neurosci. (2011) 31:8502–11. doi: 10.1523/JNEUROSCI.0208-11.2011
30. Zokaei N, Burnett Heyes S, Gorgoraptis N, Budhdeo S, Husain M. Working memory recall precision is a more sensitive index than span. J Neuropsychol. (2015) 9:319–29. doi: 10.1111/jnp.12052
31. Zokaei N, Husain M. Working memory in Alzheimer's disease and Parkinson's disease. Curr Top Behav Neurosci. (2019) 2019:325–44. doi: 10.1007/7854_2019_103
32. Parra MA. A commentary on Liang et al. 's paper with regard to emerging views of memory assessment in Alzheimer's disease. Cortex J Devoted Study Nerv Syst Behav. (2017) 88:198–200. doi: 10.1016/j.cortex.2016.06.006
33. Liang Y, Pertzov Y, Henley S, Woodward F, Husain M, Crutch S. Visual short-term memory binding deficits in Alzheimer's disease: a reply to Parra's commentary. Cortex. (2017) 88:201–4. doi: 10.1016/j.cortex.2016.11.003
34. Parra MA, Abrahams S, Logie RH, Sala SD. Age and binding within-dimension features in visual short-term memory. Neurosci Lett. (2009) 449:1–5. doi: 10.1016/j.neulet.2008.10.069
35. Rhodes S, Parra MA, Logie RH. Ageing and feature binding in visual working memory: the role of presentation time. Q J Exp Psychol. (2016) 69:654–68. doi: 10.1080/17470218.2015.1038571
36. Fan X, Wheatley EG, Villeda SA. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci. (2017) 40:251–72. doi: 10.1146/annurev-neuro-072116-031357
37. O'Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. (2016) 8:298 doi: 10.3389/fnagi.2016.00298
38. van Geldorp B, Heringa SM, van den Berg E, Olde Rikkert MGM, Biessels GJ, Kessels RPC. Working memory binding and episodic memory formation in aging, mild cognitive impairment, and Alzheimer's dementia. J Clin Exp Neuropsychol. (2015) 37:538–48. doi: 10.1080/13803395.2015.1037722
39. Logie RH, Parra MA, Della Sala S. From cognitive science to dementia assessment. Policy Insights Behav Brain Sci. (2015) 2:81–91. doi: 10.1177/2372732215601370
40. Cohrs RJ, Martin T, Ghahramani P, Bidaut L, Higgins PJ, Shahzad A. Translational medicine definition by the european society for translational medicine. New Horiz Transl Med. (2015) 2:86–8. doi: 10.1016/j.nhtm.2014.12.002
41. Perlis R. Translating biomarkers to clinical practice. Mol Psychiatry. (2011) 16:1076–87. doi: 10.1038/mp.2011.63
42. Colzato LS, Raffone A, Hommel B. What do we learn from binding features? Evidence for multilevel feature integration. J Exp Psychol Hum Percept Perform. (2006) 32:705–16. doi: 10.1037/0096-1523.32.3.705
43. Logie RH, Brockmole JR, Vandenbroucke ARE. Bound feature combinations in visual short-term memory are fragile but influence long-term learning. Vis Cogn. (2009) 17:160–79. doi: 10.1080/13506280802228411
44. Sabbagh MN, Hendrix S, Harrison JE. FDA position statement “Early Alzheimer's disease: Developing drugs for treatment, Guidance for Industry.” Alzheimers Dement. Transl Res Clin Interv. (2019) 5:13–9. doi: 10.1016/j.trci.2018.11.004
45. Vellas B, Bateman R, Blennow K, Frisoni G, Johnson K, Katz R, et al. Endpoints for Pre-Dementia AD Trials: A Report from the EU/US/CTAD Task Force. J Prev Alzheimers Dis. (2015) 2:128–35. doi: 10.14283/jpad.2015.55
46. Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimers Dement J Alzheimers Assoc. (2015) 11:415–24. doi: 10.1016/j.jalz.2014.03.005
47. Loewenstein DA, Greig MT, Curiel R, Rodriguez R, Wicklund M, Barker WW, et al. Proactive Semantic interference is associated with total and regional abnormal amyloid load in non-demented community-dwelling elders: a preliminary study. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. (2015) 23:1276–9. doi: 10.1016/j.jagp.2015.07.009
48. Papp K, Rentz D, Mormino E, Amariglio R, Burnham S, Johnson K, et al. The neuropsychology of preclinical alzheimer's disease: differential sensitivity of component processes of memory performance on biomarker evidence of amyloidosis (S41.004). Neurology. (2015) 84(14 Supplement):S41.004. Available online at: https://n.neurology.org/content/84/14_Supplement/S41.004
49. Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S. Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia. (2012) 50:833–40. doi: 10.1016/j.neuropsychologia.2012.01.018
50. Pertzov Y, Miller TD, Gorgoraptis N, Caine D, Schott JM, Butler C, et al. Binding deficits in memory following medial temporal lobe damage in patients with voltage-gated potassium channel complex antibody-associated limbic encephalitis. Brain. (2013) 136:2474–85. doi: 10.1093/brain/awt129
Keywords: binding, differential diagnosis, preclinical marker, translational research, clinical practice
Citation: Pavisic IM, Suarez-Gonzalez A and Pertzov Y (2020) Translating Visual Short-Term Memory Binding Tasks to Clinical Practice: From Theory to Practice. Front. Neurol. 11:458. doi: 10.3389/fneur.2020.00458
Received: 08 November 2019; Accepted: 29 April 2020;
Published: 10 June 2020.
Edited by:
Christopher Butler, University of Oxford, United KingdomReviewed by:
Emma Delhaye, University of Liège, BelgiumRob Udale, University of Oxford, United Kingdom
Copyright © 2020 Pavisic, Suarez-Gonzalez and Pertzov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivanna M. Pavisic, aXZhbm5hLnBhdmlzaWMuMTVAdWNsLmFjLnVr
 Ivanna M. Pavisic
Ivanna M. Pavisic Aida Suarez-Gonzalez
Aida Suarez-Gonzalez Yoni Pertzov
Yoni Pertzov