- 1“Riccardo Massa” Department of Human Sciences for Education, University of Milano-Bicocca, Milan, Italy
- 2IRCCS Fondazione don Carlo Gnocchi ONLUS, Milan, Italy
- 3Department of Psychology, Università Cattolica del Sacro Cuore, Milan, Italy
- 4Applied Technology for Neuro-Psychology Laboratory, Istituto Auxologico Italiano, IRCCS, Milan, Italy
The growing understanding of the importance of involving patients with neurological diseases in their healthcare routine either for at-home management of their chronic conditions or after the hospitalization period has opened the research for new rehabilitation strategies to enhance patient engagement in neurorehabilitation. In addition, the use of new digital technologies in the neurorehabilitation field enables the implementation of telerehabilitation systems such as virtual reality interventions, video games, web-based interventions, mobile applications, web-based or telephonic telecoach programs, in order to facilitate the relationship between clinicians and patients, and to motivate and activate patients to continue with the rehabilitation process at home. Here we present a systematic review that aims at reviewing the effectiveness of different engagement strategies and the different engagement assessments while using telerehabilitation systems in patients with neurological disorders. We used PICO's format to define the question of the review, and the systematic review protocol was designed following the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Bibliographical data was collected by using the following bibliographic databases: PubMed, EMBASE, Scopus, and Web of Science. Eighteen studies were included in this systematic review for full-text analyses. Overall, the reviewed studies using engagement strategies through telerehabilitation systems in patients with neurological disorders were mainly focused on patient self-management and self-awareness, patient motivation, and patient adherence subcomponents of engagement, that are involved in by the behavioral, cognitive, and emotional dimensions of engagement. Conclusion: The studies commented throughout this systematic review pave the way for the design of new telerehabilitation protocols, not only focusing on measuring quantitative or qualitative measures but measuring both of them through a mixed model intervention design (1). The future clinical studies with a mixed model design will provide more abundant data regarding the role of engagement in telerehabilitation, leading to a possibly greater understanding of its underlying components.
Introduction
In the field of neurorehabilitation, one of the main objectives after a brain or nerve injury is to develop rehabilitation strategies directed at the recovery of functional skills by enhancing neuroplasticity (2). Even though the type of intervention, intensity, and number of sessions are known to be important in task-specific rehabilitation trainings (3), it is known that the role of engagement is key for enhancing neuroplasticity, and to facilitate functional recovery in patients with neurological disorders (2, 4). In this regard, some studies observed that by increasing patients' attention and interest toward rehabilitation training, there is an updating and modification at a neurological level, which leads to improving functional outcomes (5). However, to achieve such positive functional outcomes in neurorehabilitation, the nervous system has to be engaged and challenged (5, 6). From a neurobiological point of view, several studies have shown how engagement may increase neural activity in different cortical areas such as (2) the orbitofrontal regions, that integrate information from sensory and motivational pathways to generate pleasure, (3) the ventral striatal dopaminergic systems, and (4) the anterior cingulate cortex, which holds attention during demanding task execution (7). Even though there are not enough studies using neuroimaging techniques to demonstrate the effects of engagement in neuroplasticity for rehabilitation, a large amount of studies using mental practice techniques, enriched environments, and attentional and motivational strategies in which patients become active actors of the rehabilitation training, corroborates the relationship between engagement and neuroplasticity (8–10). Concerning this, the growing development of technology in the last decade lead to the introduction of new digital systems in rehabilitation through which it is possible to provide different sensory stimuli enhancing patients' resources such as attention and motivation. Thus, digital technologies in rehabilitation are directed to providing information and/or support emotional, behavioral, or physiological features of the pathology within an enriched and stimulating environment (11–14). One interesting feature of digital technologies in rehabilitation is the opportunity to apply technology-based interventions to provide a rehabilitation service through digital and telecommunication technologies during the hospitalization period, or at home after discharge from the hospital (15). Such application of digital technologies for rehabilitation is commonly known as telerehabilitation (16). Moreover, through telerehabilitation systems is possible to engage patients by providing them an online (or offline) feedback of their outcomes through a double communication loop (17, 18). This type of communication combines remote monitoring of patients' performance with clinicians' appropriate responses by adapting and personalizing the planned rehabilitation activities, and empowering patients toward the targeted rehabilitation aim (18, 19). Further, through these types of telerehabilitation systems, clinicians can supply the needs of the patients in long-lasting rehabilitation programs after the hospitalization period, allowing them to remain involved in social and productive life even though of their clinical condition (17). Moreover, through telerehabilitation systems clinicians have the possibility of delivering long rehabilitation trainings in an enriched digital environment at patients' homes while saving a big amount of sanitary costs (20). Thus, the use of telerehabilitation systems can enhance the patients' engagement by conducting their rehabilitation training at home. However, how to enhance engagement and what engagement is when using telerehabilitation systems in patients with neurological disorders is not clear enough. Due to this, the following section aims to clarify some components and subcomponents of engagement at a clinical level.
Patient-Centered Medicine and Engagement
When we refer to patient engagement in the clinical field, we have to refer to patient-centered medicine (PCM). These two concepts are associated given that PCM considers a patients active participation in the clinical process as pivotal, instead of only considering the clinical professionals' point of view (21). In that context, patient engagement was considered as a concept to qualify the exchange between patients' demands and clinicians' supplies (22). Further, in healthcare, the term “engagement” came to indicate a renewed partnership between patients and healthcare providers (23). Then, the main goal of engaging patients in their clinical process can be identified in making them conscious of the management of their health status and illness, and to provide more positive outcomes in healthcare (24). Indeed, during the clinical process, patient engagement is a key factor in making them feel like participants in the therapeutic process that will lead to better adherence to the therapy, patient sensitization, and patient knowledge and empowerment (25). Even though the term “engagement” seems clear enough by itself, it involves different factors that have to take into consideration when engaging patients in a therapeutic process. Specifically, the involved factors in engagement are the following: participation and decision making, compliance and adherence, self-management, patient empowerment, and patient activation.
Participation and Decision Making
One of the main objectives for the improvement of the quality of health services defined by Entwistle and Watt (26) is the ability to involve patients in their therapeutic process by collaborating with the healthcare professionals. Two main factors have been defined for involving patients in clinical practices: patient participation and patient decision making. The first, patient participation, is considered a psychological component that focuses on identifying emotional and cognitive factors to enhance the active participation of the patients in clinical decision making (27). The second one is centered on the clinical and relational skills of the healthcare professionals in involving patients in clinical decisions (28, 29). Altogether, when referring to engagement in a clinical context, one intends to increase the communication between clinicians and patients to motivate patient participation throughout the clinical process. That means, giving the patients enough information about their illness to become more independents in their healthcare routine. Then, an engaged patient is a patient that can participate in the clinical decision making and healthcare routine, but also a patient able to actively participate in the global healthcare system promoting new forms of assistance, for example by using new technology systems (30).
Compliance and Adherence
Other factors embedded in patient engagement are “compliance” and “adherence” that refer to the adaptive behaviors of patients in following medical prescriptions or in following the healthcare routine (31). Although these two factors are often presented together, there are some differences between them. While “compliance” is related to patients' ability in adapting their life routine with a more passive/dependent attitude to the clinicians' indications (32), “adherence” is related with patients participation as an active actor in the communication exchange with the clinicians in which patients' and clinicians' plan together the patients care routine (33). Hence, the level of compliance and adherence to the clinical process depend on patients' attitudes and behaviors in accepting or disagreeing with the clinicians' prescriptions, moving the concept of patients' engagement toward a balance between patients' demands and clinicians' supplies (30).
Self-Management, Patient Empowerment, and Activation
Self-management is referred to as the patients' ability to manage symptoms, treatments, psychological, and psychosocial consequences of their pathological condition, as well as the ability to manage the cognitive, behavioral, and emotional responses, derived from their clinical condition, to reach a satisfactory quality of life (34, 35). Indeed, self-management is considered a positive outcome of patient engagement during the clinical process. Moreover, patient empowerment is also considered an important positive outcome during the patient engagement process. It is known that the term “empowerment” refers to psychological resources through which patients can control their clinical condition and the related treatments (36, 37). Thus, by providing the patients an educational healthcare process, they can recover agency and beliefs of self-efficacy over their health condition increasing their autonomy at the same time (38). Even though the concept of “empowerment” and the concept of “engagement” are strongly related, “empowerment” is considered an outcome of a mainly cognitive boosting process of patients, related to their knowledge of the clinical condition, while “engagement” also sustains the emotional aspects regarding to the acceptance of the patients clinical conditions and the behavioral skills to manage it (30). Finally, patient activation is related to the capacity of the patients in managing their clinical condition and the ability to interact with the healthcare system based on their level of knowledge (39, 40). It is suggested that an increase in patient activation leads to an increase in healthy behaviors and adherence to the clinical process (23). Patient activation has been defined by Hibbard et al. (23) as composed of four phases: (1) the passive activation level, where patients are not aware of their role in their health management; (2) where patients starts to create their resources and knowledge about their health condition; (3) where patients can elaborate ad hoc responses to the problems related to their clinical condition; and (4) where patients can maintain their new lifestyle behaviors for long-term periods, even when they are under stressful situations. Then, following the later commented phases, Hibbard et al. created the patient activation measure (PAM) to assess patient activation (23).
Hence, patient engagement considers not only the clinical environment but also the non-clinical contexts such as patients' daily routines, activity routines, and the acceptance of their clinical condition outside the hospital, by exploring the dialogue between the supplies and demands of the healthcare services (41). Concerning this, the use of new digital technologies to achieve the patients' engagement during and after the hospitalization period has been proposed (42).
Technology for Patient's Engagement in Neurorehabilitation
Today the development of new technologies has paved the way for their use for clinical purposes, especially to enhance patients' engagement in their healthcare routine (43). Recently, it has been demonstrated that the use of new digital technologies can modulate the dimensions described by Seligman (44) for positive psychology. Digital technologies have been considered essential for illness prevention such as courage, future-mindedness, optimism, interpersonal skill, faith, work ethic, hope, perseverance, flow, and joy (42). In this regard, it is known that the use of virtual environments and serious games can induce positive emotional states, creating new virtual environments for human psychological growth and well-being (45). Following the model proposed by Frome (46), four factors have to be present to induce positive emotions by using such virtual or serious games: a narrative factor, by using roleplaying through which is possible to feel the emotions of the virtual character; game-playing factor, by providing the feeling of frustration or satisfaction when winning or losing the game; the simulation factor, meaning that the game has to provide engaging activities; and the aesthetics factor, referring to the artistic features of the game. These factors can promote engagement of the users by using different technological sources such as mobile e-health (47), and e-learning platforms (48), biofeedback systems (49), virtual reality systems (50, 51), and playing videogames (45), at their own home.
In addition, new rehabilitation protocols, including the use of new technologies, have been developed in the neurorehabilitation field (52, 53). Particularly, the use of new technologies in neurorehabilitation, such as telerehabilitation systems, allows the patients to continue with their healthcare process at home (19, 54). In the field of neurorehabilitation, the rehabilitation and healthcare routine after the hospitalization period is complex, requiring a multidisciplinary coordination (55, 56). Telerehabilitation systems in neurorehabilitation allow a large number of people with neurological disorders—who often have limitations due to limited mobility and to costs associated with travel—to continue with their healthcare process at their own home, minimizing the barriers of distance, time and costs, and receiving continued support by the clinicians remotely (57, 58). The feasibility and efficacy of telerehabilitation systems in neurorehabilitation have been documented in patients with different neurological conditions such as patients in a post-stroke phase (59–61), Parkinson Disease (18, 62, 63), and Multiple Sclerosis (18, 64). Nevertheless, the role of engagement and the different factors to engage patients with neurological disorders in the telerehabilitation training during the rehabilitation period have not yet been deeply investigated. Hence, this systematic review aims at reviewing the effectiveness of different engagement strategies and the different engagement assessments while using telerehabilitation systems in patients with neurological disorders.
Methods
A systematic review of the scientific literature have been conducted in order to identify different engagement strategies, as well as studies reporting engagement assessment methods when using telerehabilitation systems in patients with neurological disorders. The systematic review protocol was designed following the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (65).
Data Sources and Search Strategy
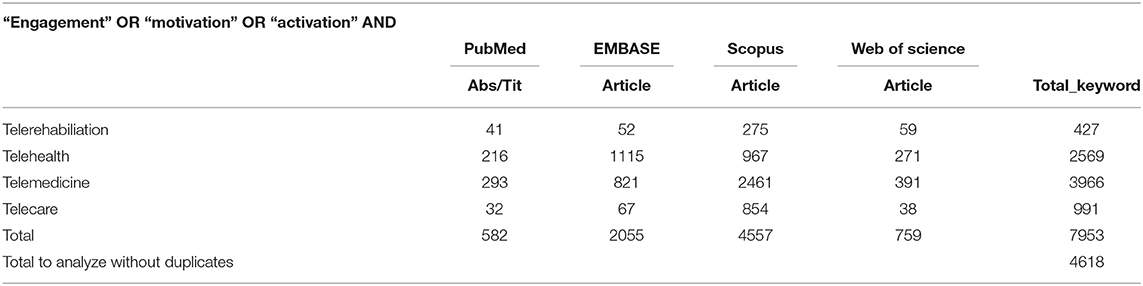
According to the PICO format to formulate the foreground question of this systematic review (66), the review question has been defined as, “in adults with neurological disorders, is the role of engagement for telerehabilitation interventions, compared to treatment as usual, effective in improving neurorehabilitation intervention.” Bibliographical data was collected on July 4, 2019, by using the following bibliographic databases: PubMed, EMBASE, Scopus, and Web of Science. For each database, we used the following combination of research keywords: (1) (“engagement” OR “motivation” OR “activation” AND “telerehabilitation”); (2) (“engagement” OR “motivation” OR “activation” AND “telehealth”); (3) (“engagement” OR “motivation” OR “activation” AND “telemedicine”); (4) (“engagement” OR “motivation” OR “activation” AND “telecare”). See the detailed search strategy in Table 1. Only full-text available articles were included in our research (conference paper were excluded), studies citation were retrieved independently for each string of keywords across all databases. Finally, the first list of the collected studies during the bibliographic research was exported to Mendeley to remove duplicated studies. Then the list of studies without duplicates was imported to Rayyan (67) for the title and abstract screening, following the specified inclusion or exclusion criteria for study selection (see section Study Selection and Data Collection) by one reviewer (M.M.G). The final list of the selected studies was sent to leading experts in the field for suggestion and identification of any missing studies, and no studies were added.
Study Eligibility Criteria
The present review aims at reviewing the effectiveness of different engagement strategies and the different engagement assessments while using telerehabilitation systems in patients with neurological disorders. Then, the selected studies had to investigate engagement while using telerehabilitation systems in adult patients with neurological disorders. Bibliographical research was limited to studies using humans and written in English. Further, the selected studies had to accomplish the following inclusion criteria:
1. Telerehabilitation interventions must have been directed to engage patients in their healthcare routine. Interventions directed to engage other stakeholders such as medical staff, hospital managers, and others were excluded.
2. Telerehabilitation interventions must have been directed to a group of patients, with a between or within-group study design. Single case studies have been excluded.
3. Telerehabilitation interventions have been directed to assess one or more components of patient engagement.
Study Selection and Data Collection
One reviewer (M.M.G.) conducted the final selection of the studies for full text analyses. The following keywords were considered as inclusion criteria for selected articles in Rayyan (67): neurorehabilitation, neurological patients, patients, participation, adherence, self-management, empowerment, activation, telerehabilitation, telehealth, telemedicine, telecare, e-health. Further the following keywords were considered as exclusion criteria: no engagement, no neurological patients, animal studies, and review studies. Then, the final selected articles that accomplished the inclusion criteria were analyzed by three reviewers (M.M.G., M.M., and J.M.) for independently full-text analyses. The final selected studies were discussed among the three reviewers in order to solve minor discrepancies about the study selection criteria that had been solved by consensus.
Risk of Bias Assessment
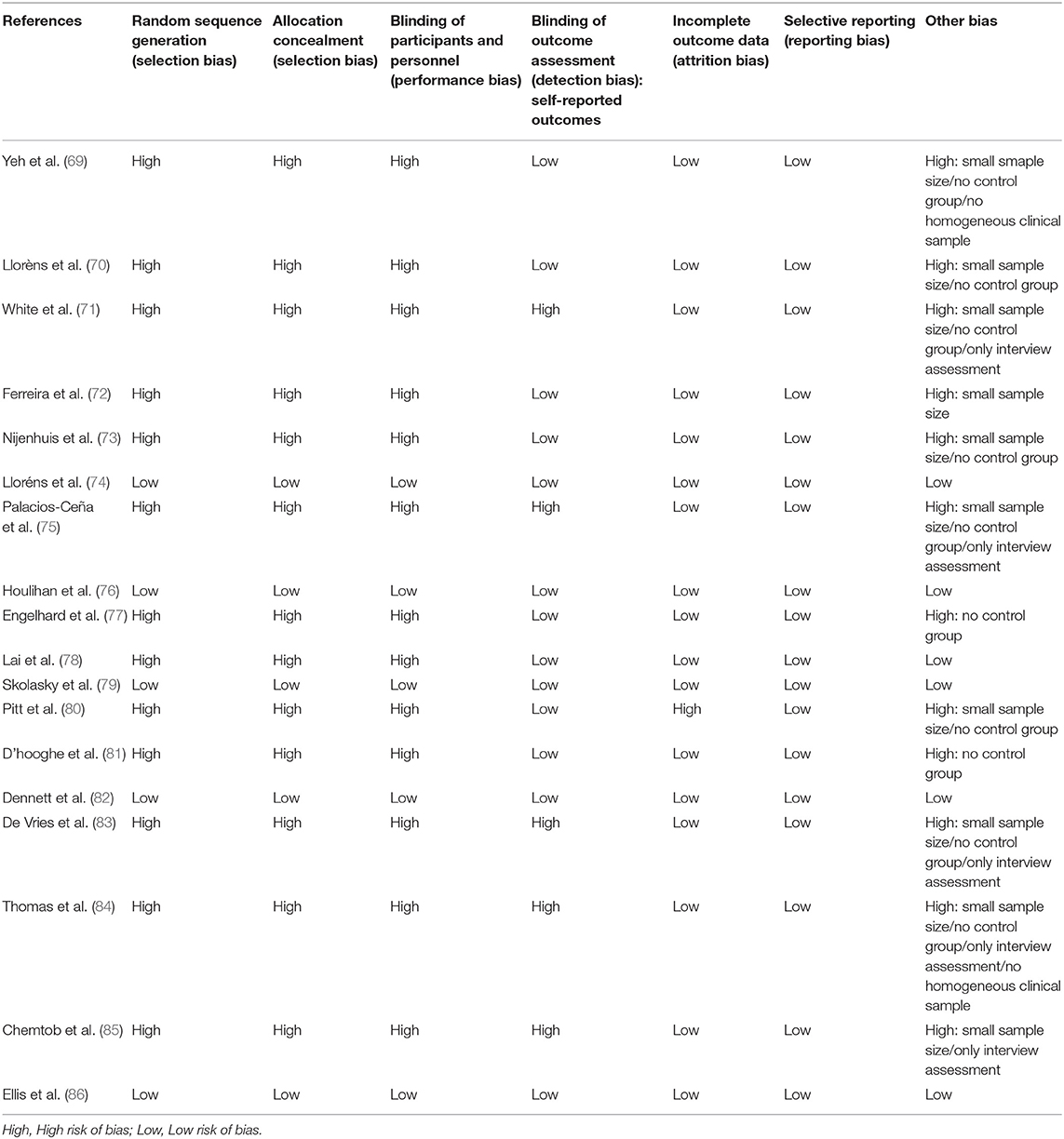
To the risk of bias assessment, the reviewers followed the guideline of the Cochrane Collaboration risk of bias tool according to the latest version of the risk of bias tool (RoB2) statement (68). All three reviewers (M.M.G, M.M, and J.M) independently evaluated the studies for risk of bias, and disagreements were resolved through consensus (Table 2).
Data Extraction
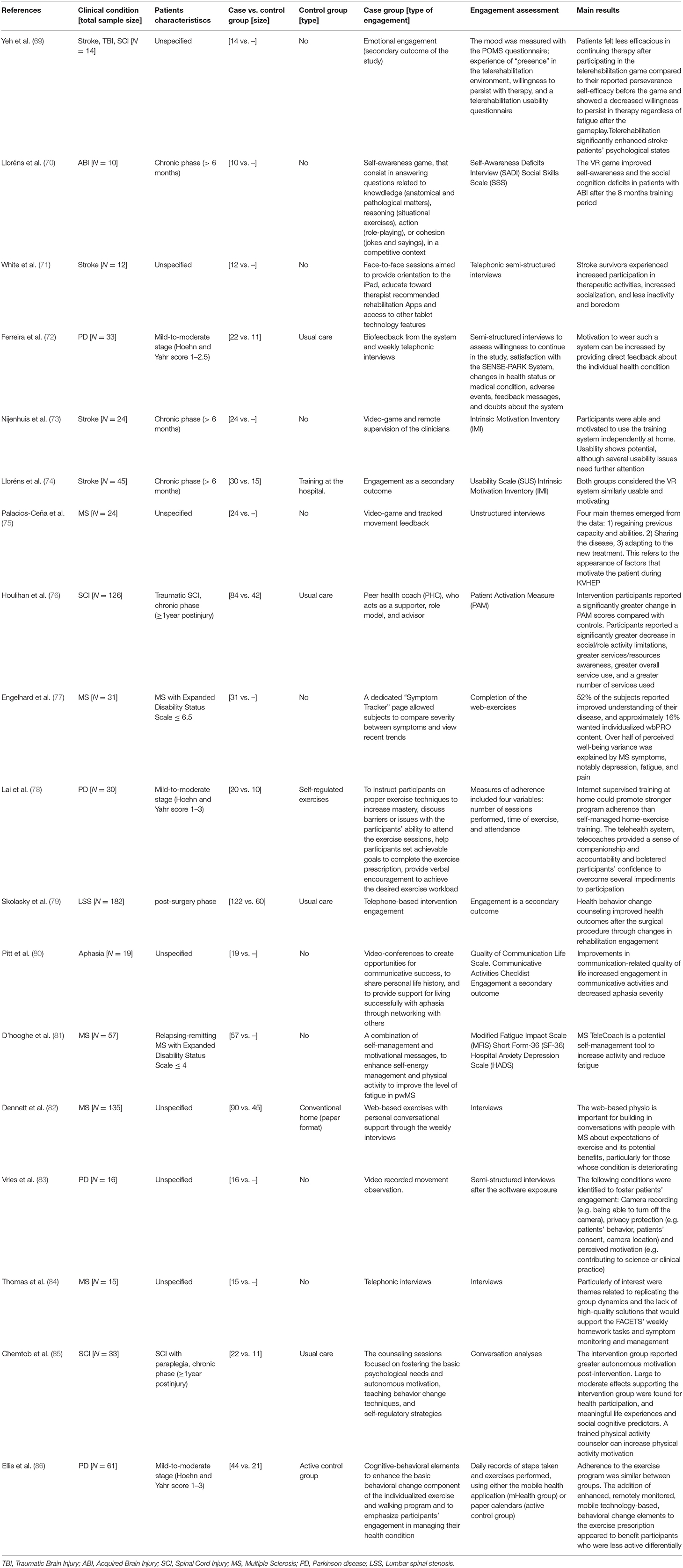
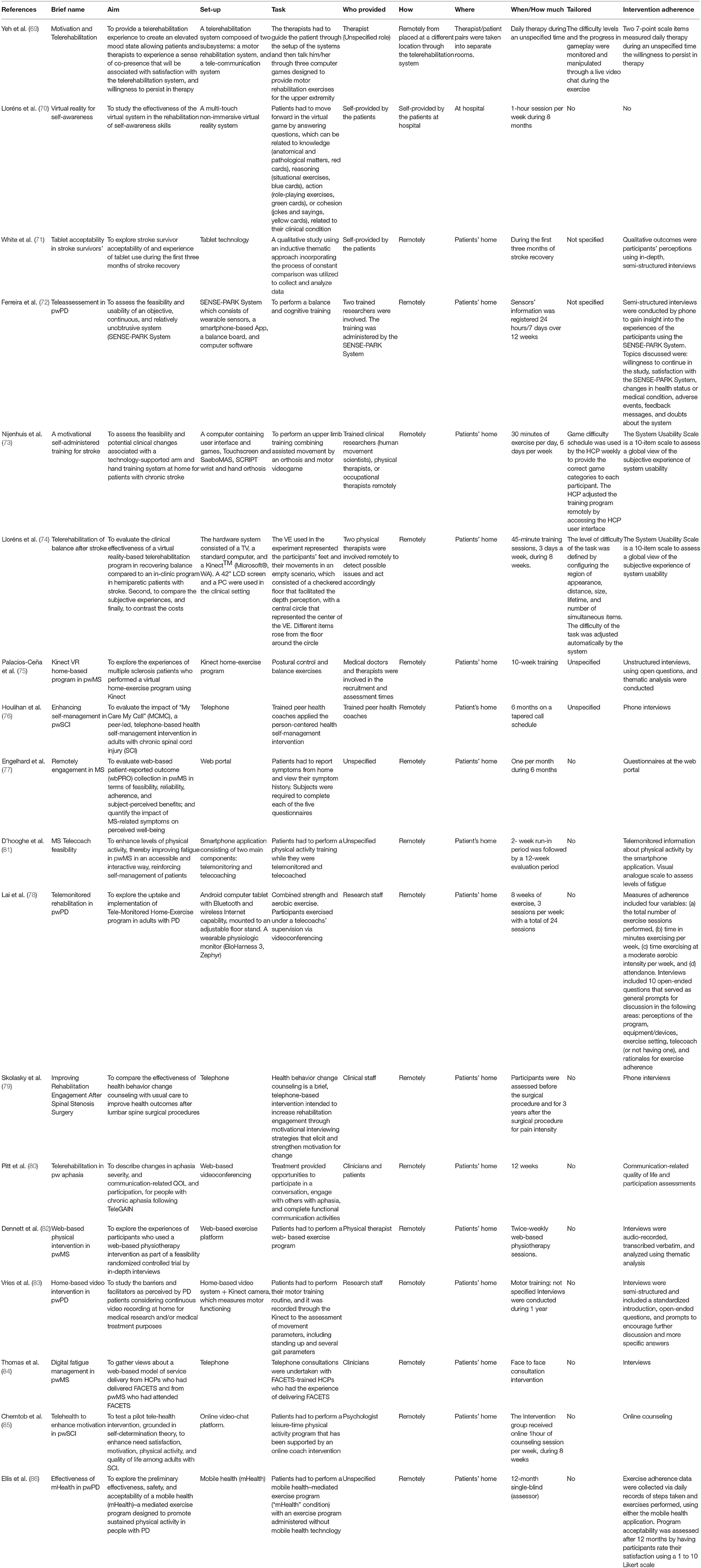
Each selected study was coded according to the following thematic categories: (1) Authors and Year of publication; (2) Clinical condition (N); (3) Patients characteristics; (4) Sample size; (5) Control group; (6) Type of engagement; (7) Engagement assessment; (8) Main results (Table 3). All three reviewers followed the coding studies criteria to analyze the final selected studies. Further, the TiDER checklist has been used for reporting detailed information about research interventions (87). Specifically, the following points of the TiDER checklist have been reported: (1) why (aim of the study), (2) what (materials), (3) who provided, (4) tailoring, and (5) intervention adherence (Table 4).
Results
Study Selection
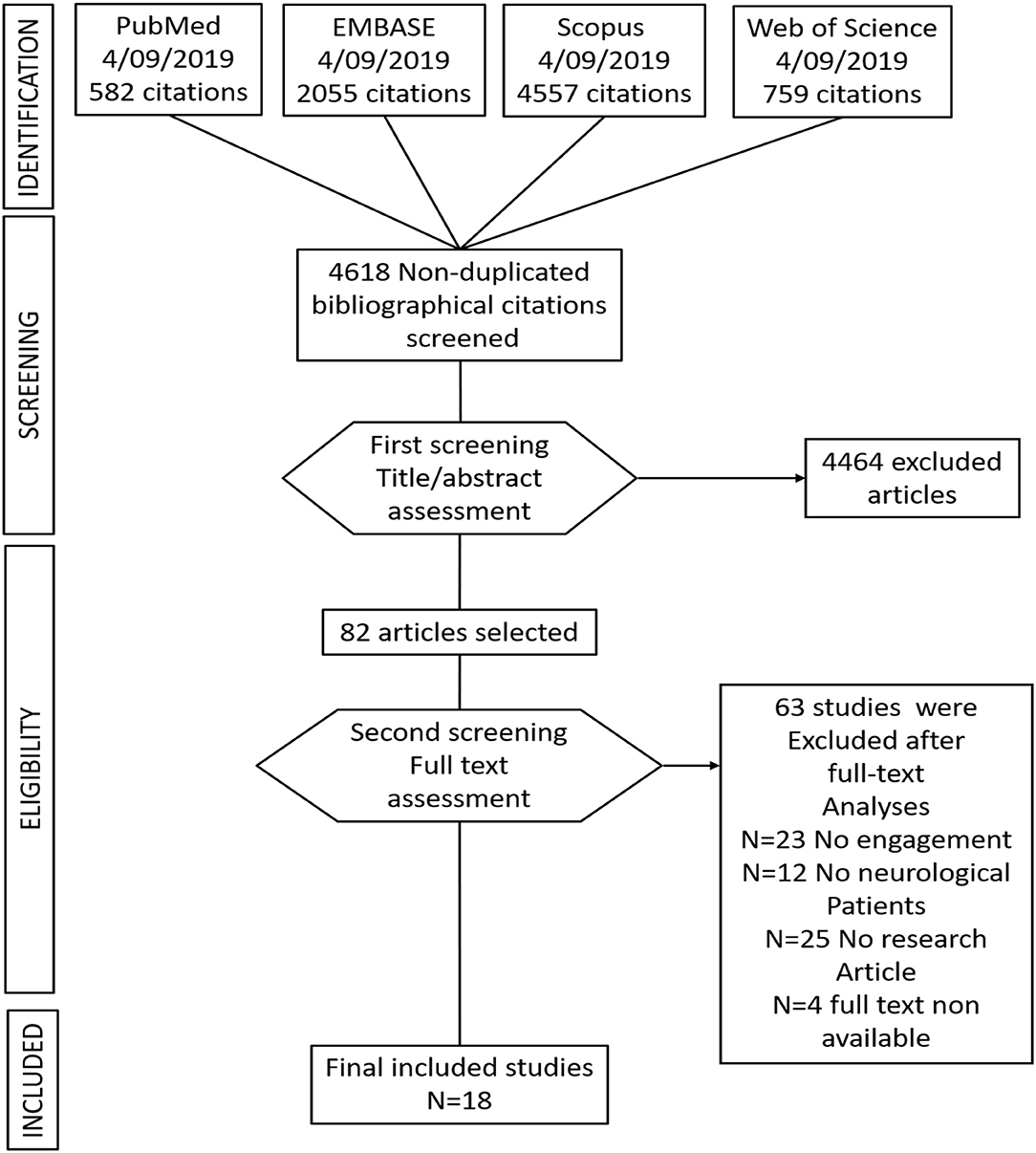
Seven thousand nine hundred and fifty three studies were found, including the above commented key words in section Data Sources and Search Strategy, and including the above-specified inclusion criteria words (section Study Selection and Data Collection). After removing duplicate studies, a total of 4,618 studies were included for the title and abstract screening into the Rayyan software. Of 4,618 non-duplicate studies, 4,464 studies did not accomplish the described study eligibility criteria. Subsequently, 82 studies were selected for full-text analyses. Of the 82 full text analyzed studies, only 18 studies were identified as suitable with the above-described inclusion criteria. See Figure 1 for a flow diagram depicting the study selection process.
Of 82 studies, only 18 studies included engagement strategies and engagement assessment either as a primary or secondary outcome after the telerehabilitation training in patients with neurological disorders.
Study Characteristics
The final eighteen selected studies were described in detail. Further, Table 3 shows the characteristics of each of the selected studies. Ten studies compared patients with neurological disorders with healthy subjects or with other group of patients (69, 72, 74, 76–79, 82, 85, 86). Among the selected studies four studies were conducted in patients with Parkinson Disease (PD) (72, 78, 83, 86), four in patients with stroke (69, 71, 73, 74), and five studies were conducted in patients with multiple sclerosis (MS) (75, 77, 81, 82, 84). All the selected studies used engagement strategies in their telerehabilitation program, as well as engagement assessment measures. Particularly, eight studies used interviews to obtain qualitative data of patient engagement (69, 71, 74, 75, 82–85), six studies used functional assessment scales (70, 72, 73, 76, 80, 81), and three studies used paper or digital diary reports (77, 78, 86).
Moreover, following the TiDER checklist for reporting research interventions (87), the following points have been reported in Table 4: (1) why (aim of the study), (2) what (materials), (3) who provided, (5) tailoring, and (6) intervention adherence. (2) Out of the eighteen analyzed studies, thirteen studies aimed at investigating the effectiveness, usability, feasibility, reliability, and acceptability of the telerehabilitation system (70–75, 77–79, 82–84, 86), one study aimed at investigating the sense of co-presence between the therapist and patients through the telerehabilitation system (69), three studies aimed at investigating changes in self-management, self-determination, and self-motivation after the telerehabilitation period (76, 81, 86), and finally one study aimed at assessing possible changes in aphasia severity after the telerehabilitation period (80). (3) Five studies used a computer-based telerehabilitation system (69, 73–75); three studies used a tablet set-up as a telerehabilitation platform (70, 71, 78); three studies used patients smart phones applications for psychological or motor telerehabilitation programs (72, 81, 86); three studies used phones as a set-up for telephone-based telerehabilitation intervention (76, 79, 82); finally, three studies used an online web-platform as an internet-based telerehabilitation intervention (77, 80, 85). (4) Out of the 18 selected studies, nine studies involved therapists (physiotherapist, psychologist, medical, coach therapist) or medical doctors in the administration of the telerehabilitation program (69, 74–76, 79, 80, 82, 84, 85); four studies involved trained researchers in the administration of the telerehabilitation program (72, 73, 78, 83), two studies described a patients self-administered telerehabilitation program (70, 71), and three studies did not specify who was involved into the telerehabilitation program (77, 81, 86). (5) Out of the 18 analyzed studies, only three studies adjusted the difficulty levels of the telerehabilitation program automatically according to the progress of the patients among the rehabilitation period (69, 73, 74). (6) Out of the 18 analyzed studies, only one study did not assess adherence to the intervention (70). Among the other 17 studies, 11 studies used semi-structured or unstructured interviews to assess patients adherence to the telerehabilitation program (71, 72, 75, 76, 78–84). Four studies used questionnaires (74, 75, 77, 86), two studies used the assessment report collected from the mobile or tablet rehabilitation application (78, 86), and one study used the online counseling feedback to assess patients adherence to the telerehabilitation program (85). In addition to the latter commented points, Table 4 shows more detailed information about the research intervention of each study.
Risk of Bias
All studies except five presented a high risk of bias in some of the assessed factors in this systematic review (74, 76, 79, 82, 86). Table 2 shows the results of the risk of bias assessment of this systematic review. All the studies included in this systematic review reported the sampling method. However, only five out of 18 studies presented a randomized control trial study design, including a control group for treatment comparisons (74, 76, 79, 82, 86). Ten studies presented an small sample size to represent the results obtained after the treatment period (69–73, 75, 80, 83–85). Five studies based their results on the analyses of interviews conducted to the patients without analyzing any other clinical measure for engagement assessment (71, 75, 83–85). All the studies included in this review reported their allocation sample method and study design. However, 12 studies did not have used random allocation methods for the sample allocation and not included a control group in the study design (70).
Engagement Interventions in Teleneurorehabilitation
Once the final 18 studies included in this systematic review have been analyzed, the studies were divided in those in which engagement was considered a primary outcome of the telerehabilitation training (n = 11) (70–72, 76–79, 81, 82, 84, 85), and those in which engagement was considered a secondary outcome of the telerehabilitation training (n = 7) (69, 73–75, 80, 83, 86).
Engagement as a Primary Outcome
Most of the 11 analyzed studies aimed at investigating the patient engagement as a primary outcome through a telerehabilitation training in patients with neurological disorders. In specific those studies involving patients' self-management, self-awareness, and self-determination strategies to enhance active patients' participation in their healthcare routine, and providing patients' empowerment. Such engagement strategies have been included in the behavioral and cognitive dimension of engagement (88). Specifically, in the present systematic review, four studies directed to enhance the behavioral and cognitive dimension of engagement while using telerehabilitation systems have been found. For instance, a non-immersive virtual reality multitouch system had been used in 10 acquired brain injury patients (ABI) at home to treat self-awareness deficit (70). Particularly, patients were engaged in a self-awareness game consisting of answering questions related to knowledge (anatomical and pathological matters), reasoning (situational exercises), action (role-playing), or cohesion (jokes and sayings), in a competitive context (70). Further, in another study, the authors used a smartphone application for both the telemonitoring and tele-coaching of 57 patients with multiple sclerosis (MS) (81). The study by D'hooghe et al. aimed at fostering patients' self-energy management and physical activity, decreasing the level of fatigue after physical activity. Regarding patients with MS, a web-based model (FACETS: Fatigue: Applying Cognitive-behavioral and Energy effectiveness Techniques to life Style) of service delivery from healthcare providers was also tested in 15 patients with MS to improve patients' behavioral and cognitive dimension of engagement (84). Further, an online video-chat platform was used as a pilot test telehealth intervention, grounded in self-determination theory, to enhance satisfaction, motivation, physical activity, and quality of life in adults with spinal cord injury (SCI) (n = 11) (85). Finally, an android application in a tablet together with a physiologic monitor was used as a telehealth system in 20 patients with PD to explore two different internet engagement trainings: a tele-coach assisted training (n = 10), and a self-regulated exercise training (n = 10) (78).
Other frequent strategies used for engagement in telerehabilitation are those directed to enhance patients' adherence and compliance to the therapy. Concerning this, in this systematic review, one study used a mobile web portal (wbPRO) to evaluate patient-reported outcomes in terms of feasibility, reliability, adherence, and subject-perceived benefits in 31 patients with MS, to quantify the impact of MS-related symptoms on the perceived patients' well-being (77). Moreover, a more sophisticated telerehabilitation system (SENSE-PARK system) including a set of wearable sensors (three to be used during the day and one at night), a Wii Balance Board software, and a smartphone application was used at patients' home to assess the feasibility and usability of the system, in 22 patients with PD (72). Further, a web-based physiotherapy platform with weekly personal, conversational support was used in patients with MS (n = 45), compared to a usual home paper format protocol (n = 45) to explore the user experience and feasibility of a web-based intervention (82).
Finally, in this systematic review, two studies directed to investigate the emotional components of the engagement strategies when using telerehabilitation systems were also found. These types of engagement strategies are embedded into the emotional dimension of engagement (88), usually implemented by using telephone and email interviews. Particularly, two studies were directed to enhance the emotional dimensions of engagement (76, 79). Specifically, in the study conducted by Houlihan et al., the therapists assessed the results obtained from a telephone-based health self-management intervention in patients with SCI (n = 42), compared with a usual care control group (n = 42). However, in the study conducted by Skolasky et al., the clinical staff involved in the study used motivational interviewing strategies to elicit and strengthen motivation for change in patients with MS (n = 31).
Engagement as a Secondary Outcome
Seven studies of this systematic review aimed to use telerehabilitation training for motor, cognitive, or logopedic interventions in patients with neurological disorders and to enhance patient engagement as a secondary outcome. Specifically, in this review, three studies were directed to investigate user experience, and system feasibility when using telerehabilitation systems for other neurorehabilitation proposes (73, 83, 86). As an example, in the study conducted by Ellis et al., they explored the preliminary effectiveness, safety, and acceptance of a mobile health (mHealth) application–a mediated exercise program– designed to promote sustained physical activity in 23 patients with PD. Moreover, in another study, the authors assessed the feasibility and potential clinical changes associated with telerehabilitation training for upper limb recovery, based in a robotic technology-supported arm, supported by a video-game training system in 24 patients with chronic stroke (73). Finally, De Vries et al. reported the opinion of 16 patients with PD when using a home-based system without video movement analysis (83).
Moreover, the other five studies aimed at investigating engagement as a secondary outcome when using telerehabilitation systems for neurorehabilitation proposes. Specifically, one study investigated changes in aphasia severity, communication-related quality of life, and participation, in 19 patients with aphasia while using the TeleGAIN telerehabilitation system (80). Moreover, another study investigated postural control and balance improvements after a 10-week of a virtual Kinect home-exercise program in 24 adults with MS, and assessed patients' adherence and motivation when using the telerehabilitation system as a secondary outcome (75). In one study conducted by Yeh et al., the authors tested a telerehabilitation system composed of two subsystems: a motor rehabilitation system and a telecommunication system to improve the mobility of patients with stroke and to motivate them to continue with the telerehabilitation training (69). Finally, in another study, the effectiveness of a virtual reality-based telerehabilitation program for balance recovery in chronic stroke patients was assessed and compared to the usual rehabilitation training (74).
Engagement Assessment
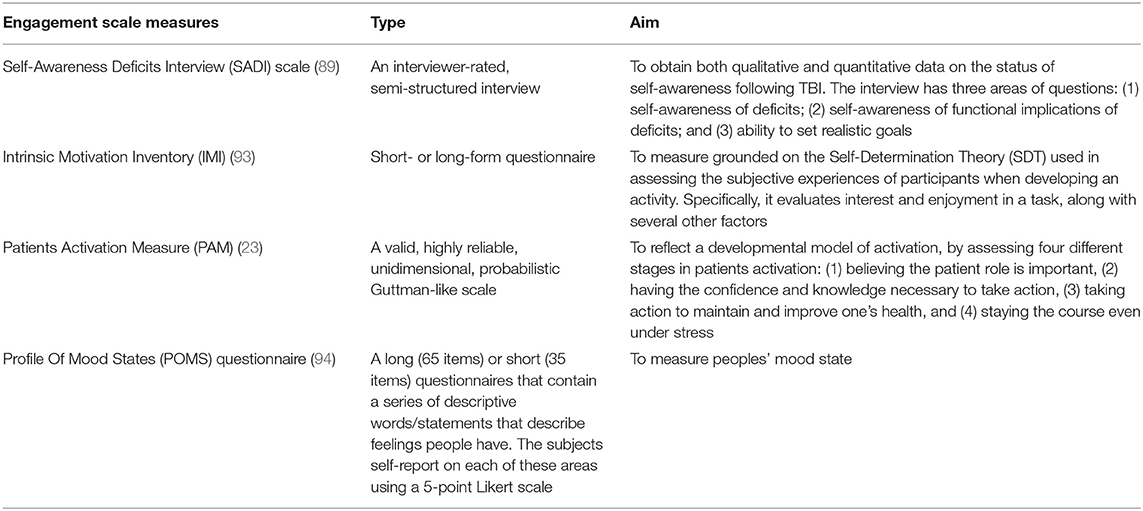
Among the analyzed studies in this systematic review, the following main three assessment methods have been found to assess patient engagement: measurement scales, telephone based-interviews, and paper diaries. Regarding the measurement scales in the study conducted by Lloréns et al. (70), the authors used the Self-Awareness Deficits Interview (SADI) scale (89), and the Social Skills Scale (SSS) (90). However, others used the Short Form-36 (SF-36) (91), and the Hospital Anxiety Depression Scale (HADS) (92) to assess engagement as a secondary outcome (81). Moreover, the Communication Life Scale and the communicative activities checklist were used in patients with aphasia to assess engagement as a secondary outcome (80). Finally, three scales directed to assess engagement as a primary outcome were used. The Intrinsic Motivation Inventory (IMI) (93), was used to assess the level of motivation in patients with stroke after the telerehabilitation period (73). The Patients Activation Measure (PAM) (23), was used to assess health self-management in patients with SCI (76). Finally, the Profile of Mood States (POMS) questionnaire (94) was used in patients with SCI or ABI after the telerehabilitation training period (69). Table 5 aims to summarize the different scale measures, and the aim of each engagement scale measure.
Engagement Outcomes
Engagement as a Primary Outcome
Regarding the outcomes observed in the analyzed studies which aimed to foster patient engagement as a primary outcome, we observed the following reported outcomes. The VR game proposed in the study conducted by Llorens et al., improved self-awareness and social cognition deficits in patients with ABI and PD after 8 months of a telerehabilitation training (70). Through a smartphone TeleCoach application, patients with MS increased activity and reduced fatigue levels after 12 weeks of training, improving patients' self-management (81). Moreover, another study demonstrated that by replicating rehabilitation group dynamics through a telerehabilitation system is possible to enhance patient engagement to the rehabilitation training in patients with MS (84). Regarding the use of telerehabilitation training in patients with stroke, one study showed that by using an iPad training stroke survivors experienced increased participation in therapeutic activities, increased socialization, as well as less inactivity and boredom (71). In addition to this, the results obtained in the study conducted by Nijenhuis et al. showed an increased motivation to participate in the rehabilitation training when using a remotely monitored training system at home (73). However, in another study conducted in patients with PD, the patients reported that direct feedback about the patients' health condition when using the telerehabilitation training system would help to increase patients' motivation (72). Another study showed that patients with PD benefit from a mobile biofeedback system that provides real feedback about patients' health conditions, and enhance patient engagement to the rehabilitation routine (86). Furthermore, in one study in which patients with stroke could feeling the sense of the co-presence of the therapist during the telerehabilitation training, the psychological state of the patients was improved (69). However, in contrast to the above-commented studies, one study reported a reduction in patients' self-efficacy and willingness regardless of patients' fatigue after the telerehabilitation training (69).
Finally, one study highlighted the importance of building in conversations by weekly interviews with people with MS about expectations of exercise and its potential benefits, particularly with those patients whose physical and mental conditions may be deteriorating while using motor telerehabilitation systems (82). In this regard, another study reported that health behavior change counseling by telephone-based interventions could improve health outcomes during the first 12 months after the surgical procedure in patients operated of spinal stenosis, improving patient engagement to the rehabilitation program (79). Moreover, 6 months of a telerehabilitation period based in a telephonic intervention program showed a more significant change in PAM scores, as well as a higher decrease in social/role activity limitations, and improvements in services/resources awareness in patients with SCI (76). Further, another telerehabilitation training using an online video-chat platform increase autonomous motivation in patients with SCI (85).
Engagement as a Secondary Outcome
Regarding the outcomes observed in the analyzed studies which aimed to foster patient engagement as a secondary outcome, we observed the following reported outcomes. One study reported improvements in communication-related quality of life in patients with aphasia, and a decrease of the aphasia severity, which lead to an increase of patient engagement in communicative activities (80). Another study conducted by Palacios-Ceña et al. highlighted the following positive factors reported by patients with MS after using a Kinect telerehabilitation systems: (1) the Kinect training increased the level of independence of the patients; (2) the patients reported to can share their illness state with their relatives'; (3) the patients reported positive effects about the incorporation of a videogame for rehabilitation, and (4) the patients reported positive effects regarding the possibility of evaluating themselves through the feedback provided by the telerehabilitation system (75).
Engagement Strategies Effectiveness
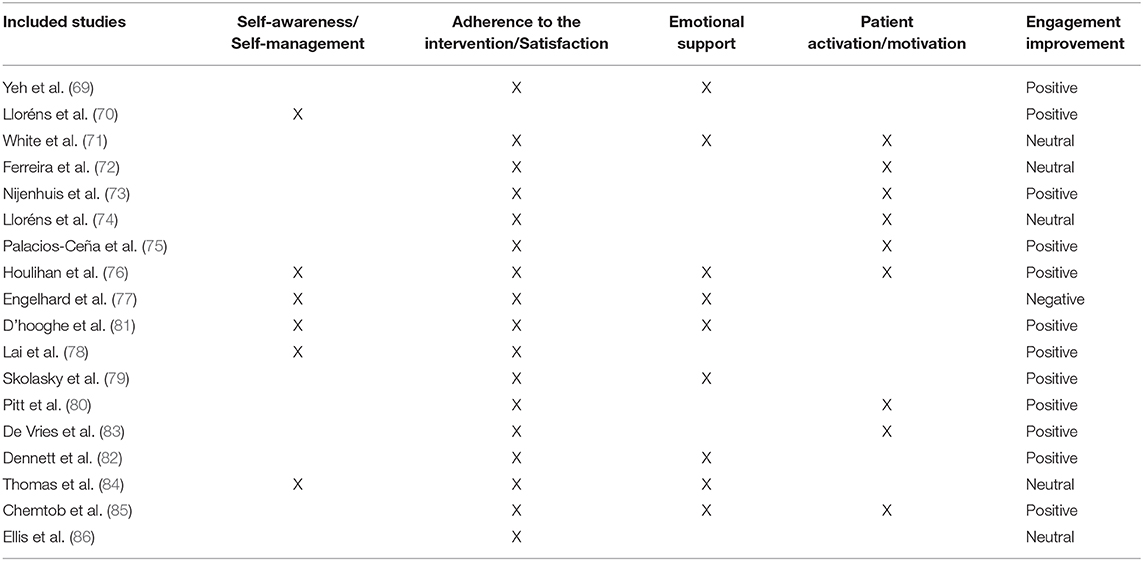
Overall, we found different patient engagement strategies throughout the 18 analyzed studies. Table 6 summarizes the different engagement strategies found among the analyzed studies, and the level of effectiveness of such engagement strategies for teleneurorehabilitation (positive, neutral, or negative). Specifically, 12 studies reported positive results when using tele-neurorehabilitation interventions for patient engagement (69, 70, 73, 75, 76, 78–83, 85). Five studies reported neutral effects in patient engagement after the tele-neurorehabilitation training period (71, 72, 74, 84, 86). Finally, only one study out of the 18 analyzed studies reported negative results in patients' adherence to the training after the telerehabilitation training period (77).
Discussion
The engagement of patients in the rehabilitation process is considered a primary aim for worldwide healthcare interventions [see (95)]. Patient engagement is considered a key component in neurorehabilitation in order to promote greater neuroplastic changes and functional outcomes (2). In this concern, digital technologies have been considered as a useful resource for enhancing patients' participation, allowing them to have an active role in their healthcare process (96, 97). The introduction of digital technologies in the field of neurorehabilitation has prompted the possibility to conduct the rehabilitation protocol at patients' homes (16, 98). Thus, telerehabilitation protocols save time for the patient by reducing displacements to the hospital, and the clinicians can follow the patients after the hospital discharge from the hospital (16, 98). However, which is the role of engagement when using tele-rehabilitation systems in neurorehabilitation? The here presented systematic review aims at reviewing the different engagement strategies and different engagement assessments while using telerehabilitation systems for neurorehabilitation.
In this systematic review, the studies were first divided into those in which patients' engagement was considered a first outcome of the telerehabilitation training, and those in which engagement was considered a secondary outcome of the telerehabilitation training. Interestingly, more studies that considered patients engagement as a primary outcome of the telerehabilitation training (N = 11), compared to those that considered patients engagement as a secondary outcome (N = 7) were found. Particularly, most of the analyzed studies that were directed to enhance patients' engagement through telerehabilitation systems in neurorehabilitation, had been conducted during the last 4 years from 2015 to 2019 (70–72, 76–79, 81, 82, 84, 85). This data indicates that fostering patients' engagement through the use of new technologies in neurorehabilitation has been a matter of interest for several years. Interestingly, this data is in line with the systematic review conducted by Barello et al. (99), in which they looked for studies using e-Health interventions for patient engagement, and highlighted the necessity of conducting more studies investigating the use of new digital technologies to enhance patient engagement. The data collected in this systematic review confirms that there was a progressive increase in the use of new technologies to engage patients, specifically those with neurological disorders, into their rehabilitation process. Secondly, our results showed an increase in interest in creating new telerehabilitation protocols in neurorehabilitation for enhancing patients' engagement by promoting patients' self-awareness and self-management (N = 6), patients' motivation (N = 9), and emotional support (N = 9). Such engagement components have been described as components of the behavioral and cognitive dimension of patients' engagement (30). Thus, in this systematic review, the studies analyzed were directed at fostering the behavioral and cognitive dimension through the use of telerehabilitation systems in patients with neurological diseases. These findings are supported by other investigations that were also directed at fostering the behavioral and cognitive dimension of engagement during the rehabilitation process of different clinical populations (100, 101). Concerning this, the results of this systematic review show that the use of telerehabilitation systems in patients with neurological disorders are useful for fostering the behavioral and cognitive dimension of engagement and for increase patients engagement with the rehabilitation program (73, 77, 78, 81, 84, 86). One explanation of this could be that through the telerehabilitation systems it is possible to give a real feedback to the patients about their physical and physiological conditions, as well as the possibility to interact with the telerehabilitation system (70, 73–75, 78, 81, 83). Concerning this, the studies of this systematic review are consistent with later investigations that demonstrated the effectiveness of digital technologies in inducing behavioral, physiological, and emotional responses by giving an immediate real feedback about such responses to the patients (22, 102–104). Moreover, such investigations were also directed at fostering the emotional dimension of the engagement, referring to the patients' acceptance of the disease, to an adequate adjustment to their illness (105), and improving the quality of the relationship between clinicians and patients (24). Specifically, in the analyzed studies of this systematic review, the emotional dimension of engagement has been tackled by using weekly telephonic interviews (72, 76, 84), using a face to face communication through on-line digital platforms (78, 80, 85), or by giving positive and motivating messages to the patients during the telerehabilitation training (78, 81).
Regarding the assessment of engagement during the telerehabilitation training in neurorehabilitation, the studies analyzed in this systematic review show that, at the moment, there are few available scales to assess the level of patient engagement and to deeply assess the different components of engagement. However, some available measures providing quantitative data about patient engagement such as the PAM (23), IMI (93), and the SADI (89), and POMS questionnaire (94) scales are available. Out of these four measures scales, the newest and the most used one is the PAM, which, as described in Table 5, enables the assessment of the patient activation during their healthcare routine in-depth. Although the PAM seems one of better measures to assess patient engagement, the POMS questionnaire could be an excellent complement to further assess the emotional state of the patients in their daily healthcare routine and during the telerehabilitation period in patients with neurological disease. The SADI is limited to patients with traumatic brain injury, and this limits the use of this scale to assess self-awareness of the illness in patients with other neurological pathologies. Finally, the IMI could be replaced by the PAM, as this is the newest measure that contemplates more aspects of patient activation in comparison to the IMI. Further, the results obtained in the PAM can reflect patient motivation to participate in their healthcare routine. Besides the quantitative engagement measures, a significant amount of studies that use interviews and diary reports for the qualitative assessment of patient engagement when using telerehabilitation systems were found. In this regard, it is known that data from motivational interviews play an essential role in evaluating patient engagement during the rehabilitation period (106, 107). Moreover, the efficacy of using semi-structured interviews to foster patients with chronic illness to participate in their healthcare routine has been demonstrated (108).
Finally, regarding the effectiveness of the engagement strategies used in the analyzed studies of this systematic review, 12 studies out of 18 reported positive outcomes in fostering patient engagement after the telerehabilitation training. In particular, the engagement strategies used in these 12 studies were mainly focused on patient participation, patient decision making, and patient self-management, all of them involved in the behavioral, cognitive, and emotional dimensions of engagement (see Table 6). Such positive results are in line with later studies in which a motivational model to foster participation in the neurorehabilitation programs was proposed (109). Moreover, others also proposed new neurorehabilitation strategies by enhancing patient self-management, self-awareness, and motivation in rehabilitation routines (2). Most of the revised studies in this systematic review presented positive results by enhancing the behavioral, cognitive, and emotional dimensions of patient engagement. However, most of them used a “monomethod” study design, directed at assessing qualitative or quantitative engagement outcomes.
Limitations
The present systematic review shows the following limitations regarding the standard protocols for systematic reviews: no registration in a public database, a librarian was not included in the bibliographic research stage, and no duplicate and independent searches of the studies were done.
Conclusions
The studies commented throughout this systematic review pave the way for the design of new telerehabilitation protocols, not only focusing on measuring quantitative or qualitative measures but measuring both of them through a mixed model intervention design (1). The future clinical studies with a mixed model design will provide more abundant data regarding the role of engagement in telerehabilitation, leading to a possibly greater understanding of its underlying components.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
MM-G and OR developed the paper concept. MM-G carried out the bibliographic review, was responsible for the methodology, and wrote the manuscript draft. MM and JM contributed to the drafting of the manuscript. FB, FR, and PM gave bibliographic suggestions and reviewed the manuscript for important intellectual content. GR, FM, and OR supervised the editing and revisions for important intellectual content. All the authors approved the final version of the manuscript for submission.
Funding
The study was co-funded by Lombardy Region (Announcement POR-FESR 2014-2020), within the project named Sidera∧B (Sistema Integrato DomiciliarE e Riabilitazione Assistita al Benessere).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Powell H, Mihalas S, Onwuegbuzie AJ, Suldo S, Daley CE. Mixed methods research in school psychology: a mixed methods investigation of trends in the literature. Psychol. Sch. (2008) 45:291–309. doi: 10.1002/pits.20296
2. Danzl MM, Etter NM, Andreatta RO, Kitzman PH. Facilitating neurorehabilitation through principles of engagement. J Allied Health. (2012) 41:35–41.
3. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. (2008) 51:S225–39. doi: 10.1044/1092-4388(2008/018)
4. Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. (2017) 2017:3589271. doi: 10.1155/2017/3589271
5. Moucha R, Kilgard MP. Chapter 7 cortical plasticity and rehabilitation. Progress in Brain Research. Elsevier (2006) 157:111–389. doi: 10.1016/S0079-6123(06)57007-4
6. Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PMandR. (2010) 2:S208–19. doi: 10.1016/j.pmrj.2010.10.016
7. Matthews G, Warm JS, Reinerman LE, Langheim LK, Saxby DJ. Task engagement, attention, and executive control. In: Gruszka A, Matthews G, Szymura B, editors. Handbook of Individual Differences in Cognition. The Springer Series on Human Exceptionality. New York, NY: Springer (2010). p. 205–30.
8. Nadeau SE. A paradigm shift in neurorehabilitation. Lancet Neurol. (2002) 1:126–30. doi: 10.1016/S1474-4422(02)00044-3
9. Bovend'eerdt TJH, Dawes H, Wade DT. Practical research-based guidance for motor imagery practice in neurorehabilitation. Disabil Rehabil. (2012) 34:2192–200. doi: 10.3109/09638288.2012.676703
10. Miller KL. Patient centered care: a path to better health outcomes through engagement and activation. NeuroRehabilitation. (2016) 39:465–70. doi: 10.3233/NRE-161378
11. Lupton D. Digital Health: Critical and Cross-Disciplinary Perspectives. Critical Approaches to Health. New York, NY: Routledge/Taylor and Francis Group (2018). Available online at: https://search.proquest.com/docview/2009246760?accountid=16562
12. Foster CH, Richards J, Thorogood M, Hillsdon M, Kaur A, Wickramasinghe KK, et al. Remote and Web 2.0 interventions for promoting physical activity. Cochrane Database Syst Rev. (2013) CD010395. doi: 10.1002/14651858.CD010395
13. Serino S, Baglio F, Rossetto F, Realdon O, Cipresso P, Parsons TD, et al. Picture Interpretation Test (PIT) 360°: an innovative measure of executive functions. Sci Rep. (2017) 7:16000. doi: 10.1038/s41598-017-16121-x
14. Realdon O, Serino S, Savazzi F, Rossetto F, Cipresso P, Parsons TD, et al. An ecological measure to screen executive functioning in MS: the Picture Interpretation Test (PIT) 360°. Sci Rep. (2019) 9:5690. doi: 10.1038/s41598-019-42201-1
15. Piron L, Turolla A, Agostini M, Zucconi C, Cortese F, Zampolini M, et al. Exercises for paretic upper limb after stroke: a combined virtual-reality and telemedicine approach. J Rehabil Med. (2009) 41:1016–20. doi: 10.2340/16501977-0459
16. Agostini M, Lorenzo M, Rita B, Vanna P, Paolo T, Annalena V, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. (2015) 21:202–13. doi: 10.1177/1357633X15572201
17. Tella SD, Blasi V, Mendozzi L, Rovaris M, Baglio F. Integrated telerehabilitation approach in multiple sclerosis: a systematic review and meta-analysis. J Telemed Telecare. (2019) 1–15. doi: 10.1177/1357633X19850381
18. Isernia S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, Gramigna C, et al. Efficiency and patient-reported outcome measures from clinic to home: the human empowerment aging and disability program for digital-health rehabilitation. Front Neurol. (2019) 10:1206. doi: 10.3389/fneur.2019.01206
19. Realdon O, Rossetto F, Nalin M, Baroni I, Cabinio M, Fioravanti R, et al. Technology-enhanced multi-domain at home continuum of care program with respect to usual care for people with cognitive impairment: the ability-telerehabilitation study protocol for a randomized controlled trial. BMC Psychiatry. (2016) 16:425. doi: 10.1186/s12888-016-1132-y
20. Palsbo SE, Bauer D. Telerehabilitation: Managed Care's New Opportunity. Managed Care Quarterly (2000).
21. Bensing J. Bridging the gap: the separate worlds of evidence-based medicine and patient-centered medicine. Pat Educ Couns. (2000) 39:17–25. doi: 10.1016/S0738-3991(99)00087-7
22. Graffigna G, Barello S, Riva G, Bosio AC. Patient engagement: the key to redesign the exchange between the demand and supply for healthcare in the era of active ageing. Stud Health Technol Inform. (2014) 203:85–95. doi: 10.3233/978-1-61499-425-1-85
23. Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. (2004) 39:1005–26. doi: 10.1111/j.1475-6773.2004.00269.x
24. Graffigna G, Barello S. Innovating healthcare in the era of patient engagement: challenges, opportunities and new trends. In: Patient Engagement: A Consumer-Centered Model to Innovate Healthcare. Berlin, Boston: De Gruyter (2016). p. 1–12. doi: 10.1515/9783110452440
25. Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. N Engl J Med. (2011) 365:2445–7. doi: 10.1056/NEJMp1112442
26. Entwistle VA, Watt IS. Patient involvement in treatment decision-making: the case for a broader conceptual framework. Pat Educ Couns. (2006) 63:268–78. doi: 10.1016/j.pec.2006.05.002
27. Marteau TM, Dormandy E. Facilitating informed choice in prenatal testing: how well are we doing? Am J Med Genet. (2001) 106:185–90. doi: 10.1002/ajmg.10006
28. Charles C, Gafni A, Whelan T. How to improve communication between doctors and patients. Br Med J. (2000) 320:1220. doi: 10.1136/bmj.320.7244.1220
29. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. (1999) 49:651–61. doi: 10.1016/S0277-9536(99)00145-8
30. Graffigna G, Barello S, Triberti S. Giving (Back) a role to patients in the delivery of healthcare services: theoretical roots of patient engagement. In: Patient Engagement: A Consumer-Centered Model to Innovate Healthcare. Berlin, Boston, MA: De Gruyter (2016). p. 13–26.
31. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Pat Prefer Adher. (2012) 6:38–48. doi: 10.2147/PPA.S24752
32. Ingram TL. Compliance: a concept analysis. Nursing Forum. (2009) 44:189–94. doi: 10.1111/j.1744-6198.2009.00142.x
33. Stewart M. Towards a global definition of patient centred care: the patient should be the judge of patient centred care. Br Med J. (2001) 322:444–5. doi: 10.1136/bmj.322.7284.444
34. Barlow J. How to use education as an intervention in osteoarthritis. Best Pract Res Clin Rheumatol. (2001) 15:545–58. doi: 10.1053/berh.2001.0172
35. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Pat Educ Couns. (2002) 48:177–87. doi: 10.1016/S0738-3991(02)00032-0
36. Aujoulat I, d'Hoore W, Deccache A. Patient empowerment in theory and practice: polysemy or cacophony?” Pat Educ Couns. (2007) 66:13–20. doi: 10.1016/j.pec.2006.09.008
37. Feste C, Anderson RM. Empowerment: from philosophy to practice. Pat Educ Couns. (1995) 26:139–44. doi: 10.1016/0738-3991(95)00730-N
38. Anderson RM, Martha MF. Patient empowerment: myths and misconceptions. Pat Educ Couns. (2010) 79:277–82. doi: 10.1016/j.pec.2009.07.025
39. Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. (2012) 27:520–6. doi: 10.1007/s11606-011-1931-2
40. Greene J, Hibbard JH, Tusler M. How Much Do Health Literacy and Patient Activation Contribute to Older Adults' Ability to Manage Their Health? AARP Public Policy Institute (2005).
41. Graffigna G, Barello S, Bonanomi A, Lozza E. Measuring patient engagement: development and psychometric properties of the Patient Health Engagement (PHE) scale. Front Psychol. (2015). 6:274. doi: 10.3389/fpsyg.2015.00274
42. Riva G, Baños RM, Botella C, Wiederhold BK, Gaggioli A. Positive technology: using interactive technologies to promote positive functioning. Cyberpsychol Behav Soc Netw. (2012) 15:69–77. doi: 10.1089/cyber.2011.0139
43. Triberti S, Riva G. Positive technology for enhancing the patient engagement experiences. Patient Engagement: A Consumer-Centered Model to Innovate Healthcare. Berlin; Boston, MA: De Gruyter (2016). doi: 10.1515/9783110452440-005
44. Seligman MEP. Positive psychology, positive prevention, and positive therapy. Handbook of Positive Psychology (2002). p. 3–12.
45. Argenton L, Triberti S, Serino S, Muzio M, Riva G. Serious games as positive technologies for individual and group flourishing. Stud. Comput. Intell. (2014) 536:221–244. doi: 10.1007/978-3-642-45432-5_11
46. Frome J. Eight ways videogames generate emotion. In: 3rd Digital Games Research Association International Conference: “Situated Play”, DiGRA 2007. Tokyo (2007).
47. Tachakra S, Wang XH, Istepanian RSH, Song YH. Mobile E-health: the unwired evolution of telemedicine. Telemed J E-Health. (2003) 9:247–57. doi: 10.1089/153056203322502632
48. Sinclair P, Kable A, Levett-Jones T. The effectiveness of internet-based e-learning on clinician behavior and patient outcomes: a systematic review protocol. JBI Database Syst Rev Implement Rep. (2015) 13:52–64. doi: 10.11124/jbisrir-2015-1919
49. Giggins OM, Persson UMC, Caulfield B. Biofeedback in rehabilitation. J Neuroeng Rehabil. (2013) 10:60. doi: 10.1186/1743-0003-10-60
50. Repetto C, Gorini A, Algeri D, Vigna C, Gaggioli A, Riva G. The use of biofeedback in clinical virtual reality: the intrepid project. Stud Health Technol Inform. (2009) 7:128–32. doi: 10.3791/1554
51. Turolla A, Dam M, Ventura L, Tonin P, Agostini M, Zucconi C, et al. Virtual reality for the rehabilitation of the upper limb motor function after stroke: a prospective controlled trial. J Neuroeng Rehabil. (2013) 10:85. doi: 10.1186/1743-0003-10-85
52. Salisbury DB, Dahdah M, Driver S, Parsons TD, Richter KM. Virtual reality and brain computer interface in neurorehabilitation. Baylor Univ Med Center Proc. (2017) 29:124–7. doi: 10.1080/08998280.2016.11929386
53. Rinne P, Mace M, Nakornchai T, Zimmerman K, Fayer S, Sharma P, et al. Democratizing neurorehabilitation:how accessible are low-cost mobile-gaming technologies for self-rehabilitation of arm disability in stroke? PLoS ONE. (2016) 11:413. doi: 10.1371/journal.pone.0163413
54. Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: a literature overview of technologies and clinical applications. NeuroRehabilitation. (2010) 27:287–304. doi: 10.3233/NRE-2010-0612
55. Barnes MP, Radermacher H. Neurological rehabilitation in the community. J Rehabil Med. (2001) 33:244–8. doi: 10.1080/165019701753236419
56. Atwal A, Kirsty T, Kay C, Christine C. Multidisciplinary perceptions of the role of nurses and healthcare assistants in rehabilitation of older adults in acute health care. J Clin Nurs. (2006) 15:1418–25. doi: 10.1111/j.1365-2702.2005.01451.x
57. Hailey D, Roine R, Ohinmaa A, Dennett L. Evidence of benefit from telerehabilitation in routine care: a systematic review. J Telemed Telecare. (2011) 17:281–7. doi: 10.1258/jtt.2011.101208
58. Huijgen BC, Vollenbroek-Hutten MMR, Zampolini M, Opisso E, Bernabeu M, van Nieuwenhoven J, et al. Feasibility of a home-based telerehabilitation system compared to usual care: arm/hand function in patients with stroke, traumatic brain injury and multiple sclerosis. J Telemed Telecare. (2008) 14:249–56. doi: 10.1258/jtt.2008.080104
59. Chen J, Liu M, Sun D, Jin Y, Wang T, Ren C. Effectiveness and neural mechanisms of home-based telerehabilitation in patients with stroke based on FMRI and DTI: a study protocol for a randomized controlled trial. Medicine. (2018) 97:e9605. doi: 10.1097/MD.0000000000009605
60. Piron L, Tonin P, Trivello E, Battistin L, Dam M. Motor tele-rehabilitation in post-stroke patients. Inform Health Soc Care. (2004) 29:119–25. doi: 10.1080/14639230410001723428
61. Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomized, controlled trial. Stroke. (2012) 43:2168–74. doi: 10.1161/STROKEAHA.111.646943
62. Giansanti D, Macellari V, Maccioni G. Telemonitoring and telerehabilitation of patients with Parkinson's disease: health technology assessment of a novel wearable step counter. Telemed e-Health. (2008) 14:76–83. doi: 10.1089/tmj.2007.0019
63. Gandolfi M, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual reality telerehabilitation for postural instability in Parkinson's disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. (2017) 2017:7962826. doi: 10.1155/2017/7962826
64. Khan F, Amatya B, Kesselring J, Galea M. Telerehabilitation for persons with multiple sclerosis. Cochrane Database Syst Rev. (2015) 4:CD010508. doi: 10.1002/14651858.CD010508.pub2
65. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 64:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
66. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching pubmed for clinical questions. BMC Med Informat Decis Mak. (2007) 7:16. doi: 10.1186/1472-6947-7-16
67. Elmagarmid A, Fedorowicz Z, Hammady H, Ilyas I, Khabsa M, Ouzzani M. Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for cochrane reviews. In: Evidence-Informed Public Health: Opportunities and Challenges. Abstracts of the 22nd Cochrane Colloquium. Hyderabad: John Wiley & Sons (2014).
68. Sterne JAC, Savovi? J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:14898. doi: 10.1136/bmj.l4898
69. Yeh SC, McLaughlin M, Nam Y, Sanders S, Chang C, Kennedy B, et al. Emotions and telerebabilitation: pilot clinical trials for virtual telerebabilitation application using haptic device and its impact on post stroke patients' mood and motivation. In: Shumaker R, editor. International Conference on Virtual and Mixed Reality. VMR 2011: Virtual and Mixed Reality - Systems and Applications. Berlin; Heidelberg: Springer (2011). p. 119–128.
70. Lloréns R, Navarro MD, Alcañiz M, Noé E. Therapeutic effectiveness of a virtual reality game in self-awareness after acquired brain injury. Ann Rev CyberTher Telemed. (2012) 10:297–301. doi: 10.3233/978-1-61499-121-2-297
71. White JH, Janssen H, Jordan L, Pollack M. Tablet technology during stroke recovery: a survivor's perspective. Disabil Rehabil. (2015) 37:1186–92. doi: 10.3109/09638288.2014.958620
72. Ferreira JJ, Godinho C, Santos AT, Domingos J, Abreu D, Lobo R, et al. Quantitative home-based assessment of parkinson's symptoms: the SENSE-PARK feasibility and usability study. BMC Neurol. (2015) 15:89. doi: 10.1186/s12883-015-0343-z
73. Nijenhuis SM, Prange GB, Amirabdollahian F, Sale P, Infarinato F, Nasr N, et al. Feasibility study into self-administered training at home using an arm and hand device with motivational gaming environment in chronic stroke. J Neuroeng Rehabil. (2015) 12:89. doi: 10.1186/s12984-015-0080-y
74. Lloréns R, Noé E, Colomer C, Alcañiz M. Effectiveness, usability, and cost-benefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2015) 96:418–25.e2. doi: 10.1016/j.apmr.2014.10.019
75. Palacios-Ceña D, Ortiz-Gutierrez RM, Buesa-Estellez A, Galân-Del-Rio F, Cachón-Pérez JM, Martinez-Piedrola R, et al. Multiple sclerosis patients' experiences in relation to the impact of the kinect virtual home-exercise programme: a qualitative study. Eur J Phys Rehabil Med. (2016) 52:347–55.
76. Houlihan BV, Brody M, Everhart-Skeels S, Pernigotti D, Burnett S, Zazula J, et al. Randomized trial of a peer-led, telephone-based empowerment intervention for persons with chronic spinal cord injury improves health self-management. Arch Phys Med Rehabil. (2017) 98:1067–76.e1. doi: 10.1016/j.apmr.2017.02.005
77. Engelhard MM, Patek SD, Sheridan K, Lach JC, Goldman MD. Remotely engaged: lessons from remote monitoring in multiple sclerosis. Int J Med Inform. (2017) 100:26–31. doi: 10.1016/j.ijmedinf.2017.01.006
78. Lai B, Bond K, Kim Y, Barstow B, Jovanov E, Bickel CS. Exploring the uptake and implementation of tele-monitored home-exercise programmes in adults with Parkinson's disease: a mixed-methods pilot study. J Telemed Telecare. (2018) 26:53–63. doi: 10.1177/1357633X18794315
79. Skolasky RL, Maggard AM, Wegener ST, Riley LH. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am Vol. (2018) 100:21–30. doi: 10.2106/JBJS.17.00418
80. Pitt R, Theodoros D, Hill AJ, Russell T. The impact of the telerehabilitation group aphasia intervention and networking programme on communication, participation, and quality of life in people with aphasia. Int J Speech Lang Pathol. (2018) 21:1–11. doi: 10.1080/17549507.2018.1488990
81. D'hooghe M, Van Gassen G, Kos D, Bouquiaux O, Cambron M, Decoo D, et al. Improving fatigue in multiple sclerosis by smartphone-supported energy management: the MS TeleCoach Feasibility Study. Mult Scler Relat Disord. (2018) 22:90–6. doi: 10.1016/j.msard.2018.03.020
82. Dennett R, Coulter E, Paul L, Freeman J. A qualitative exploration of the participants' experience of a web-based physiotherapy program for people with multiple sclerosis: does it impact on the ability to increase and sustain engagement in physical activity? Disabil Rehabil. (2019) 1–8. doi: 10.1080/09638288.2019.1582717
83. De Vries NM, Smilowska K, Hummelink J, Abramiuc B, Van Gilst MM, Bloem BR, et al. Exploring the Parkinson patients' perspective on home-based video recording for movement analysis: a qualitative study. BMC Neurol. (2019) 19:71. doi: 10.1186/s12883-019-1301-y
84. Thomas S, Pulman A, Thomas P, Collard S, Jiang N, Dogan H, et al. Digitizing a face-to-face group fatigue management program: exploring the views of people with multiple sclerosis and health care professionals via consultation groups and interviews. JMIR Form Res. (2019) 3:e10951. doi: 10.2196/10951
85. Chemtob K, Rocchi M, Arbour-Nicitopoulos K, Kairy D, Fillion B, Sweet SN. Using tele-health to enhance motivation, leisure time physical activity, and quality of life in adults with spinal cord injury: a self-determination theory-based pilot randomized control trial. Psychol Sport Exerc. (2019) 43:243–52. doi: 10.1016/j.psychsport.2019.03.008
86. Ellis TD, Cavanaugh JT, DeAngelis T, Hendron K, Thomas CA, Saint-Hilaire M, et al. Comparative effectiveness of mhealth-supported exercise compared with exercise alone for people with parkinson disease: randomized controlled pilot study. Phys Ther. (2019) 99:203–16. doi: 10.1093/ptj/pzy131
87. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687
88. Graffigna G, Barello S, Riva G. Technologies for patient engagement. Health Aff. (2013) 32:1172. doi: 10.1377/hlthaff.2013.0279
89. Fleming JM, Strong J, Ashton R. Self-awareness of deficits in adults with traumatic brain injury: how best to measure? Brain Injury. (1996) 10:1–15. doi: 10.1080/026990596124674
90. Riggio RE. Assessment of basic social skills. J Pers Soc Psychol. (1986) 51:649–60. doi: 10.1037/0022-3514.51.3.649
91. Stewart M. The medical outcomes study 36-item short-form health survey (SF-36). Aust J Physiother. (2007) 53:208. doi: 10.1016/S0004-9514(07)70033-8
92. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
93. Ryan R, Deci E. Intrinsic Motivation Inventory (IMI) [Measurement Instrument]. The Intrinsic Motivation Inventory, Scale Description (1994). Available online at: http://selfdeterminationtheory.org/intrinsic-motivation-inventory (accessed January 01, 2018).
94. McNair DM, Lorr M, Droppleman LF. Manual for the POMS. Educational and Industrial Testing Service (1971). p. 205–30.
95. Hardyman W, Daunt KL, Kitchener M. Value co-creation through patient engagement in health care: a micro-level approach and research agenda. Public Manage Rev. (2015) 17:90–107. doi: 10.1080/14719037.2014.881539
96. Maye F, Cox M. Digital revolution – implementation of an electronic assistive technology pilot project in a neuro-rehabilitation setting. Int J Integr Care. (2017) 17:A112. doi: 10.5334/ijic.3417
97. O'Neil O, Fernandez MM, Herzog J, Beorchia M, Gower V, Gramatica F, et al. Virtual reality for neurorehabilitation: insights from 3 European clinics. PM R. (2018) S198–206. doi: 10.1016/j.pmrj.2018.08.375
98. Perry JC, Ruiz-Ruano JA, Keller T. Telerehabilitation: toward a cost-efficient platform for post-stroke neurorehabilitation. In” IEEE International Conference on Rehabilitation Robotics, Zurich (2011).
99. Barello S, Triberti S, Graffigna G, Libreri C, Serino S, Hibbard J, et al. EHealth for patient engagement: a systematic review. Front Psychol. (2015) 6:2013. doi: 10.3389/fpsyg.2015.02013
100. Sharry J, Davidson R, McLoughlin O, Doherty G. A service-based evaluation of a therapist-supported online cognitive behavioral therapy program for depression. J Med Internet Res. (2013) 15:e121. doi: 10.2196/jmir.2248
101. Robertson L, Smith M, Castle D, Tannenbaum D. Using the internet to enhance the treatment of depression. Aust Psychiatry. (2006) 14:413–7. doi: 10.1111/j.1440-1665.2006.02315.x
102. Riva G. Is presence a technology issue? Some insights from cognitive sciences. Virtual Real. (2009) 13:159–69. doi: 10.1007/s10055-009-0121-6
103. Gaggioli A, Raspelli S, Grassi A, Pallavicini F, Cipresso P, Wiederhold BK, et al. Ubiquitous health in practice: the interreality paradigm. Studies in Health Technology and Informatics. In MMVR (2011). p. 185–191.
104. Serino S, Triberti S, Villani D, Cipresso P, Gaggioli A, Riva G. Toward a validation of cyber-interventions for stress disorders based on stress inoculation training: a systematic review. Virtual Real. (2014) 18:73–87. doi: 10.1007/s10055-013-0237-6
105. Barello S, Graffigna G. Engaging patients to recover life projectuality: an Italian cross-disease framework. Qual Life Res. (2015) 24:1087–96. doi: 10.1007/s11136-014-0846-x
106. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. (2003) 71:843–61. doi: 10.1037/0022-006X.71.5.843
107. Öhman A. Qualitative methodology for rehabilitation research. J Rehabil Med. (2005) 37:273–80. doi: 10.1080/16501970510040056
108. Pomey MP, Ghadiri DP, Karazivan P, Fernandez N, Clavel N. Patients as partners: a qualitative study of patients' engagement in their health care. PLoS ONE. (2015) 10:e0122499. doi: 10.1371/journal.pone.0122499
Keywords: engagement, self-management, patient activation, digital technologies, teleneurorehabilitation
Citation: Matamala-Gomez M, Maisto M, Montana JI, Mavrodiev PA, Baglio F, Rossetto F, Mantovani F, Riva G and Realdon O (2020) The Role of Engagement in Teleneurorehabilitation: A Systematic Review. Front. Neurol. 11:354. doi: 10.3389/fneur.2020.00354
Received: 03 December 2019; Accepted: 09 April 2020;
Published: 06 May 2020.
Edited by:
Paolo Tonin, Sant'Anna Institute, ItalyReviewed by:
Janne Marieke Veerbeek, University of Zurich, SwitzerlandHannes Devos, University of Kansas, United States
Copyright © 2020 Matamala-Gomez, Maisto, Montana, Mavrodiev, Baglio, Rossetto, Mantovani, Riva and Realdon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Matamala-Gomez, bWFydGEubWF0YW1hbGExMEBnbWFpbC5jb20=; bWFydGEubWF0YW1hbGFnb21lekB1bmltaWIuaXQ=
 Marta Matamala-Gomez
Marta Matamala-Gomez Marta Maisto1
Marta Maisto1 Petar Aleksandrov Mavrodiev
Petar Aleksandrov Mavrodiev Francesca Baglio
Francesca Baglio Federica Rossetto
Federica Rossetto Fabrizia Mantovani
Fabrizia Mantovani Giuseppe Riva
Giuseppe Riva Olivia Realdon
Olivia Realdon