- 1Department of Neurology, University Hospital Basel and University of Basel, Basel, Switzerland
- 2Department of Intensive Care, University Hospital Basel and University of Basel, Basel, Switzerland
Background: Venous thromboembolism (VTE) can occur simultaneously with a cryptogenic stroke (CS) linked to patent foramen ovale (PFO), given paradox thromboembolism as potential stroke cause. However, little is known on the frequency of concomitant VTE and CS. We aimed to review the literature on the frequency of VTE in patients with CS linked to PFO (primary aim) and of ischemic stroke (IS) among patients with pulmonary embolism (PE) (secondary aim).
Methods: We performed a Medline search for cohort studies, written in English, with the following characteristics: (a) enrolling patients hospitalized for an acute ischemic stroke undergoing a work-up for deep venous thrombosis (DVT) and/or PE. To be included in this review, a study had to have at least a subgroup of patients with PFO; (b) the time interval between the index stroke and the work-up had to be within 40 days and the studies had to differentiate between DVT and PE. For the secondary aim, studies had to include patients with acute PE, known PFO-status and routine brain imaging on admission or within 1 year.
Results: We found eight studies reporting on the frequency of VTE after an acute CS linked to PFO. Concerning DVT, the reported frequency ranged between 7 and 27%; concerning PE, it lied between 4.4 and 37%. Six studies assessed the frequency of ischemic brain lesions among patients with an acute PE. In all studies, the presence of PFO was associated with ischemic brain lesions, both at baseline and follow-up.
Conclusion: VTE can be detected in patients with CS linked to PFO. While –based on the presented literature–routine screening for VTE in patients with CS linked to PFO does not appear justified, history taking, and clinical exam should consider concomitant VTE. Whenever clinically suspected, the threshold to trigger ancillary testing for VTE should be low. Among patients with an acute PE and PFO, vigilance for new neurologic deficits should be increased, with a low threshold for brain imaging.
Background
Up to date, ~25% of ischemic stroke are described as cryptogenic (CS) (1). Even though a prospective follow up study did not describe a PFO as an independent risk factor for ischemic stroke in general (2), various studies demonstrated an association between PFO and CS (3–6). The suspected pathophysiological mechanism is paradox embolism, enabling a passage of the venous thrombus through the PFO into the arterial circulation (7). Imaging demonstrating the migration of a thrombus was also described (8). The source of venous thromboembolism (VTE) is often suspected in the peripheral venous system. An acute rise of the right atrial pressure—for example through a Valsalva maneuver—could facilitate the passage through a PFO. Ozcan et al. (9) described an association between Valsalva maneuver and a history of VTE with a PFO related ischemic stroke. Four trials demonstrated—after PFO closure—a reduced incidence of recurrent ischemic stroke compared to antithrombotic therapy (antiplatelet or anticoagulation) (10–13). However, none of the trials mandated screening for VTE, and all had anticoagulation as an exclusion criterion. In clinical practice, detection of VTE leads to anticoagulation, potentially postponing PFO closure as long as anticoagulation is needed, given the lack of data on concomitant anticoagulation linked to PFO closure.
In addition, patients with PFO and a diagnosed PE may be at increased risk for ischemic stroke, further underlying the role of paradox embolism (14).
In this work, we aim to review the literature on the frequency of VTE in patients with CS linked to PFO, and the frequency of ischemic stroke in patients with PE.
Methods
For this narrative review, we performed a Medline search using the keyword “deep vein thrombosis,” “patent foramen ovale” and “ischemic stroke.” Two reviewers (AZ, GMDM) evaluated the included studies. We searched for cohort studies, written in English after 1990, enrolling patients hospitalized for an acute ischemic stroke undergoing a work-up for deep venous thrombosis (DVT) and/or pulmonary embolism (PE). To be included in this review, a study had to have at least a subgroup of patients with PFO and had to differentiate between DVT and PE. The time interval between the index stroke and the work-up did not have to exceed 40 days, to increase chances of finding VTE linked to paradox embolism rather than secondary to immobilization due to the index stroke.
Concerning the secondary aim, we included cohort studies written in English who (a) enrolled patients with acute pulmonary embolism (b) performed a search for patent foramen ovale and (c) carried out a brain imaging after the diagnosis of an acute PE. In our Medline search we used the keyword “patent foramen ovale,” “pulmonary embolism” and “stroke.”
Results
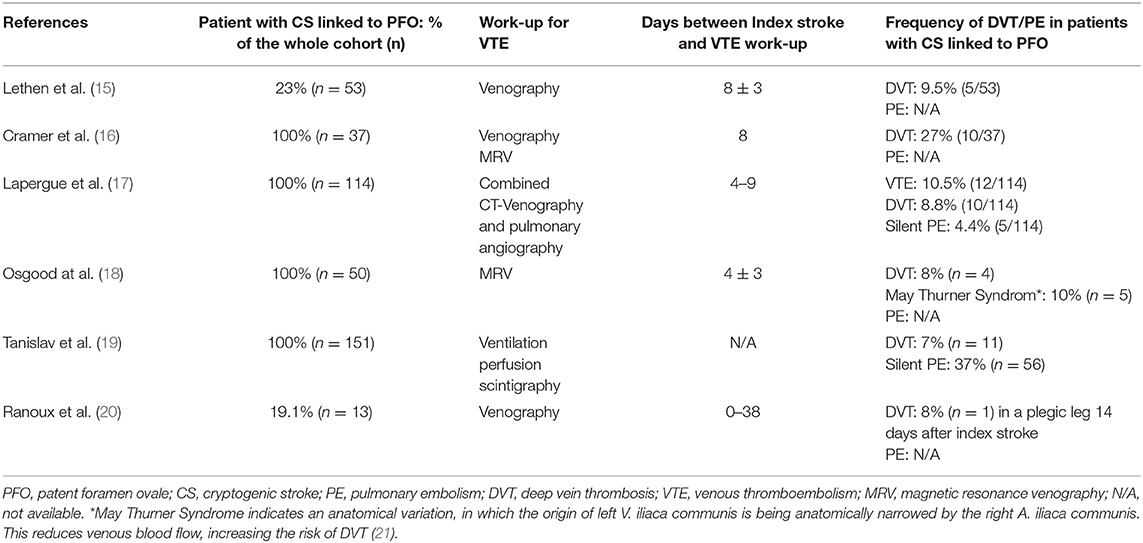
Our review identified eight studies reporting the frequency of VTE in patients with CS linked to PFO. Six of these studies did not compare the frequency of DVT between CS and non-CS patients (Table 1) (15–20), two studies did (Table 2) (22, 23).
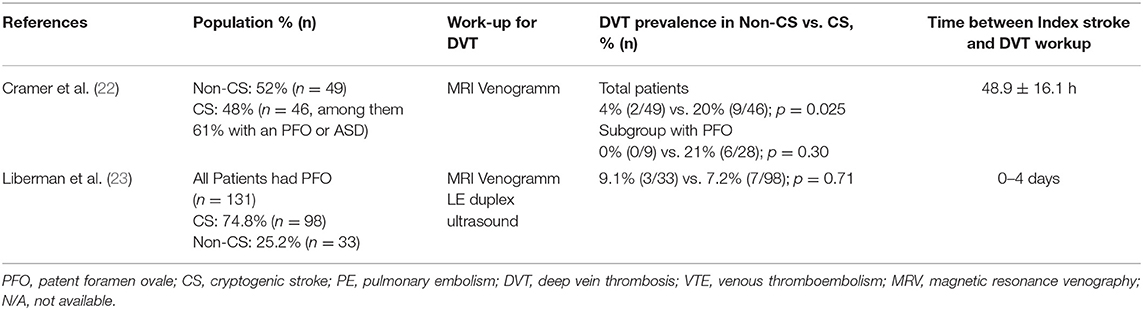
Table 2. Summary of studies comparing the frequency of DVT among patients with cryptogenic vs. known-cause stroke.
Studies Not Comparing the Frequency of DVT in Patients With CS Vs. Non-CS
Investigation regarding the emergence of VTE were performed within 0 to 38 days after index stroke. Concerning DVT, the reported frequency ranged between 7 and 27%; concerning PE, it lied between 4.4 and 37% (15–20). Concomitant DVT in patients with PE were described in two studies: Lapergue et al. (17) found a DVT in 3 out of 5 patients with silent PE, Tanislav et al. (19) in 8 out of 56 patients. In a study by Osgood et al. (18) four pelvic DVT were diagnosed (8%), as well as 5 cases of May Thurner Syndrom. The latter describes an anatomical variation, in which the left V. iliaca communis is being anatomically narrowed by the right A. iliaca communis. This reduces venous blood flow, increasing the risk of DVT (21).
Studies Comparing the Frequency of DVT in Patients With CS Vs. Non-CS
The prospective PELVIS study found—in patients with CS—more MR-venograms with pelvic DVT compared to non-CS (20 vs. 4%, p = 0.025), suggesting the source of paradox embolism may be located in the pelvic veins in a subset of patients with CS. Notably—when looking at the subgroup with PFO—there was no significant difference between CS and non-CS in the frequency of DVT (21 vs. 0%, p = 0.30) (22). In the retrospective study of Liberman et al. (23), contrast enhanced MR-venograms were used, and patients with CS vs. non-CS were compared. All patients, both CS and non-CS, had PFO. No significant difference in the frequency of DVT—both pelvic and lower extremity—was found between CS and non-CS (7.2 vs. 9.1%, p = 0.71), calling for further research before implementing routine pelvic MR-venograms. Clinical evidence of a PE was found in one patient with chronic lower extremity DVT.
Studies on the Frequency of Ischemic Strokes in Patients With Acute PE and PFO
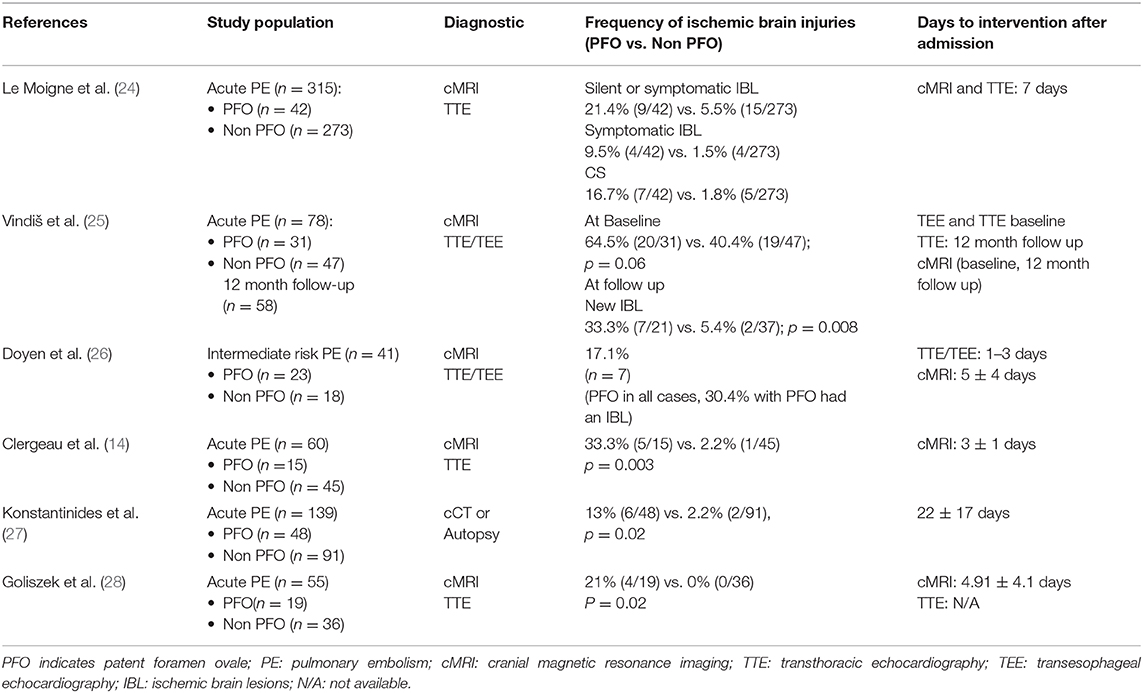
We found six studies; detailed analyses regarding population characteristics, diagnostic measures and time to interventions after admission are outlined in Table 3 (14, 24–28). Overall, ischemic stroke was reported to be diagnosed within 2–22 days following after admission and was more frequent in patients with overt PFO with four studies revealing statistical significance (14, 25, 27, 28). In the study of Konstantinides et al. (27) all investigations were performed during the hospital stay (22 ± 17 days).
Discussion
Studies demonstrated a wide range in the reported frequency of VTE in patients with CS linked to PFO, likely because the diagnostic of lower extremity DVT depends on the investigator and expertise in using duplex sonography (15, 20). In asymptomatic patients, a lower sensitivity (60%) of venous duplex sonography is described (29).
The two studies comparing the frequency of DVT between patients with CS vs. non-CS yielded conflicting results. In PELVIS (22)—but not in the study by Liberman et al. (23)—a higher frequency of DVT was observed among patients with CS than among those with non-CS. Differences in the DVT screening protocols as well as baseline characteristics may explain the conflicting results. In contrast to PELVIS, in the study of Liberman et al. (23) MR-venograms were contrast-based (i.e., less prone to artifacts), all patients had PFO, were older (mean age 46 years vs. 57 years, respectively) and had a higher burden of cardiovascular risk factors. To note, neither the subgroup of PFO patients in the PELVIS study nor the patients in the study of Libermann at al. (23) showed significant differences on the DVT frequency. Before implementing routine MR-Venography in clinical practice, further research is needed.
Liberman et al. (23) used the Causative Classification System to retrospectively classify the etiology of the ischemic stroke. Of note, patients with transient ischemic attacks were also included. In PELVIS, a stroke neurologist was responsible to identify and classify the cause of the ischemic stroke, based on the TOAST criteria.
In the three pivotal trials on PFO-closure (10, 11, 13), a search for VTE was not part of the routine diagnostic work up. In the follow up examinations, the occurrence of PE or DVT in the PFO closure group and the medical therapy group were reported as adverse events. Suspecting the frequency of underdiagnosed VTE, the risk of PE could even rise after PFO closure and without an effective oral anticoagulation. However, only the long-term evaluation of the RESPECT trial showed a higher detection rate of PE in the PFO closure group (24% vs 0.6%, p = 0.03) (12).
The CLOSE study compared PFO closure to oral anticoagulation. Three recurrent ischemic strokes were reported in the oral anticoagulation arm, whereas no recurrent stroke was described in the PFO closure arm (30). Since no trial allowed for PFO closure under concomitant oral anticoagulation, there are no data concerning PFO closure under oral anticoagulation. Thus, the diagnosis of DVT/PE—indicating oral anticoagulation for at least 3 months—could postpone PFO-closure leaving patients at risk of a stroke recurrence even under oral anticoagulation. To note, the early start of an oral anticoagulation could also lead to hemorrhagic transformation (7).
The reported association between PE and IS in patients with PFO further underlines the role of paradox embolism. Particularly in patients with intermediate-risk PE, PFO related ischemic brain lesions were frequent, up to 17.1% (26). Of note, none of these patients had a significant carotid stenosis or suspected cardioembolic source of ischemic stroke. Even under effective oral anticoagulation, Vindiš et al. (25) reported a significant difference in recurrent ischemic lesions in patients with PFO after PE, raising the question if PFO closure should be considered in some patients with PE (25).
Conclusion and Clinical Implications
Since VTE calls for therapeutic anticoagulation, the clinically important question arises if a baseline search for DVT in patients with CS linked to PFO is necessary. The reported frequency of DVT in two studies using MRI Venogram showed a large range of up to 20% (22, 23) while other studies described lower frequencies (15, 17). In patients with CS linked to PFO, the focus of medical history and physical exam should be intensified on the search for DVT/PE. The threshold for DVT/PE screening should be low, giving the potential subsequent indications for oral anticoagulation linked to PFO screening. Further prospective studies are needed to establish the optimal diagnostic work up for VTE/PE in patients with CS linked to PFO, as well as the safety of combining anticoagulation to PFO-closure.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
GD formulated the research question. AZ and GD summarized and extracted the data manually from published papers for this review. GD, AZ, and RS drafted the article and reviewed it critically.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13:429–38. doi: 10.1016/S1474-4422(13)70310-7
2. Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. (2006) 47:440–5. doi: 10.1016/j.jacc.2005.10.044
3. Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. (2000) 55:1172–9. doi: 10.1212/WNL.55.8.1172
4. Michael H, Andreas H, Manfred O, Andreas H, Annette G. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. (2007) 357:2262–8. doi: 10.1056/NEJMoa071422
5. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. (1988) 318:1148–52. doi: 10.1056/NEJM198805053181802
6. Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. (1992) 117:461–5. doi: 10.7326/0003-4819-117-6-461
7. Schwamm LH, Jaff MR, Dyer KS, Gonzalez RG, Huck AE. Case 13-2016: a 49-year-old woman with sudden hemiplegia and aphasia during a transatlantic flight. N Engl J Med. (2016) 374:1671–80. doi: 10.1056/NEJMcpc1501151
8. Srivastava TN, Payment MF. Images in clinical medicine. Paradoxical embolism–thrombus in transit through a patent foramen ovale. N Engl J Med. (1997) 337:681. doi: 10.1056/NEJM199709043371005
9. Ozcan Ozdemir A, Tamayo A, Munoz C, Dias B, David Spence J. Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism. J Neurol Sci. (2008) 275:121–7. doi: 10.1016/j.jns.2008.08.018
10. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377:1033–42. doi: 10.1056/NEJMoa1707404
11. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol. (2018) 71:2335–42.
12. Saver JL, Carroll JD, Thaler DE, Smalling RW, Macdonald LA, Marks DS, et al. Longterm outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. (2017) 377:1022–32. doi: 10.1056/NEJMoa1610057
13. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. (2017) 377:1011–21. doi: 10.1056/NEJMoa1705915
14. Clergeau MR, Hameon M, Morello R, Saloux E, Viader F, Hamon M. Silent cerebral infarcts in patients with pulmonary embolism and a patent foramen ovale: a prospective diffusion-weighted mri study. Stroke. (2009) 40:3758–62. doi: 10.1161/STROKEAHA.109.559898
15. Lethen H, Flachskampf FA, Schneider R, Sliwka U, Köhn G, Noth J, et al. Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack. Am J Cardiol. (1997) 80:1066–9. doi: 10.1016/S0002-9149(97)00604-8
16. Cramer SC, Maki JH, Waitches GM, Souza ND', Grotta JC, Burgin WS, et al. Paradoxical emboli from calf and pelvic veins in cryptogenic stroke. J Neuroimaging. (2003) 13:218–23. doi: 10.1111/j.1552-6569.2003.tb00181.x
17. Lapergue B, Decroix JP, Evrard S, Wang A, Bendetowicz D, Offroy MA, et al. Diagnostic yield of venous thrombosis and pulmonary embolism by combined CT venography and pulmonary angiography in patients with cryptogenic stroke and patent foramen ovale. Eur Neurol. (2015) 74:69–72. doi: 10.1159/000437261
18. Osgood M, Budman E, Carandang R, Goddeau RP, Henninger N. Prevalence of pelvic vein pathology in patients with cryptogenic stroke and patent foramen ovale undergoing MRV pelvis. Cerebrovasc Dis. (2015) 39:216–23. doi: 10.1159/000376613
19. Tanislav C, Puille M, Pabst W, Reichenberger F, Grebe M, Nedelmann M, et al. High frequency of silent pulmonary embolism in patients with cryptogenic stroke and patent foramen ovale. Stroke. (2011) 42:822–4. doi: 10.1161/STROKEAHA.110.601575
20. Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke. (1993) 24:31–4. doi: 10.1161/01.STR.24.1.31
21. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. (1957) 8:419–27. doi: 10.1177/000331975700800505
22. Cramer SC, Rordorf G, Maki JH, Kramer LA, Grotta JC, Burgin WS, et al. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study. Stroke. (2004) 35:46–50. doi: 10.1161/01.STR.0000106137.42649.AB
23. Liberman AL, Daruwalla VJ, Collins JD, Maas MB, Botelho MPF, Ayache JB, et al. Diagnostic yield of pelvic magnetic resonance venography in patients with cryptogenic stroke and patent foramen ovale. Stroke. (2014) 45:2324–9. doi: 10.1161/STROKEAHA.114.005539
24. Le Moigne E, Timsit S, Salem D Ben, Didier R, Jobic Y, Paleiron N, et al. Patent foramen ovale and ischemic stroke in patients with pulmonary embolism: a prospective cohort study. Ann Intern Med. (2019) 170:756–63. doi: 10.7326/M18-3485
25. Vindiš D, Hutyra M, Šanák D, Král M, Cecháková E, Littnerová S, et al. Patent foramen ovale and the risk of cerebral infarcts in acute pulmonary embolism—a prospective observational study. J Stroke Cerebrovasc Dis. (2018) 27:357–64. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.004
26. Doyen D, Castellani M, Moceri P, Chiche O, Lazdunski RÃ, Bertora D, et al. Patent foramen ovale and stroke in intermediate-risk pulmonary embolism. Chest. (2014) 146:967–73. doi: 10.1378/chest.14-0100
27. Konstantinides S, Geibel A, Kasper W, Olschewski M, Blümel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. (1998) 97:1946–51. doi: 10.1161/01.CIR.97.19.1946
28. Goliszek S, Wisniewska M, Kurnicka K, Lichodziejewska B, Ciurzynski M, Kostrubiec M, et al. Patent foramen ovale increases the risk of acute ischemic stroke in patients with acute pulmonary embolism leading to right ventricular dysfunction. Thromb Res. (2014) 134:1052–6. doi: 10.1016/j.thromres.2014.09.013
29. Tomkowski WZ, Davidson BL, Wisniewska J, Malek G, Kober J, Kuca P, et al. Accuracy of compression ultrasound in screening for deep venous thrombosis in acutely ill medical patients. Thromb Haemost. (2007) 97:191–4. doi: 10.1160/TH06-10-0601
30. Turc G, Calvet D, Guérin P, Sroussi M, Chatellier G, Mas JL, et al. Closure, anticoagulation, or antiplatelet therapy for cryptogenic stroke with patent foramen ovale: systematic review of randomized trials, sequential meta-analysis, and new insights from the CLOSE study. J Am Heart Assoc. (2018) 7:e008356. doi: 10.1161/JAHA.117.008356
Keywords: cryptogenic stroke, patent foramen ovale, deep vein thrombosis, pulmonary embolism, venous thromboembolism
Citation: Zietz A, Sutter R and De Marchis GM (2020) Deep Vein Thrombosis and Pulmonary Embolism Among Patients With a Cryptogenic Stroke Linked to Patent Foramen Ovale—A Review of the Literature. Front. Neurol. 11:336. doi: 10.3389/fneur.2020.00336
Received: 28 February 2020; Accepted: 07 April 2020;
Published: 05 May 2020.
Edited by:
Aristeidis H. Katsanos, McMaster University, CanadaReviewed by:
Georgios Tsivgoulis, National and Kapodistrian University of Athens, GreeceLina Palaiodimou, University General Hospital Attikon, Greece
Ava L. Liberman, Montefiore Medical Center, United States
Copyright © 2020 Zietz, Sutter and De Marchis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annaelle Zietz, YW5uYWVsbGV2YWxlcmllLnppZXR6QHVzYi5jaA==
 Annaelle Zietz
Annaelle Zietz Raoul Sutter2
Raoul Sutter2