- Department of Neurology and Institute of Neurology, First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Background: Spinocerebellar ataxia type 3 (SCA3) is an inherited form of ataxia that leads to progressive neurodegeneration. Fatigue is a common non-motor symptom in SCA3 and other neurodegenerative diseases, such as Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS). Although risk factors to fatigue in these diseases have been thoroughly studied, whether or not fatigue can affect clinical phenotypes has yet to be investigated.
Methods: Ninety-one molecularly confirmed SCA3 patients and 85 age- and sex-matched controls were recruited for this study. The level of fatigue was measured using the 14-item Fatigue Scale (FS-14), and the risk factors to fatigue and how fatigue correlates with clinical phenotypes were studied using multivariable linear regression models.
Results: We found that the severity was significantly higher in the SCA3 group than in the control group (9.30 ± 3.04% vs. 3.94 ± 2.66, P = 0.000). Daytime somnolence (β = 0.209, P = 0.002), severity of ataxia (β = 0.081, P = 0.006), and poor sleep quality (β = 0.187, P = 0.037) were found to have a positive relationship with fatigue. Although fatigue had no relationship with age at onset or ataxic progression, we found that it did have a positive relationship with the severity of ataxia (β = 7.009, P = 0.014).
Conclusions: The high level of fatigue and the impact of fatigue on the clinical manifestation of SCA3 patients suggest that fatigue plays a large role in the pathogenesis of SCA3, thus demonstrating the need for intervention and treatment options in this patient cohort.
Introduction
Spinocerebellar ataxia type 3 (SCA3), which was initially known as Machado–Joseph disease (MJD), is the most common subtype of spinocerebellar ataxias (SCAs) worldwide and one of autosomal dominant neurodegenerative disorders. SCA3 is caused by the dynamic mutation involving CAG repeat expansion in exon 10 of the ATXN3 gene on chromosome 14q32.1, which results in a pathologically glutamine tract within the encoded ataxin-3 (1). It is clinically characterized by progressive cerebellar ataxia, dysphagia, oculomotor disturbances, and extrapyramidal signs. In addition to the cardinal motor manifestations, SCA3 may also exhibit several non-motor clinical manifestations such as sleep behavior disorders, cognitive deficits, olfactory dysfunction, psychiatric symptoms, nutritional problems, autonomic nervous function disturbances, and fatigue (2, 3).
Fatigue, which is clinically defined as difficulty in the initiation of or sustainment of voluntary mental and physical activities (4), is a subjective experience and common in neurodegenerative disorders such as Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS) (5–12). As a common symptom, fatigue was found to have great impact on the quality of life in PD (13–16). Fatigue is also a pronounced non-motor symptom in SCAs (17–19), especially in SCA3 patients, with a high prevalence around 52.9–63.5% (3, 20, 21). The significant predictors of fatigue, such as length of expanded CAG repeats, excessive daytime somnolence, and mood disorders such as anxiety and major depression have been explored (3, 19, 20, 22, 23). Given that the frequency of fatigue is so high and severity worsens over time with disease progression (4), the question is raised as to whether or not it could affect the clinical symptoms seen in neurodegenerative disorders, which have never been investigated.
For this study, we recruited 91 molecularly confirmed SCA3 patients and 85 age- and sex-matched controls to study the frequency of fatigue and the risk factors to fatigue in our subjects. And then, correlation between fatigue and clinical manifestations such as age at onset (AAO), severity, and progression of ataxia was further investigated.
Subjects and Methods
Study Subjects
Ninety-one molecular-confirmed SCA3 patients and 85 healthy individuals were recruited between October 2014 and January 2018. In order to be eligible to participate, patients had to meet our inclusion criteria as follows: (1) the presence of ataxia, (2) definite genetic diagnosis of SCA3, (3) willingness to participate, and (4) age of 20 years and older. Exclusion criteria were the following: (1) the presence of homozygotes for SCA3, (2) met exclusion criteria of SCA3 by a previous genetic test, and (3) had concomitant disorder(s) that affect the International Cooperative Ataxia Rating Scale (ICARS), which is the ataxia measurement used in this study. The control group was matched for age, gender, and environmental characteristics including mostly spouses, caregivers of SCA3/MJD patients, and unrelated healthy person. Relatives at risk were excluded from the control group.
This study was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University, and written informed consents were signed by all participants prior to study commencement.
Genotype and Phenotype Analysis
Genomic DNA was extracted from peripheral blood using QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany) following standard procedures. The numbers of subjects' CAG repeats were determined by polymerase chain reaction (PCR) amplification combined with Sanger sequencing as reported in our previous studies (24, 25). Based on the CAG repeat numbers, alleles of ATXN3 could be divided into normal alleles (CAG repeat numbers ≤44) and expanded alleles (CAG repeat numbers ≥50) (25).
Each patient participated in a face-to-face interview with ataxia specialists (SRG, HLX, and JSY) to collect demographic data and neurological features. The severity of ataxia was assessed using semiquantitative International Cooperative Ataxia Rating Scale (ICARS), which involves four subscales of postural and stance disorders, limb ataxia, dysarthria, and oculomotor disorders, with a sum score ranging from 0 (absence of ataxia) to 100 (severe ataxia) (26). AAO was defined as the time when the patient or close relatives/caregivers could recall the first appearance of any symptoms related to SCA3. Disease duration was defined as the time span between AAO and the age at first visit. Progression degree of ataxia was measured by a patient's ICARS score divided by duration.
In order to rate the severity of fatigue, the 14-item Fatigue Scale (FS-14) was used. The FS-14, which is a 14-item questionnaire, reflects the severity of two different kinds of fatigue (physical fatigue and mental fatigue), and a higher score suggests severer fatigue (27). Since the study subjects were all Chinese, we adopted a Chinese version of FS-14, which was proved to exhibit optimal sensitivity and specificity in recognizing fatigue among the Chinese-speaking population (28) and was widely used for valuing the severity of fatigue (29–34). To value the severity of daytime somnolence, sleep disturbances, and depression, which were reported to related to the severity of fatigue (19, 20, 22), patients were asked to complete the Chinese version of questionnaires of the Epworth Sleepiness Scale (ESS), the Pittsburgh Sleep Quality Index (PSQI), and the Beck Depression Inventory (BDI). The ESS assesses sleep propensity in eight different daily situations with a total score ranging from 0 (no excessive daytime sleepiness) to 24 (severe excessive daytime sleepiness). Patients were classified as having excess daytime somnolence (EDS) if the ESS score was at least 10 (35). The PSQI measures sleep quality and disturbances using seven components, resulting in a total score ranging from 0 to 21. Poor sleep quality was indicated if the PSQI score was >5 (36). BDI is a 21-question, self-rating tool that measures symptom of depression. Standard cutoff scores range from 0 to 63, with a higher score indicating severe depression. For the purposes of this study, a BDI score of ≥19 was defined as clinically relevant depression (37).
Statistical Analyses
Variables with normal distribution valued by Kolmogorov–Smirnov test were expressed as the mean ± SD and evaluated by Student's independent sample t-test. Variables with skewed distribution were expressed as mean ± SD and median and evaluated by one-way analysis of variance (ANOVA). Gender difference between SCA3 patients and controls was analyzed with chi-square test.
To determine possible factors relative to fatigue, we used a multivariable logistic regression model. The FS-14 score was dichotomized into two groups based on the value of median. The FS-14 score (binary) was the dependent variable. The AAO, gender (binary), disease duration, the length of normal and expanded CAG repeats, ICARS, ESS, BDI, and PSQI scores were considered independent variables.
To analyze whether fatigue was correlated with clinical phenotypes (e.g., AAO, severity of ataxia, and progression of ataxia) of SCA3 patients, a multivariable linear regression model was performed. To determine the influence of fatigue on AAO, it was set as the dependent variable. Scores from the FS-14 (binary), ESS, BDI, and PSQI, along with gender (binary), CAG repeat numbers of normal alleles, and expanded alleles, were regarded as the independent variables. In the model of analyzing the relationship between fatigue and severity of ataxia, ICARS score was considered the dependent variable. Scores from the FS-14 (binary), ESS, BDI, and PSQI along with gender (binary), AAO, disease duration, and CAG repeat numbers of normal alleles and expanded alleles were considered independent variables. To explore the relationship between fatigue and progression of ataxia, we used the progression degree of ataxia as the dependent variable and scores of FS-14 (binary), ESS, BDI, and PSQI along with gender (binary), AAO, and the length of normal and expanded CAG repeats as the independent variables.
To detect whether the data were suitable for logistic or linear regression model, the goodness of fit was tested by calculating R2. The closer the value of R2 to 1, the better goodness of fit the equation had.
All statistical analyses were performed using SPSS version 27.0 (SPSS Inc., Chicago, IL, USA). The results were considered statistically significant at P < 0.05.
Results
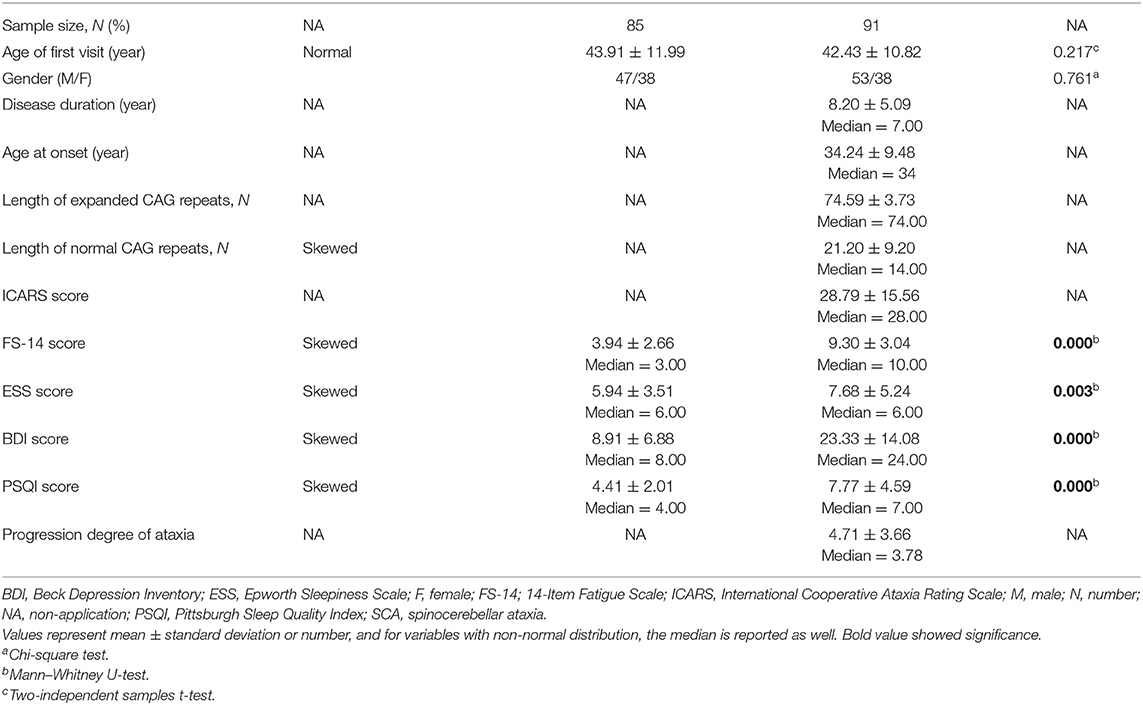
The demographic features of 91 SCA3 patients and 85 controls are summarized in Table 1. Of the 91 subjects with SCA3, 53 (58.24%) were men and 38 (41.76%) were women. The mean AAO was 34.24 ± 9.48 years, and average disease duration was 8.20 ± 5.09 years. The average numbers of CAG repeat in normal and expanded allele were 21.20 ± 9.20 and 74.59 ± 3.73, respectively. The control group was composed of 85 healthy normal person. The gender and age distribution between SCA3 patients and controls showed no significant differences (P = 0.761 for gender; P = 0.217 for age).
The mean FS-14 score was significantly higher in the SCA3 group than in the control group (9.30 ± 3.04, median = 10 vs. 3.94 ± 2.66, median = 3, P = 0.000). Otherwise, ESS, PSQI, and BDI scores were also higher in SCA3 patients than in the control subjects (7.68 ± 5.24 vs. 5.94 ± 3.51, P = 0.003; 7.77 ± 4.59 vs. 4.41 ± 2.01, P = 0.000; 23.33 ± 14.08 vs. 8.91 ± 6.88, P = 0.000, respectively) (Table 1). The proportion of SCA3 patients with EDS (ESS score ≥10), poor sleep quality (PSQI score ≥5), as well as depression (BDI score ≥19) were also higher than controls (37.36 vs. 11.76%, P = 0.000, 72.53 vs. 48.24%, P = 0.001, 59.34 vs. 4.71%, P = 0.000, respectively).
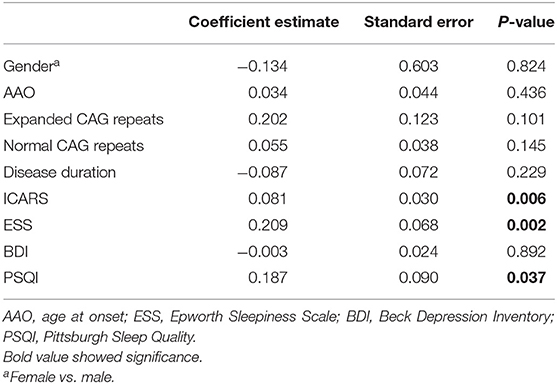
To investigate the factors associated with fatigue, a multivariable logistic regression model was performed. After adjusting for AAO, gender, disease duration, length of normal CAG repeats, and scores of BDI and PSQI, we found that daytime somnolence (β = 0.209, P = 0.002), severity of ataxia (β = 0.081, P = 0.006), and the poor sleep quality (β = 0.187, P = 0.037) were positively correlated with fatigue (Table 2). The R2 for the regression model of fatigue was 0.509.
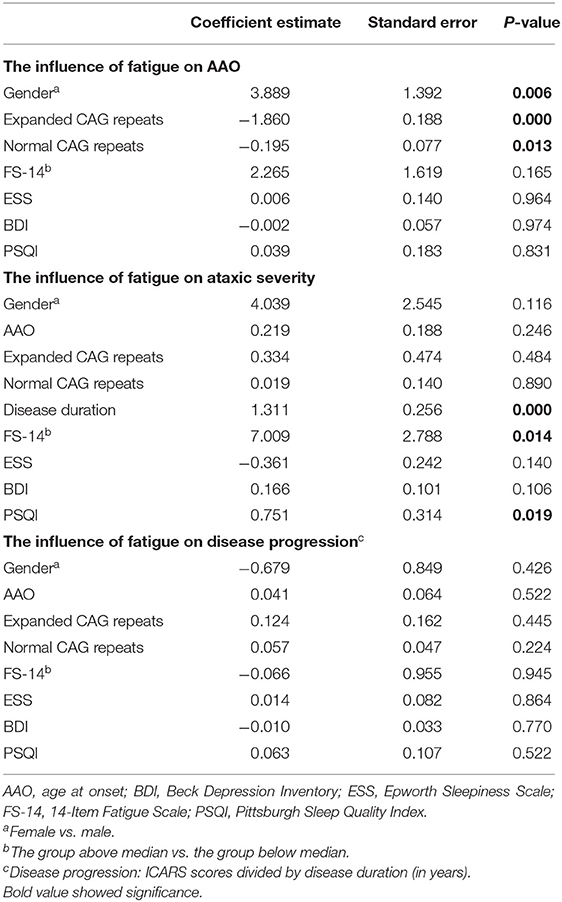
The multivariable linear regression model was further utilized to evaluate correlative factors of SCA3 clinical phenotypes. After adjusting for AAO, gender, disease duration, length of normal and expanded CAG repeats, and scores of ESS, BDI, and PSQI, we found that fatigue was positively correlated with severity of ataxia (β = 7.009, P = 0.014). There was no significant correlation between fatigue and AAO (β = 2.265, P = 0.165) or fatigue and disease progression (β = 0.066, P = 0.945) (Table 3). The R2 for the regression model of ataxia severity was 0.551.
Discussion
In the present study, we found that SCA3 patients have significant higher level of fatigue, compared to the normal controls. We also found that daytime somnolence and ataxic severity are risk factors associated with fatigue. While daytime somnolence, the length of expanded CAG repeats, anxiety, and depression have been reported to be associated with fatigue (3, 19, 20, 22, 23), it was the first time that ataxic severity was found to be associated with fatigue. The significant correlation between fatigue and disease severity was also revealed in a PD cohort (8). The positive relationship between fatigue and clinical features was initially investigated in SCA3 in the present study. We found that although fatigue had no correlations with AAO and ataxic progression, it did have a significant positive relationship with the severity of ataxia.
Our findings suggest that there is a bidirectional relationship between ataxia and fatigue in SCA3 patients. However, the physiological mechanisms to this close connection remains unknown and is typically considered multifactorial. Normally, fatigue can be divided into either central fatigue, which is caused by lesions in pathways associated with arousal and attention, reticular and limbic systems, thalamus, and the basal ganglia or peripheral fatigue, which is caused by neuromuscular junction disorders or metabolic myopathy (4). Previous studies have demonstrated that dopaminergic dysfunction of the basal ganglia is one of the potential mechanisms for central fatigue in PD patients (38, 39). Similarly, abnormalities of the dopamine transporter were observed in SCA3 patients (40). Additionally, one study showed that there was widespread microstructural white matter damage in the thalamus, bilateral cerebral white matter, and the frontal and temporal lobes in SCA3 patients (24). Therefore, we speculated that abnormalities in the dopamine transporter and white matter of cerebra may contribute to the central fatigue observed in SCA3 patients. It is tangible that the peripheral fatigue noted in SCA3 patients may be due to peripheral neuropathy, since symptoms such as muscle cramps, weakness, numbness, muscle wasting, neuropathic pain, and disturbance of skeletal muscle energy metabolism are common in SCA3 patients (41, 42).
The influence of fatigue on ataxic severity highlights the need to prevent fatigue in SCA3. Until now, a treatment for fatigue in SCA3 patients has not yet been reported. However, standardized treatment for fatigue in PD patients does exist, inclusive of a balanced medication regime targeting movement disorder (e.g., dopaminergic agents) and depression (e.g., psychostimulants and antidepressants) combined with a rehabilitation program (e.g., cognitive behavior therapy and graded exercise) (5). Furthermore, research has demonstrated that pharmacological (modafinil) and non-pharmacological (respiratory exercise and repetitive transcranial magnetic stimulation) management may be possible treatment options for fatigue in ALS patients (43). These reports indicate that the appropriate combination of pharmacological and non-pharmacological treatments could be promising treatment options for alleviating fatigue and the severity of ataxia in SCA3 patients.
Conclusions
In summary, the high frequency of fatigue and its impact on the clinical manifestation in SCA3 patients suggests that it plays a large role in the pathogenesis of SCA3 and necessitates the development of an intervention and treatment plan in SCA3 patients.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by First Affiliated Hospital of Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
J-SY and H-LX were in charge of study concept and design, statistical analysis and interpretation, and writing of the manuscript. HW and S-RG were in charge of study concept and design and critical revision of the manuscript for important intellectual content. W-JC and NW designed and supervised the research. P-PC, AS, M-ZQ, H-XL, and M-TL collected and analyzed data.
Funding
This work was supported by The National Natural Science Foundation of China to S-RG (81100851, Beijing), J-SY (81901209, Beijing), and NW (U1505222, Beijing). This work was also supported by the Natural Science Foundation of Fujian Province to S-RG (2018J01156, Fuzhou).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors sincerely thank the participants for their help and willingness to participate in this study. We also thank the reviewers for their helpful comments.
References
1. Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. (1994) 8:221–8. doi: 10.1038/ng1194-221
2. Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. (2010) 9:885–94. doi: 10.1016/S1474-4422(10)70183-6
3. Yuan X, Ou R, Hou Y, Chen X, Cao B, Hu X, et al. Extra-cerebellar signs and non-motor features in Chinese patients with spinocerebellar ataxia type 3. Front Neurol. (2019) 10:110. doi: 10.3389/fneur.2019.00110
4. Chaudhuri P, Behan O. Fatigue in neurological disorders. Lancet. (2004) 363:978–88. doi: 10.1016/S0140-6736(04)15794-2
5. Nassif DV, Pereira JS. Fatigue in Parkinson's disease: concepts and clinical approach. Psychogeriatrics. (2018) 18:143–50. doi: 10.1111/psyg.12302
6. Siciliano M, Trojano L, Santangelo G, De Micco R, Tedeschi G, Tessitore A. Fatigue in parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2018) 33:1712–23. doi: 10.1002/mds.27461
7. Fu R, Cui SS, Du JJ, He YC, Gao C, Huang P, et al. Fatigue correlates with LRRK2 G2385R variant in Chinese parkinson's disease patients. Parkinsonism Relat Disord. (2017) 44:101–5. doi: 10.1016/j.parkreldis.2017.09.016
8. Stocchi F, Abbruzzese G, Ceravolo R, Cortelli P, D'Amelio M, De Pandis MF, et al. Prevalence of fatigue in Parkinson disease and its clinical correlates. Neurology. (2014) 83:215–20. doi: 10.1212/WNL.0000000000000587
9. Nazemi M, Raad MH, Arzoomanian CS, Ghasemzadeh A. Fatigue and depression in Iranian amyotrophic lateral sclerosis patients in tehran in 2012. Electron Physician. (2016) 8:2194–8. doi: 10.19082/2194
10. Lo Coco D, La Bella V. Fatigue, sleep, and nocturnal complaints in patients with amyotrophic lateral sclerosis. Eur J Neurol. (2012) 19:760–3. doi: 10.1111/j.1468-1331.2011.03637.x
11. Lou JS. Fatigue in amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am. (2008) 19:533–43. doi: 10.1016/j.pmr.2008.02.001
12. Ramirez C, Piemonte ME, Callegaro D, Da Silva HC. Fatigue in amyotrophic lateral sclerosis: frequency and associated factors. Amyotroph Lateral Scler. (2008) 9:75–80. doi: 10.1080/17482960701642502
13. Serrano-Duenas M, Bravo R, Merchan T, Serrano M. Fatigue in parkinson's disease: metric properties of the fatigue impact scale for daily use (D-FIS), and its impact on quality of life. Clin Neurol Neurosurg. (2018) 169:12–15. doi: 10.1016/j.clineuro.2018.03.020
14. Kluger BM, Parra V, Jacobson C, Garvan CW, Rodriguez RL, Fernandez HH, et al. The prevalence of fatigue following deep brain stimulation surgery in Parkinson's disease and association with quality of life. Parkinsons Dis. (2012) 2012:769506. doi: 10.1155/2012/769506
15. Dogan VB, Koksal A, Dirican A, Baybas S, Dirican A, Dogan GB. Independent effect of fatigue on health-related quality of life in patients with idiopathic Parkinson's disease. Neurol Sci. (2015) 36:2221–6. doi: 10.1007/s10072-015-2340-9
16. Elbers RG, van Wegen EE, Verhoef J, Kwakkel G. Impact of fatigue on health-related quality of life in patients with parkinson's disease: a prospective study. Clin Rehabil. (2014) 28:300–11. doi: 10.1177/0269215513503355
17. Moro M, Moscovich M, Farah CH, Camargo F, Teive HAG, Munhoz RP. Nonmotor symptoms in spinocerebellar ataxias (SCAs). Cerebellum Ataxias. (2019) 6:12. doi: 10.1186/s40673-019-0106-5
18. Moro R, Munhoz P, Moscovich M, Arruda WO, Raskin S, Silveira-Moriyama L, et al. Nonmotor symptoms in patients with spinocerebellar ataxia type 10. Cerebellum. (2017) 16:938–944. doi: 10.1007/s12311-017-0869-2
19. Brusse E, Brusse-Keizer MG, Duivenvoorden HJ, van Swieten JC. Fatigue in spinocerebellar ataxia: patient self-assessment of an early and disabling symptom. Neurology. (2011) 76:953–9. doi: 10.1212/WNL.0b013e31821043a4
20. Martinez R, Nunes MB, Faber I, D'Abreu A, Lopes-Cendes I, Franca MC Jr. Fatigue and its associated factors in spinocerebellar ataxia type 3/machado-joseph disease. Cerebellum. (2017) 16:118–21. doi: 10.1007/s12311-016-0775-z
21. D'Abreu M, Franca C Jr, Paulson HL, Lopes-Cendes I. Caring for Machado-Joseph disease: current understanding and how to help patients. Parkinsonism Relat Disord. (2010) 16:2–7. doi: 10.1016/j.parkreldis.2009.08.012
22. Friedman JH, Amick MM. Fatigue and daytime somnolence in machado joseph disease (spinocerebellar ataxia type 3). Mov Disord. (2008) 23:1323–4. doi: 10.1002/mds.22122
23. Saute JA, da Silva AC, Donis KC, Vedolin L, Saraiva-Pereira ML, Jardim LB. Depressive mood is associated with ataxic and non-ataxic neurological dysfunction in SCA3 patients. Cerebellum. (2010) 9:603–5. doi: 10.1007/s12311-010-0205-6
24. Gan SR, Shi SS, Wu JJ, Wang N, Zhao GX, Weng ST, et al. High frequency of machado-joseph disease identified in southeastern Chinese kindreds with spinocerebellar ataxia. BMC Med Genet. (2010) 11:47. doi: 10.1186/1471-2350-11-47
25. Gan SR, Ni W, Dong Y, Wang N, Wu ZY. Population genetics and new insight into range of CAG repeats of spinocerebellar ataxia type 3 in the Han Chinese population. PLoS ONE. (2015) 10:e0134405. doi: 10.1371/journal.pone.0134405
26. Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The ataxia neuropharmacology committee of the world federation of neurology. J Neurol Sci. (1997) 145:205–11.
27. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. (1993) 37:147–53.
28. Jing MJ, Lin WQ, Wang Q, Wang JJ, Tang J, Jiang ES, et al. Reliability and construct validity of two versions of chalder fatigue scale among the general population in Mainland China. Int J Environ Res Public Health. (2016) 13:147. doi: 10.3390/ijerph13010147
29. Lin W, Chen XL, Chen Q, Wen J, Chen X. Jin's three-needle acupuncture technique for chronic fatigue syndrome: a study protocol for a multicentre, randomized, controlled trial. Trials. (2019) 20:155. doi: 10.1186/s13063-019-3243-5
30. Guo J, Huang W, Tang CY, Wang GL, Zhang F, Wang LP. Effect of acupuncture on sleep quality and hyperarousal state in patients with primary insomnia: study protocol for a randomised controlled trial. BMJ Open. (2016) 6:e009594. doi: 10.1136/bmjopen-2015-009594
31. Wang J, Yin G, Li G, Liang W, Wei Q. Efficacy of physical activity counseling plus sleep restriction therapy on the patients with chronic insomnia. Neuropsychiatr Dis Treat. (2015) 11:2771–8. doi: 10.2147/NDT.S94724
32. Zhang Q, Yao D, Yang J, Zhou Y. Factors influencing sleep disturbances among spouse caregivers of cancer patients in Northeast China. PLoS ONE. (2014) 9:e108614. doi: 10.1371/journal.pone.0108614
33. Lian TH, Guo P, Zuo LJ, Hu Y, Yu SY, Liu L, et al. An investigation on the clinical features and neurochemical changes in parkinson's disease with depression. Front Psychiatry. (2018) 9:723. doi: 10.3389/fpsyt.2018.00723
34. Liu L, Xu P, Zhou K, Xue J, Wu H. Mediating role of emotional labor in the association between emotional intelligence and fatigue among Chinese doctors: a cross-sectional study. BMC Public Health. (2018) 18:881. doi: 10.1186/s12889-018-5817-7
35. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5.
36. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213.
37. Cecchin R, Pires AP, Rieder CR, Monte TL, Silveira I, Carvalho T, et al. Depressive symptoms in machado-joseph disease (SCA3) patients and their relatives. Community Genet. (2007) 10:19–26. doi: 10.1159/000096276
38. Friedman JH, Abrantes A, Sweet LH. Fatigue in parkinson's disease. Expert Opin Pharmacother. (2011) 12:1999–2007. doi: 10.1517/14656566.2011.587120
39. Chaudhuri P, Behan O. Fatigue and basal ganglia. J Neurol Sci. (2000) 179:34–42. doi: 10.1016/s0022-510x(00)00411-1
40. Braga-Neto P, Felicio AC, Hoexter MQ, Pedroso JL, Dutra LA, Alessi H, et al. Cognitive and olfactory deficits in machado-joseph disease: a dopamine transporter study. Parkinsonism Relat Disord. (2012) 18:854–8. doi: 10.1016/j.parkreldis.2012.04.015
41. Franca JC Jr, D'Abreu MA, Nucci A, Cendes F, Lopes-Cendes I. Prospective study of peripheral neuropathy in machado-joseph disease. Muscle Nerve. (2009) 40:1012–8. doi: 10.1002/mus.21396
42. Yabe K, Tha K, Yokota T, Sato K, Soma H, Takei A, et al. Estimation of skeletal muscle energy metabolism in machado-joseph disease using (31)P-MR spectroscopy. Mov Disord. (2011) 26:165–8. doi: 10.1002/mds.23335
Keywords: fatigue, spinocerebellar ataxia type 3, neurodegeneration, clinical manifestation, ataxic severity
Citation: Yang J-S, Xu H-L, Chen P-P, Sikandar A, Qian M-Z, Lin H-X, Lin M-T, Chen W-J, Wang N, Wu H and Gan S-R (2020) Ataxic Severity Is Positively Correlated With Fatigue in Spinocerebellar Ataxia Type 3 Patients. Front. Neurol. 11:266. doi: 10.3389/fneur.2020.00266
Received: 19 November 2019; Accepted: 20 March 2020;
Published: 22 April 2020.
Edited by:
Emilia Mabel Gatto, Sanatorio de la Trinidad Mitre, ArgentinaReviewed by:
Peng Lei, Sichuan University, ChinaSantiago Perez-Lloret, National Council for Scientific and Technical Research (CONICET), Argentina
Copyright © 2020 Yang, Xu, Chen, Sikandar, Qian, Lin, Lin, Chen, Wang, Wu and Gan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Wu, Nzc5ODQ3NTAzQHFxLmNvbQ==; Shi-Rui Gan, Z2Fuc2hpcnVpQGZqbXUuZWR1LmNu
†These authors have contributed equally to this work
 Jin-Shan Yang
Jin-Shan Yang Hao-Ling Xu
Hao-Ling Xu Ping-Ping Chen
Ping-Ping Chen Arif Sikandar
Arif Sikandar Mei-Zhen Qian
Mei-Zhen Qian Hui-Xia Lin
Hui-Xia Lin Min-Ting Lin
Min-Ting Lin Wan-Jin Chen
Wan-Jin Chen Ning Wang
Ning Wang Hua Wu
Hua Wu Shi-Rui Gan
Shi-Rui Gan