- 1Unit of Neurology, Neurophysiology, Department of Medicine, Università Campus Bio-Medico di Roma, Rome, Italy
- 2NeXT: Neurophysiology and Neuroengineering of Human-Technology Interaction Research Unit, Campus Bio-Medico University, Rome, Italy
Fatigue is a very common symptom among people with multiple sclerosis (MS), but its management in clinical practice is limited by the lack of clear evidence about the pathogenic mechanisms, objective tools for diagnosis, and effective pharmacological treatments. In this scenario, neurophysiology could play a decisive role, thanks to its ability to provide objective measures and to explore the peripheral and the central structures of the nervous system. We hereby review and discuss current evidence about the potential role of neurophysiology in the management of MS-related fatigue. In the first part, we describe the use of neurophysiological techniques for exploring the pathogenic mechanisms of fatigue. In the second part, we review the potential application of neurophysiology for monitoring the response to pharmacological therapies. Finally, we show data about the therapeutic implications of neurophysiological techniques based on non-invasive brain stimulation.
Introduction
Fatigue is a very common symptom in multiple sclerosis (MS) and produces significant detrimental effects on the quality of life (1). Despite its prevalence and impact, the management of fatigue in clinical practice is often challenging since the underlying pathophysiological mechanisms have not been well-elucidated (2), pharmacological treatments have limited efficacy (3), and fatigue assessment is commonly based exclusively on self-report questionnaires (4).
Although the advent of magnetic resonance imaging (MRI) significantly changed the overall management of MS, the role of neurophysiology remains of great importance in the functional evaluation of specific pathways such as visual, somatosensory, auditory, and motor systems and in the study of the central and the peripheral mechanisms of sensorimotor integration. Fatigue is a complex symptom including motor, cognitive, and psychological aspects, but through neurophysiological techniques, it is possible to evaluate mainly motor fatigue, from both research and clinical perspectives. Motor fatigue can be classified as central or peripheral. By definition, peripheral fatigue is the inability to generate force at the muscle level, while central fatigue refers to changes arising from the neural networks in the brain and the spinal cord, causing a lack of drive to the muscles.
The alterations occurring at the neuromuscular level cannot fully explain the phenomenon of fatigue (5), and in the last few years, different studies have speculated over the meaning and magnitude of the contribution of the central nervous system (CNS). In particular, in MS, fatigue seems to arise from the disruption of a complex neural network involving the cerebral cortex, the thalamus, and the basal ganglia (6–8). Similarly also in other neurological conditions such as Parkinson's disease and stroke, different supraspinal structures are considered to be key players in fatigue generation (9).
In this scenario, neurophysiological techniques can play a decisive role in the assessment of the pathophysiology of MS-related fatigue, thanks to their ability to provide objective measures and to explore the peripheral and the central structures of the nervous system, with excellent time resolution. Besides that, various studies have also demonstrated good correlations between neurophysiological parameters and disability measures (10), highlighting the usefulness of neurophysiology in monitoring disease evolution and response to therapy.
Finally, several studies have evaluated the therapeutic implications of neurophysiological techniques based on non-invasive brain stimulation (NIBS) in different neuropsychiatric diseases such as stroke, depression, dementia, and movement disorders (11–13). In particular, in MS, promising results have been obtained in the treatment of disabling symptoms such as spasticity (14) and fatigue (15).
In this review, we will provide an outline of the current evidence about the potential role of neurophysiology in the management of MS-related fatigue. In the first part, we will describe the potential application of neurophysiological techniques for exploring the pathogenic mechanisms of fatigue. Then, we will report on the potential use of neurophysiology for measuring fatigue and monitoring the response to symptomatic therapies. In the third part, we will review the potential application of neuromodulation as an innovative treatment for fatigue. Eventually, we will discuss the limitations and the shortcomings of available data, highlighting the key challenges in the field and suggesting some directions for future research.
Neurophysiology as Investigating Tool for The Pathogenic Mechanisms of Fatigue
During a physical effort, there is a progressive decline of firing rate of spinal motoneurons (16), but the significance of such phenomenon is not clear as it can be interpreted as exhaustion or as fatigue adaptation.
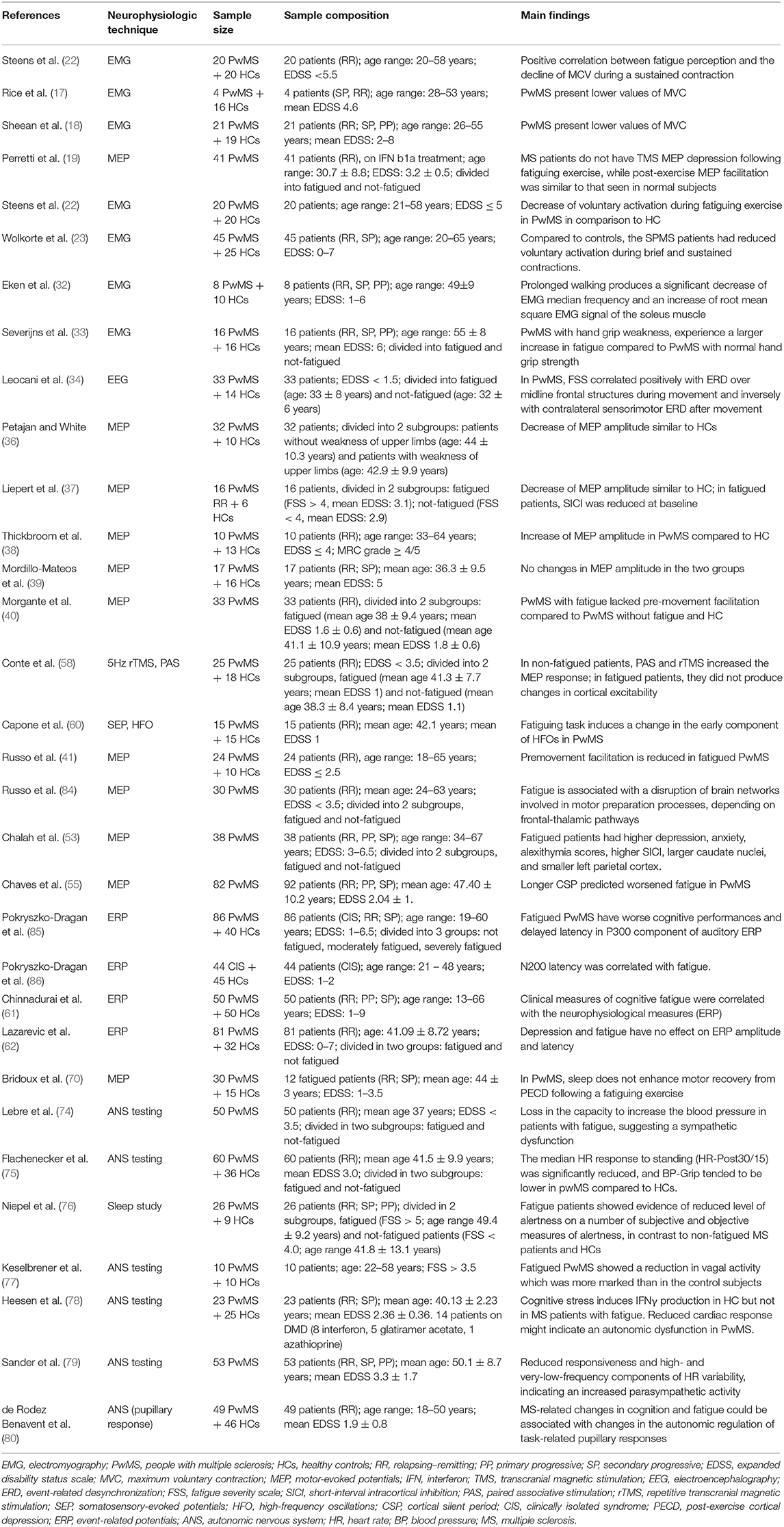
Most studies reported that MS patients present lower strength values of maximal voluntary contraction (MVC) in comparison to healthy subjects (17–20), and the decrease of these values is positively correlated with fatigue perception (21). The fall of muscle force (and MVC as well) could be related to a submaximal voluntary drive, which is known as central activation failure (CAF) (9). CAF can be evaluated by the twitch-interpolated technique, in which the subjects are asked to perform a MVC in a given muscle and an electrical stimulus is subsequently applied to the motor nerve supplying the tested muscle. If there is a further increase of muscle force after electrical stimulation, then the muscle's voluntary central drive was not at its maximum, thus demonstrating CAF. Using this technique, Steens et al. (22) showed a decrease of voluntary activation during fatiguing exercise in people with MS (PwMS) in comparison to healthy subjects, probably due to insufficient CNS compensatory mechanisms. The reduction of voluntary activation seems to be particularly important in the pathogenesis of fatigue in patients with secondary-progressive MS as compared to relapsing–remitting MS (23).
Electromyography (EMG) allows quantifying the reduction of amplitude or frequency of muscle action potentials (MAP) during a fatiguing task. Surface EMG (sEMG) is a non-invasive technique in which electrodes placed on the skin record electrical muscle activity (24, 25). In particular, the amplitude of the sEMG signal is considered as a measure of voluntary drive to peripheral structures (9). Muscle contraction is characterized by the progressive recruitment of different motor units, depending on their size, biochemical features, and fatigability (26, 27). The development of muscular fatigue produces specific changes in EMG signal, consisting in an initial increase and then in the decrease of MAP amplitude (28, 29), a reduction of median frequency of discharge, and a reduction of motor conduction velocity along fatigued muscle fibers (28, 30, 31).
These phenomena, also present in healthy subjects, are more evident in PwMS. For instance, Eken et al. found that prolonged walking produces a significant decrease of EMG median frequency with a corresponding increase of the root mean square of the EMG signal of the soleus muscle (32). Similar changes of EMG parameters have also been found in the upper limb by Severijns et al. (33) in a cohort of PwMS after a protocol of repetitive shoulder anteflexion movements. Interestingly, these changes in EMG parameters are present even without a clear performance decline and are not directly correlated with the level of perceived fatigue. These findings suggest that peripheral mechanisms cannot fully explain the development of fatigue and that central mechanisms could also be involved. In this regard, different neurophysiological methods can be used to study the contribution of CNS.
Electroencephalography (EEG) allows evaluating the role of cortico-cortical connections. Using this technique, Leocani et al. (34) investigated the correlation between fatigue severity [measured through the Fatigue Severity Scale (FSS) questionnaire] and EEG parameters consisting of event-related desynchronization (ERD) and event-related synchronization (ERS). They found that, in PwMS compared to healthy controls, FSS correlated positively with ERD over midline frontal structures during movement and inversely with contralateral sensorimotor ERS after movement. These findings suggest an overactivation of the frontal regions in fatigued patients, a possible expression of a compensatory mechanism for the subcortical dysfunction causing fatigue.
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique that can be used to explore the contribution of the different structures of the CNS to fatigue generation. Indeed single-pulse TMS allows evaluating the functionality of the corticospinal tract by recording the amplitude and the latency of motor-evoked potentials (MEP), while paired-pulse TMS provides insight into the cortico-cortical connections. Moreover, repetitive TMS (rTMS) protocols are known to induce short- and long-term modifications of cortical excitability, thus reflecting plasticity changes at the cortical level.
In healthy subjects, MEP amplitude increases during a fatiguing exercise and reduces after its end (35). In MS patients, results are more variable because some studies reported a decrease of MEP amplitude similar to healthy subjects (36, 37), while others reported an increase (19, 38) or no changes (39). Also, in the premovement phase, a significant lack of MEP facilitation after a sustained motor task was shown in fatigued PwMS compared to controls and not-fatigued patients (40, 41), suggesting a disruption of the brain networks involved in motor preparation which has been correlated to structural and functional changes in frontal-thalamic pathways (41).
Different paired-pulse TMS studies have demonstrated, in healthy subjects, physiological modifications of cortical excitability as a result of fatigue development. Paired-pulse TMS protocols are used to test different cortical circuits (42) and include short-interval cortical inhibition (SICI) (43), a protocol related to inhibitory gamma-aminobutyric acid (GABA)-A interneurons, in which a subthreshold conditioning first pulse inhibits the response to a suprathreshold second pulse delivered 1–5 ms later (44); intracortical facilitation (ICF) (45), linked to glutamatergic intracortical circuits in which a subthreshold conditioning first pulse enhances the response to a suprathreshold second pulse delivered 7–20 ms later (46); and late intracortical inhibition (LICI) (47), mediated by GABA-B receptors in which two suprathreshold pulses at long-interstimulus intervals of 50–200 ms are delivered (48). Benwell et al. (49) showed that SICI initially increases and then decreases as force declines during a fatiguing exercise involving the first dorsal interosseous (FDI) muscle. Similarly, Maruyama et al. (50) found a transient reduction of SICI in FDI muscle after isometric contractions, while there was no change in ICF. By contrast, Hunter et al. (51) likewise found a reduction of SICI, while ICF decreased during a sustained submaximal voluntary muscle contraction. Besides that, changes of ICF or SICI seem to depend also on the type of fatiguing motor task used in the experimental protocol—for instance, being different during handwriting compared to isometric finger abduction (52).
In PwMS, different alterations in cortical excitability parameters have been described. Liepert et al. (37) found that, compared to healthy controls and to PwMS without fatigue, SICI was reduced in PwMS with fatigue, already at baseline, before the fatiguing exercise. In contrast, Morgante et al. (40) found similar values of SICI and ICF in PwMS with and without fatigue and in healthy controls, while Chalah et al. found a significant reduction of SICI in non-fatigued compared to fatigued PwMS and no significant difference in ICF and other TMS measures (53).
Another neurophysiological measure which can be assessed through TMS is the cortical silent period (CSP) that is an interruption of the voluntary muscle contraction after a TMS pulse over the contralateral motor cortex and is thought to be mediated by GABA-B inhibitory neurotransmission, (54). CSP duration in PwMS predicted fatigue and was associated with poor cardiovascular fitness (55).
Several studies have investigated the changes of cortical plasticity of PwMS through rTMS protocols (56, 57), but only a few of them have explored their role in fatigue pathogenesis.
Morgante et al. (40) found that PwMS have reduced plasticity demonstrated by the lack of MEP increase after the 5-Hz rTMS protocol, without any difference between fatigued and not-fatigued patients. Conte et al. (58) found instead that, during an attention-demanding task, the response to 5-Hz rTMS and paired associative stimulation (PAS)—a neuromodulatory protocol consisting of repetitive peripheral nerve stimulation combined with TMS over the contralateral motor cortex (59)—significantly differs between PwMS with or without fatigue. Indeed in fatigued patients both PAS and 5-Hz stimulation did not produce the expected changes in cortical excitability, while in not-fatigued patients they both increased the MEP response, although less efficiently than in healthy subjects.
TMS techniques do not allow a complete evaluation of brain subcortical structures, the role of which seems to be crucial in fatigue generation. In a recent study, Capone et al. (60) evaluated how high-frequency oscillations (HFOs)—a burst of fast oscillations that overlies the cortical response of median nerve somatosensory-evoked potentials—are influenced by a fatiguing exercise in a cohort of 15 PwMS and 15 healthy controls. They showed a significant change of the early component of HFOs, reflecting the possible primary role played by the thalamus in the pathogenesis of MS-related fatigue, while the latter component reflects that the cortico-cortical network activity in the somatosensory cortex was not modified significantly. Furthermore, increasing evidence from neuroimaging studies is supporting the hypothesis that the thalamus is a key player in fatigue generation (6).
Fatigue is a complex symptom involving both cognitive and motor domains and multiple factors, in addition to sensorimotor dysfunction as assessed by EEG and EPs, which can contribute to its pathogenesis and/or exacerbate its manifestations (demographics, comorbidity, genetics, diet, exercise, depression, cognitive impairment, pain, and sleep disorders) (6). Neurophysiology can also play an important role in defining and quantifying some of these factors. For instance, event-related potentials (ERP) could be a useful tool to investigate the mechanisms involved in the pathogenesis of cognitive fatigue.
Pokryszko-Dragan et al. found that fatigued PwMS have worse cognitive performances and delayed latency in the P300 component of the auditory ERP and also in the early stage of the disease. These results were confirmed by Chinnadurai et al. (61) in a sample of 50 PwMS using a modified version of auditory ERP. However, a recent study by Lazarevic et al. (62) did not find any effect of depression and fatigue on the ERP parameters. Thus, further research is needed to clarify the role of ERP in the assessment of cognitive impairment in PwMS.
It has been demonstrated that sleep disorders such as obstructive sleep apnea (63), restless leg syndrome (64, 65), periodic limb movements (66), and rapid eye movement behavior disorders (67) are more frequent in PwMS than in the general population and can contribute to the development of motor (2) and cognitive fatigue (68). In all these disorders, overnight polysomnography is essential to make a diagnosis and to quantify the consequent reduction of sleep efficiency (69).
Moreover, the disease itself can produce pathological and functional modifications in the CNS that alter the restorative sleep capacity and thus exacerbate fatigue perception. This phenomenon was investigated by Bridoux et al. using TMS for assessing the reduction of MEP amplitude induced by an exercise (post-exercise corticomotor depression or PECD). They demonstrated that, in healthy subjects, sleep enhances recovery from PECD, while in PwMS, the restorative effect of sleep is reduced or lost (70).
Autonomic dysfunction is very common among PwMS and can occur since the earliest stages of the disease. It is mainly caused by demyelinating lesions located in the periventricular region of the fourth ventricle, in the brainstem, and in the spinal cord (69). Autonomic dysfunction can produce different symptoms affecting the bowel, the bladder, the heart, and the blood vessels.
The functionality of the autonomic nervous system can be tested by the Quantitative Sudomotor Axon Reflex testing (71) and the study of cardiovascular parameters such as blood pressure and heart rate response to Valsalva maneuver, heart rate variability during deep breathing, and blood pressure and heart rate changes during tilt test (72).
In particular, cardiac autonomic dysfunction has been associated to fatigue in PwMS (73), but the mechanisms and significance of this association remain unclear.
Some authors have hypothesized that MS-related fatigue is caused by a sympathetic vasomotor dysfunction with a normal parasympathetic activity (74–76).
On the contrary, other studies found that fatigued PwMS have a reduction in vagal activity compared to controls (77–79).
Recent evidence suggests that pupillometry could be an alternative method to evaluate the involvement of the autonomic nervous system in PwMS. Indeed the pupil size depends on the balance between the sympathetic and parasympathetic components of the autonomic nervous system. For instance, de Rodez Benavent et al. (80) investigated the changes in pupil size during problem-solving in MS patients (with and without fatigue) vs. controls. They found that MS-related changes in cognition and fatigue could be associated with changes in the autonomic regulation of task-related pupillary responses.
Taken together, the neurophysiologic data demonstrated that MS-related fatigue seems to have a central origin. The changes in EMG parameters, described in MS patients (32, 33), are thought to be more a consequence of alterations in CNS structures rather than a primary determinant of fatigue. However, it cannot completely be ruled out that such changes could be the epiphenomenon of peripheral alterations occurring at the neuromuscular level.
Neuroimaging studies (60, 81, 82) demonstrated that the main pathogenic substrate of MS-related fatigue could be a dysfunction of the circuits between the thalamus, the basal ganglia, and the cortex, and neurophysiological findings support this hypothesis. Indeed single-pulse TMS studies demonstrated that in MS patients the pathogenesis of fatigue is not driven by mechanisms directly related to corticospinal functioning but is due to alterations in structures located upstream to the primary motor cortex (39). In particular, both EEG (34) and TMS studies (37, 40, 58) pointed out the role of cortical areas involved in movement preparation and attention. For instance, Sandroni et al. (83) found that, in PwMS, fatiguing tasks are associated with a change in ERP without significant modifications in MEP parameters, thus suggesting that fatigue affects neural processes acting after stimulus evaluation and before the activation of the primary motor cortex.
More recently, Capone et al. (60) explored the contribution of the thalamus by means of HFOs obtained from the median nerve SEP, demonstrating that a dysfunction of the thalamo-cortical axons contributes to fatigability in MS patients.
Although CNS functional alterations are consistently reported by neurophysiological studies, their significance remains largely unknown because they were considered by some authors as pathogenic factors (40) and by others as the epiphenomena of adaptive processes (60). According to the first hypothesis, neurophysiologic techniques measure the change in the activity of CNS networks caused by the MS-related damage of gray and white matter. On the other side, according to the alternative hypothesis, this damage produces compensatory/adaptive mechanisms that can be recorded by means of neurophysiological techniques.
More broadly, several structural and functional abnormalities in various cortico-subcortical neural networks (e.g., fronto-striatal network, cortico-striato-thalamo-cortical loop) occur during MS as a result of inflammation, neurodegeneration, and compensatory neuroplasticity processes. From this perspective, the development of fatigue could depend on the dynamic balance between damage and restorative processes during the disease's course (8). Indeed the latter can be predominant in the initial phase of the disease, thus masking the clinical occurrence of fatigue, while, later on, the damage could prevail so that patients experience clinically relevant fatigue. Accordingly, the heterogeneity in the results of neurophysiological studies can depend on the stage of the disease in which the recording has been done.
Interestingly, the neurophysiological markers of fatigue at different levels, such as changes in EMG parameters (33), in HFO features (60), or in cortical plasticity (40), can also be observed in MS patients without fatigue. This finding could suggest that an impairment in fatigability mechanisms (expressed by neurophysiological alterations) does exist in MS since the earliest phases of the disease, independently from the level of fatigue in everyday life measured through questionnaires. This is not surprising because fatigue is a multifactorial and complex symptom, and different factors, in addition to thalamo-cortical dysfunction, could be necessary to make it clinically relevant.
MS can cause extensive damage of the CNS, so it is not surprising that autonomic nervous system involvement or subtle alterations of cognitive functioning may occur at any stage of the disease. Thus, these are other factors that need to be considered as potential players in fatigue generation, but evidences are not unambiguous. Sleep disorders should also be taken into account since the impairment of a restorative process can exacerbate—or even be one of the main generators— fatigue (2, 68, 70).
Longitudinal studies involving patients at different stages of the disease (from clinically isolated syndrome to advanced progressive MS) and investigating possible factors involved in fatigue perception (such as genetics, comorbidity, cognitive impairment, depression, and sleep disorders) could contribute to corroborate such hypothesis. In Table 1, we have summarized the studies that have used neurophysiological techniques for investigating fatigue pathogenesis.
Neurophysiology for Monitoring the Response to Therapies for Fatigue
The most frequently used pharmacological treatments for fatigue are amantadine, 4-aminopyridine, and modafinil. The non-pharmacological interventions include physical (e.g., aerobic exercises, resistance training, yoga, and tai-chi) and psychological/cognitive approaches (e.g., cognitive behavioral therapy, education programs, and mindfulness interventions). However, evidence supporting the efficacy of these interventions is still preliminary and, sometimes, conflicting (87).
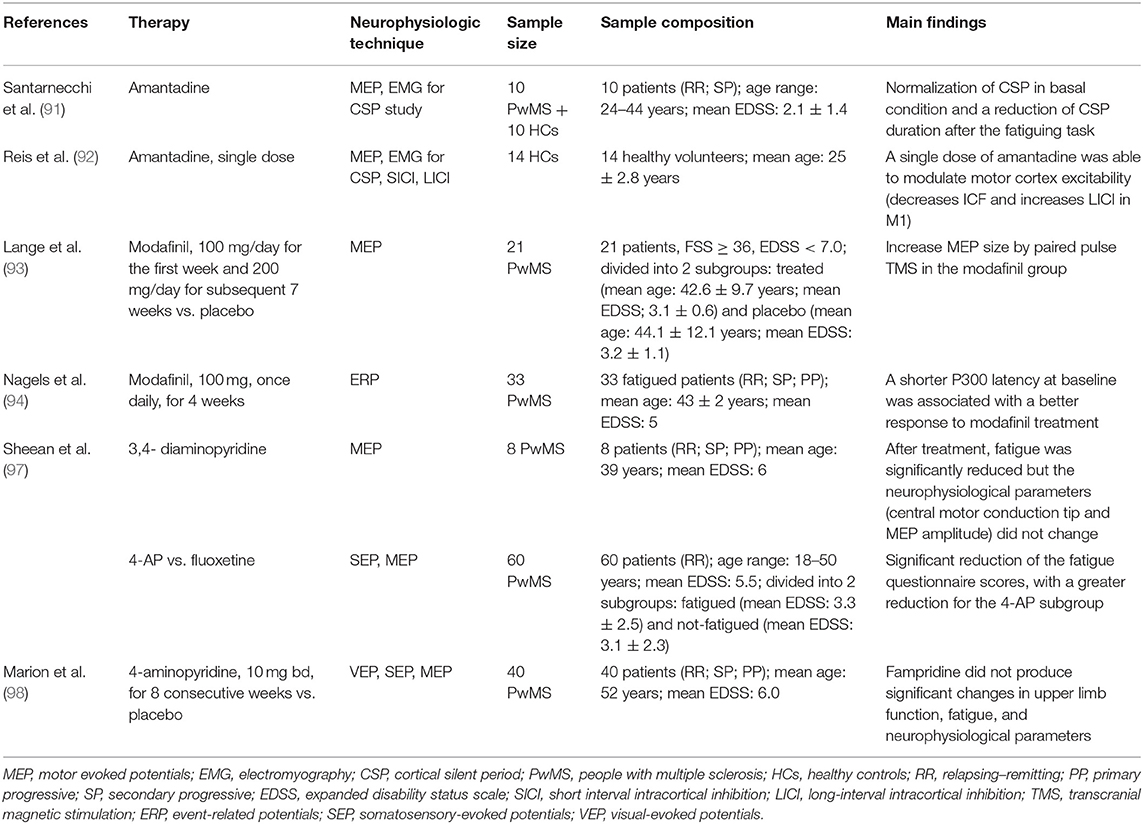
Amantadine is an antiviral agent firstly introduced to prevent and treat flu viruses. Animal models have shown that amantadine induces the release of dopamine from nerve endings (88). Moreover, one clinical trial has shown an increased level of beta-endorphin and beta-lipoprotein after amantadine assumption, with clear clinical improvement (89). The real mechanism of action of amantadine as fatigue therapy is not yet clear, but the fact that amantadine acts as a dopaminergic factor supports the dopamine imbalance theory for fatigue generation (90). One relevant study, addressing the neurophysiological effects of amantadine in MS-related fatigue, was conducted by Santarnecchi et al. (91). They found that chronic treatment with this drug improves clinical fatigue (assessed through questionnaires) and restores GABAergic inhibitory mechanisms in the motor cortex of PwMS, as indicated by the normalization of CSP in basal condition and by the reduction of CSP duration after a fatiguing task. Reis et al. (92) evaluated the effect of a single dose of amantadine on human motor cortex excitability in healthy subjects. They showed that a single dose of amantadine significantly decreases ICF and increases LICI in the motor cortex. MEP recruitment curves, motor thresholds, and duration of CSP remained unchanged after treatment. These data suggested that a single dose of amantadine is able to modulate motor cortex excitability, possibly involving GABAergic and glutamatergic neurotransmission.
Another drug, tested for MS-related fatigue, was modafinil, a central alpha-adrenergic agonist approved for the treatment of attention-deficit hyperactivity disorder and narcolepsy. Lange et al. (93) reported a significant improvement of fatigue questionnaire scores and in the nine-hole peg test, after modafinil administration, in a group of 21 PwMS. Furthermore, they tested different TMS protocols before and after 8 weeks of treatment, showing an increase of MEP size by paired pulse TMS, in the modafinil group.
Nagels et al. (94) evaluated visual- and auditory-evoked potentials (EP) for predicting the response to modafinil treatment (100 mg, once daily, for 4 weeks), in 33 PwMS with fatigue. They found that the latency of auditory P300 predicted the treatment response with a good specificity and sensitivity. In particular, a shorter latency at baseline was associated with a better response to modafinil treatment.
In order to better clarify the mechanisms of action of modafinil in fatigue relief, Niepel et al. (76) investigated the effect of a single dose (200 mg) of modafinil on measures of alertness and autonomic function in fatigued PwMS compared to not-fatigued PwMS and healthy controls.
They found that fatigued patients had a reduced level of alertness and cardiovascular sympathetic activation compared to the other two groups, and modafinil was able to reverse these deficiencies. On the basis of these findings, they hypothesized that the anti-fatigue effect of modafinil was related to the activation of the noradrenergic locus coeruleus (76).
Despite these interesting data, at present, there is no indication, in clinical practice, for the use of modafinil for fatigue relief.
Potassium channel blockers—e.g., 4-aminopyridine (4-AP)—belong to a group of drugs able to restore conduction propriety in demyelinating axons as shown in animal models (95). Different trials have also explored the central effect of 4-AP, speculating on a potential role in optimizing neurotransmitter release at the synaptic level (dopamine, acetylcholine, noradrenaline, and serotonin). This latter hypothesis is supported by the observation of an increase BOLD signal during a motor task following a 3,4-diaminopyridine administration compared with a placebo dose assumption (96).
Sheean et al. (97) evaluated changes in TMS-evoked corticospinal excitability parameters in eight PwMS with fatigue before and after treatment with 3,4-diaminopyridine. The motor performance of adductor pollicis muscle was evaluated by TMS, rapid voluntary movements, and a fatiguing exercise test consisting of a sustained isometric contraction. After 3 weeks, fatigue was significantly reduced but neurophysiological parameters (central motor conduction time and MEP amplitude) did not change in the treated patients compared to the untreated ones. These findings suggest that the effect of 3,4-diaminopyridine on fatigue could be linked with mechanisms and structures other than corticospinal tract functionality. Moreover, methodological factors should be considered in the interpretation of these results. Indeed only upper limbs spared from the disease were evaluated, thus representing a major limitation of the study.
More recently, Marion et al. designed a randomized double-blind placebo-controlled trial to investigate the effect of modified-release 4-aminopyridine (fampridine) on upper limb function, fatigue, and several neurophysiological parameters such as visual-evoked potentials (latency and amplitude), somatosensory-evoked potentials (latency and amplitude), motor-evoked potentials (latency), central motor conduction time, resting motor threshold, MEP recruitment curves, and paired-pulse TMS protocols. They found that fampridine (10 mg bd, for eight consecutive weeks) did not produce significant changes in upper limb function, fatigue, and neurophysiological parameters (98).
Over the last years, various studies have demonstrated that neurophysiology can be helpful in measuring and predicting response to treatment. However, the results are not definitive since data are scarce and sometimes not conclusive. Studies greatly differ from each other in variables such as outcome measures, treatment and follow-up duration, neurophysiological techniques, and clinical features of patients. Moreover, to the best of our knowledge, no study has evaluated, through neurophysiological tools, the effectiveness of non-pharmacological interventions such as physical, psychological, and cognitive approaches. Anyway, it still seems reasonable to assume that neurophysiology can have a role in monitoring the response to fatigue treatment, and more studies on the matter are warranted.
In Table 2, we have summarized the studies that have used neurophysiological techniques for monitoring the treatment for fatigue in PwMS.
Neurophysiology as Innovative Treatment for Fatigue in MS Patients
Neurophysiological studies are being carried out not only to identify objective and measurable markers of fatigue, as previously illustrated, but also to find neuromodulation protocols able to reduce this disabling symptom.
NIBS approaches are playing a major role in this research setting, following a large neurophysiological evidence of central abnormalities in PwMS with fatigue (34, 39, 56, 99).
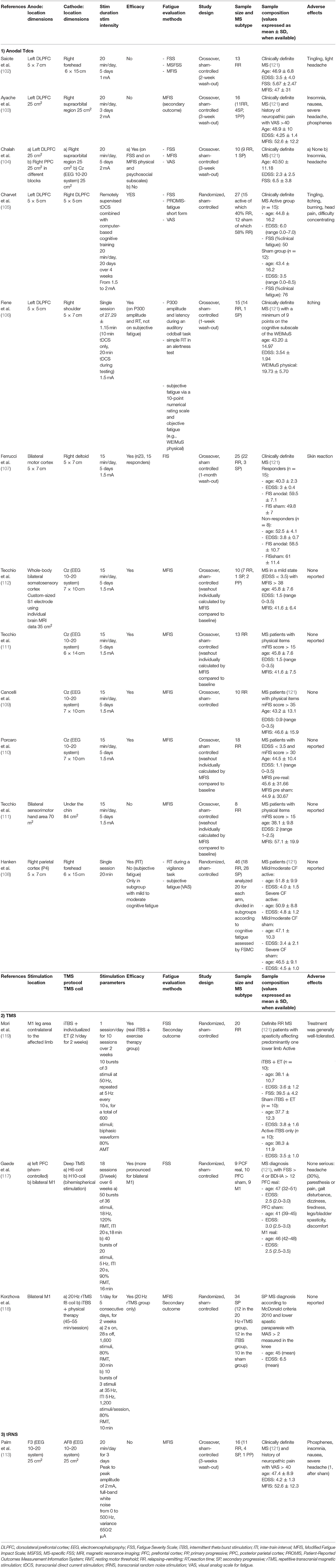
In Table 1, the results of a MEDLINE research on sham-controlled NIBS studies for the treatment of fatigue in PwMS is presented, and the stimulation parameters are described for each study.
Transcranial direct current stimulation (tDCS) is the NIBS technique mostly used so far (cf. Table 1). It is classically assumed that tDCS can modulate human brain activity with effects that could outlast the period of stimulation by inducing a subthreshold shift of the resting membrane potential toward depolarization (anodal tDCS) or hyperpolarization (cathodal tDCS) (15). Beyond local effects, connectional (axonal) and non-neuronal effects have also been described (15). The tDCS mechanisms of action are still incompletely understood; an effect on calcium-dependent synaptic plasticity of glutamatergic neurons and a local reduction in GABA neurotransmission have been hypothesized (15).
Anodal tDCS applied to the motor cortical areas reduced motor fatigue in healthy subjects (100, 101). In patients with MS-related fatigue, anodal tDCS has been used with variable effects, depending on the parameters of stimulation and the clinical characteristics of the patients included in the studies.
As shown in Table 1, different targets have been stimulated by anodal tDCS. The evidence of functional alterations in the frontal areas in PwMS with fatigue (8) focused the attention of some researchers on the stimulation of the left dorsolateral prefrontal cortex (DLPFC).
Among these studies, negative results were reported by Saiote et al. (102) and Ayache et al. (103). Some methodological factors such as the wash-out duration and the stimulation intensity (102), the stimulation duration, and the heterogeneity of the population included (103) could have played a role in these results. Other three studies reported positive results on fatigue after anodal tDCS was applied over the left DLPFC (104–106). Among these, worthy of note are the use of a remotely supervised tDCS system in combination with a computer-based cognitive training (105) and the use of objective outcome measures, such as the P300 evoked potential and the reaction time (106). The application of anodal tDCS to the motor cortex bilaterally (107) and to the right parietal cortex (108) also gave a preliminary evidence of efficacy.
The group of Tecchio et al. focused on a personalized anodal tDCS approach targeting the whole-body primary somatosensory areas (S1) bilaterally, following the evidence of S1 reduced excitability and M1 hyperexcitability in PwMS with fatigue (109–112). They used a tailored procedure with personalized electrodes based on the patients' brain MRI located in place through an MRI-guided neuronavigation system. In a more recent study of this group, the importance of the individual baseline neural networks activity has been outlined as a further parameter for individualized treatment (110). The results of their studies support the efficacy of personalized tDCS approaches.
Only one sham-controlled study has explored the effects on MS-related fatigue of another NIBS technique called transcranial random-noise stimulation (tRNS). This stimulation was applied on frontal regions but produced negative results (113).
The other NIBS technique introduced in the research setting for the treatment of fatigue in PwMS is TMS. (114). Repetitive protocols of TMS showed long-lasting effects on cortical excitability in patients with stroke (115), MS-related spasticity (116) and major depression (13).
Regarding MS-related fatigue, three sham-controlled studies using TMS showed promising results (117–119). Two of these studies used TMS in combination with physical therapy and enrolled patients affected by spasticity (118, 119). Different TMS protocols were used: intermittent theta-burst stimulation (iTBS) applied to the M1 leg area (119), deep TMS, delivered with specific H-coils to the left prefrontal cortex and to bilateral M1 (117), and 20-Hz repetitive TMS and iTBS applied to bilateral M1 (118). Preliminary evidence of efficacy was described for all the protocols excepted for iTBS on bilateral M1 (118).
In a recent systematic meta-analysis, Liu et al. reviewed the efficacy and safety of NIBS specifically for the treatment of MS-related fatigue (120). They performed a literature search for sham-controlled brain stimulation studies based on tDCS, rTMS, tRNS, and transcranial alternating current stimulation (tACS). A total of 14 eligible studies published from 2011 to 2018, for a total of 207 MS patients, were found: 11 tDCS studies, one rTMS study, one iTBS (combined with exercise therapy) study, and one tRNS study. A significant improvement in fatigue scores compared to sham was found after tDCS treatment. A subgroup analysis demonstrated significance for the intensity of 1.5 mA and for bilateral S1 stimulation location. The two TMS studies and the tRNS study did not reach statistical significance.
Several data are available about the therapeutic use of NIBS for reducing MS-related fatigue (Table 3). These techniques—and in particular tDCS and some TMS protocols—have shown to be effective as add-on therapy for fatigue management, and more studies are needed to explore their further implementation. The mechanisms by which NIBS could improve fatigue are still unclear (8, 15, 104). Different hypothesis have been proposed such as presynaptic increase of spinal drive from motor cortex, modulation of premotor areas, increase in motivation, decrease in muscle pain, increase in muscle coupling, promotion of changes in cortical resting state activity and cortico-cortical connectivity, and induction of long-term potentiation-like and long-term depression-like neuroplastic changes at a local and/or network level. The potential role of altered oscillatory activity in the pathogenesis of MS-related cognitive fatigue and the potential advantage of tACS application have also been outlined (122). A better comprehension of the pathogenesis could be useful to develop therapies that specifically target the mechanisms of fatigue generation in MS.
The studies published so far are greatly heterogeneous, differing in many variables such as the NIBS technique used, the cortical targets, the stimulation intensity, and the characteristics of the populations included. Indeed although most of the studies enrolled patients with EDSS ≤ 6, other population characteristics were more heterogeneous among studies, such as the MS subtype, the presence of comorbidities, the measured outcome in addition to spasticity (e.g., neuropathic pain), and the baseline fatigue scores.
Other important limitations to the use of NIBS for therapeutic purpose remain the still heterogeneous definition of fatigue, the limited comprehension of its complex and multifactorial pathophysiology, and the limited use of objective measures other than self-report questionnaires.
Because of this methodological heterogeneity and the low sample sizes, the level of evidence for NIBS efficacy resulted too low to draw any robust conclusion to support its use in clinical practice (15) but encourages further studies on NIBS as a treatment for fatigue (120).
Conclusions
Several studies have used neurophysiological tools to evaluate MS-related fatigue. Until now, this possibility has been mainly exploited for investigating the pathogenic mechanisms of fatigue and for modulating brain circuits for therapeutic purposes. The potential role of neurophysiology for quantifying fatigue and predicting and/or monitoring response to treatment has been evaluated in only a few studies.
From a methodological perspective, the most used techniques are TMS and tDCS. TMS is a very versatile method that allows both to assess, non-invasively, the functionality of corticospinal tract and cortico-cortical connections and, when delivered in repetitive protocols, to modulate brain activity (114). On the other side, tDCS is the most investigated technique as a potential treatment for fatigue because it is safe, well-tolerated, low-cost, and portable (13, 15).
Other neurophysiological techniques have been used, although in a relatively small number of studies. In particular, EEG has been used for exploring the role of cortico-cortical connections (34), EMG for evaluating the contribution of peripheral structures (9), evoked potentials for investigating the pathogenetic mechanisms (60) and predicting response to pharmacological treatment (94) and autonomic nervous system testing and polysomnography for assessing additional factors that can produce or exacerbate fatigue in PwMS.
Most part of the studies have been conducted in small samples by comparing the findings obtained in fatigued MS patients with those obtained in healthy controls or not-fatigued MS patients. Usually, each study used a single neurophysiological technique, while few studies combined different neurophysiological techniques (83) or neurophysiology with MRI (58).
Overall the literature data presented in this review demonstrate that neurophysiology could play a role in the management and evaluation of MS-related fatigue. Despite of heterogeneity in results and methodological limitations, current evidence supports further studies on the role of neurophysiology in the management of fatigue. In particular, for therapeutic purpose, tailored approaches based on individual network dysfunctions, individual plasticity impairment, and other neurophysiological variables should be explored.
Author Contributions
FC, FM, EF, and MR wrote the manuscript. FC, FM, and VD critically revised the manuscript.
Conflict of Interest
FC has received travel grants from Biogen, Merck, and Sanofi-Genzyme.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis — a brief review. J Neurol Sci. (2012) 323:9–15. doi: 10.1016/j.jns.2012.08.007
2. Ayache SS, Chalah MA. Fatigue in multiple sclerosis - insights into evaluation and management. Neurophysiol Clin. (2017) 47:139–71. doi: 10.1016/j.neucli.2017.02.004
3. Yang TT, Wang L, Deng XY, Yu G. Pharmacological treatments for fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. (2017) 380:256–61. doi: 10.1016/j.jns.2017.07.042
4. Rottoli M, La Gioia S, Frigeni B, Barcella V. Pathophysiology, assessment and management of multiple sclerosis fatigue: an update. Expert Rev Neurother. (2017) 17:373–9. doi: 10.1080/14737175.2017.1247695
5. Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. (2008) 104:542–50. doi: 10.1152/japplphysiol.01053.2007
6. Capone F, Collorone S, Cortese R, Di Lazzaro V, Moccia M. Fatigue in multiple sclerosis: the role of thalamus. Mult Scler. (2020) 26:6–16. doi: 10.1177/1352458519851247
7. Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. (2004) 363:978–88. doi: 10.1016/S0140-6736(04)15794-2
8. Chalah MA, Riachi N, Ahdab R, Créange A, Lefaucheur JP, Ayache SS. Fatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulation. Front Cell Neurosci. (2015) 9:460. doi: 10.3389/fncel.2015.00460
9. Zwarts MJ, Bleijenberg G, van Engelen BG. Clinical neurophysiology of fatigue. Clin Neurophysiol. (2008) 119:2–10. doi: 10.1016/j.clinph.2007.09.126
10. Leocani L, Comi G. Neurophysiological investigations in multiple sclerosis. Curr Opin Neurol. (2000) 13:255–61. doi: 10.1097/00019052-200006000-00004
11. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127:1031–48. doi: 10.1016/j.clinph.2015.11.012
12. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. (2016) 9:336–46. doi: 10.1016/j.brs.2016.03.010
13. Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2019.11.002
14. Iodice R, Manganelli F, Dubbioso R. The therapeutic use of non-invasive brain stimulation in multiple sclerosis - a review. Restor Neurol Neurosci. (2017) 35:497–509. doi: 10.3233/RNN-170735
15. Lefaucheur JP, Chalah MA, Mhalla A, Palm U, Ayache SS, Mylius V. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol Clin. (2017) 47:173–84. doi: 10.1016/j.neucli.2017.03.003
16. Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. (1984) 7:691–9. doi: 10.1002/mus.880070902
17. Rice CL, Vollmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve. (1992) 15:1123–32. doi: 10.1002/mus.880151011
18. Sheean GL, Murray NM, Rothwell JC, Miller DH, Thompson AJ. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain. (1997) 120:299–315. doi: 10.1093/brain/120.2.299
19. Perretti A, Balbi P, Orefice G, Trojano L, Marcantonio L, Brescia-Morra V, et al. Post-exercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation: a study in multiple sclerosis. Clin Neurophysiol. (2004) 115:2128–33. doi: 10.1016/j.clinph.2004.03.028
20. Jørgensen M, Dalgas U, Wens I, Hvid LG. Muscle strength and power in persons with multiple sclerosis - a systematic review and meta-analysis. J Neurol Sci. (2017) 376:225–41. doi: 10.1016/j.jns.2017.03.022
21. Steens A, de Vries A, Hemmen J, Heersema T, Heerings M, Maurits N, et al. Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabil Neural Repair. (2012) 26:48–57. doi: 10.1177/1545968311416991
22. Steens A, Heersema DJ, Maurits NM, Renken RJ, Zijdewind I. Mechanisms underlying muscle fatigue differ between multiple sclerosis patients and controls: a combined electrophysiological and neuroimaging study. Neuroimage. (2012) 59:3110–8. doi: 10.1016/j.neuroimage.2011.11.038
23. Wolkorte R, Heersema DJ, Zijdewind I. Reduced voluntary activation during brief and sustained contractions of a hand muscle in secondary-progressive multiple sclerosis patients. Neurorehabil Neural Repair. (2016) 30:307–16. doi: 10.1177/1545968315593809
24. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. (2000) 10:361–74. doi: 10.1016/S1050-6411(00)00027-4
25. Drost G, Stegeman DF, van Engelen BG, Zwarts MJ. Clinical applications of high-density surface EMG: a systematic review. J Electromyogr Kinesiol. (2006) 16:586–602. doi: 10.1016/j.jelekin.2006.09.005
26. Kandel ER, Schwartz JH. (editors). Principles of Neural Science. 4th ed. New York, NY: McGraw-Hill (2000).
27. Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol. (1985) 76:2411–9. doi: 10.1152/jappl.1994.76.6.2411
28. Merletti R, Knaflitz M, De Luca CJ. Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol. (1985) 69:1810–20. doi: 10.1152/jappl.1990.69.5.1810
29. Behm DG. Force maintenance with submaximal fatiguing contractions. Can J Appl Physiol. (2004) 29:274–90. doi: 10.1139/h04-019
30. De Luca CJ. Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng. (1984) 11:251–79.
31. Kallenberg LA, Hermens HJ. Behaviour of a surface EMG based measure for motor control: motor unit action potential rate in relation to force and muscle fatigue. J Electromyogr Kinesiol. (2008) 18:780–8. doi: 10.1016/j.jelekin.2007.02.011
32. Eken MM, Richards R, Beckerman H, van der Krogt M, Gerrits K, Rietberg M, et al. Quantifying muscle fatigue during walking in people with multiple sclerosis. Clin Biomech. (2019) 72:94–101. doi: 10.1016/j.clinbiomech.2019.11.020
33. Severijns D, Octavia JR, Kerkhofs L, Coninx K, Lamers I, Feys P. Investigation of fatigability during repetitive robot-mediated arm training in people with multiple sclerosis. PLoS ONE. (2015) 10:e0133729. doi: 10.1371/journal.pone.0133729
34. Leocani L, Colombo B, Magnani G, Martinelli-Boneschi F, Cursi M, Rossi P, et al. Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement–EEG evidence. Neuroimage. (2001) 13(Pt 1):1186–92. doi: 10.1006/nimg.2001.0759
35. Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. (1996) 490:529–36. doi: 10.1113/jphysiol.1996.sp021164
36. Petajan JH, White AT. Motor-evoked potentials in response to fatiguing grip exercise in multiple sclerosis patients. Clin Neurophysiol. (2000) 111:2188–95. doi: 10.1016/S1388-2457(00)00469-7
37. Liepert J, Mingers D, Heesen C, Baumer T, Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler. (2005) 11:316–21. doi: 10.1191/1352458505ms1163oa
38. Thickbroom GW, Sacco P, Faulkner DL, Kermode AG, Mastaglia FL. Enhanced corticomotor excitability with dynamic fatiguing exercise of the lower limb in multiple sclerosis. J Neurol. (2008) 255:1001–5. doi: 10.1007/s00415-008-0818-6
39. Mordillo-Mateos L, Soto-Leon V, Torres-Pareja M, Peinado-Palomino D, Mendoza-Laiz N, Alonso-Bonilla C, et al. Fatigue in multiple sclerosis: general and perceived fatigue does not depend on corticospinal tract dysfunction. Front Neurol. (2019) 10:339. doi: 10.3389/fneur.2019.00339
40. Morgante F, Dattola V, Crupi D, Russo M, Rizzo V, Ghilardi MF, et al. Is central fatigue in multiple sclerosis a disorder of movement preparation? J Neurol. (2011) 258:263–72. doi: 10.1007/s00415-010-5742-x
41. Russo M, Crupi D, Naro A, Avanzino L, Buccafusca M, Dattola V, et al. Fatigue in patients with multiple sclerosis: from movement preparation to motor execution. J Neurol Sci. (2015) 351:52–7. doi: 10.1016/j.jns.2015.02.031
42. Di Lazzaro V, Rothwell J, Capogna M. Noninvasive Stimulation of the Human Brain: Activation of Multiple Cortical Circuits. Neuroscientist. (2018) 24:246–60. doi: 10.1177/1073858417717660
43. Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. (1993) 471:501–19. doi: 10.1113/jphysiol.1993.sp019912
44. Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. (2000) 111:794–9. doi: 10.1016/S1388-2457(99)00314-4
45. Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. (1996) 496:873–81. doi: 10.1113/jphysiol.1996.sp021734
46. Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. (2006) 96:1765–71. doi: 10.1152/jn.00360.2006
47. Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. (1992) 85:355–64.
48. McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. (2006) 173:86–93. doi: 10.1007/s00221-006-0365-2
49. Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW. Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res. (2006) 170:191–8. doi: 10.1007/s00221-005-0195-7
50. Maruyama A, Matsunaga K, Tanaka N, Rothwell JC. Muscle fatigue decreases short-interval intracortical inhibition after exhaustive intermittent tasks. Clin Neurophysiol. (2006) 117:864–70. doi: 10.1016/j.clinph.2005.12.019
51. Hunter SK, McNeil CJ, Butler JE, Gandevia SC, Taylor JL. Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Exp Brain Res. (2016) 234:2541–51 doi: 10.1007/s00221-016-4658-9
52. Cinelli K, Green LA, Kalmar JM. The task at hand: fatigue-associated changes in cortical excitability during writing. Brain Sciences. (2019) 9:353. doi: 10.3390/brainsci9120353
53. Chalah MA, Kauv P, Créange A, Hodel J, Lefaucheur JP, Ayache SS. Neurophysiological, radiological and neuropsychological evaluation of fatigue in multiple sclerosis. Mult Scler Relat Disord. (2019) 28:145–52. doi: 10.1016/j.msard.2018.12.029
54. Epstein C, Wassermann E, Ziemann U. Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press (2012).
55. Chaves AR, Kelly LP, Moore CS, Stefanelli M, Ploughman M. Prolonged cortical silent period is related to poor fitness and fatigue, but not tumor necrosis factor, in multiple sclerosis. Clin Neurophysiol. (2019) 130:474–83. doi: 10.1016/j.clinph.2018.12.015
56. Leocani L, Chieffo R, Gentile A, Centonze D. Beyond rehabilitation in MS: insights from non-invasive brain stimulation. Mult Scler. (2019) 25:1363–71. doi: 10.1177/1352458519865734
57. Stampanoni Bassi M, Buttari F, Maffei P, De Paolis N, Sancesario A, Gilio L, et al. Practice-dependent motor cortex plasticity is reduced in non-disabled multiple sclerosis patients. Clin Neurophysiol. (2020) 131:566–73. doi: 10.1016/j.clinph.2019.10.023
58. Conte A, Li Voti P, Pontecorvo S, Quartuccio ME, Baione V, Rocchi L, et al. Attention-related changes in short-term cortical plasticity help to explain fatigue in multiple sclerosis. Mult Scler. (2016) 22:1359–56. doi: 10.1177/1352458515619780
59. Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, et al. Paired associative stimulation. Suppl Clin Neurophysiol. (2004) 57:563–9. doi: 10.1016/S1567-424X(09)70395-2
60. Capone F, Motolese F, Rossi M, Musumeci G, Insola A, Di Lazzaro V. Thalamo-cortical dysfunction contributes to fatigability in multiple sclerosis patients and neurophysiological study. Mult Scler Relat Disord. (2019) 39:101897. doi: 10.1016/j.msard.2019.101897
61. Chinnadurai SA, Venkatesan SA, Shankar G, Samivel B, Ranganathan LN. A study of cognitive fatigue in multiple sclerosis with novel clinical and electrophysiological parameters utilizing the event related potential P300. Mult Scler Relat Disord. (2016) 10:1–6. doi: 10.1016/j.msard.2016.08.001
62. Lazarevic S, Azanjac Arsic A, Aleksic D, Toncev G, Miletic-Drakulic S. Depression and fatigue in patients with multiple sclerosis have no influence on the parameters of cognitive evoked potentials. J Clin Neurophysiol. (2019). doi: 10.1097/WNP.0000000000000640. [Epub ahead of print].
63. Braley TJ, Segal BM, Chervin RD. Sleep-disordered breathing in multiple sclerosis. Neurology. (2012) 79:929–36. doi: 10.1212/WNL.0b013e318266fa9d
64. Deriu M, Cossu G, Molari A, Murgia D, Mereu A, Ferrigno P, et al. Restless legs syndrome in multiple sclerosis: a case–control study. Mov Disord. (2009) 24:697–701. doi: 10.1002/mds.22431
65. Manconi M, Ferini-Strambi L, Filippi M, Bonanni E, Iudice A, Murri L, et al. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep. (2008) 31:944–52.
66. Ferini-Strambi L, Filippi M, Martinelli V, Oldani A, Rovaris M, Zucconi M, et al. Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci. (1994) 125:194–7. doi: 10.1016/0022-510X(94)90035-3
67. Gómez-Choco MJ, Iranzo A, Blanco Y, Graus F, Santamaría J, Saiz A. Prevalence of restless legs syndrome, and, REM sleep behavior disorder in multiple sclerosis. Mult Scler. (2007) 13:805–8. doi: 10.1177/1352458506074644
68. Chinnadurai SA, Gandhirajan D, Pamidimukala V, Kesavamurthy B, Venkatesan SA. Analysing the relationship between polysomnographic measures of sleep with measures of physical and cognitive fatigue in people with multiple sclerosis. Mult Scler Relat Disord. (2018) 24:32–7. doi: 10.1016/j.msard.2018.05.016
69. Habek M, Adamec I, Barun B, Crnošija L, Gabelić T, Krbot Skorić M. Clinical neurophysiology of multiple sclerosis. Adv Exp Med Biol. (2017) 958:129–39. doi: 10.1007/978-3-319-47861-6_8
70. Bridoux A, Créange A, Sangare A, Ayache SS, Hosseini H, Drouot X, et al. Impaired sleep-associated modulation of post-exercise corticomotor depression in multiple sclerosis. J Neurol Sci. (2015) 354:91–6. doi: 10.1016/j.jns.2015.05.006
71. Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. (1983) 14:573–80. doi: 10.1002/ana.410140513
72. Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. (2006) 48:342–62. doi: 10.1016/j.pcad.2005.11.003
73. Findling O, Hauer L, Pezawas T, Rommer PS, Struhal W, Sellner J. Cardiac autonomic dysfunction in multiple sclerosis: a systematic review of current knowledge and impact of immunotherapies. J Clin Med. (2020) 24:9. doi: 10.3390/jcm9020335
74. Lebre AT, Mendes MF, Tilbery CP, Almeida AL, Scatolini Neto A. Relation between fatigue and autonomic disturbances in multiple sclerosis. Arq Neuro Psiquiatr. (2007) 65:663–8. doi: 10.1590/S0004-282X2007000400023
75. Flachenecker P, Rufer A, Bihler I, Hippel C, Reiners K, Toyka K, et al. Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology. (2003) 61:851–3. doi: 10.1212/01.WNL.0000080365.95436.B8
76. Niepel G, Bibani RH, Vilisaar J, Langley RW, Bradshaw CM, Szabadi E, et al. Association of a deficit of arousal with fatigue in multiple sclerosis: effect of modafinil. Neuropharmacology. (2013) 64:380–8. doi: 10.1016/j.neuropharm.2012.06.036
77. Keselbrener L, Akselrod S, Ahiron A, Eldar M, Barak Y, Rotstein Z. Is fatigue in patients with multiple sclerosis related to autonomic dysfunction? Clin Auton Res. (2000) 10:169–75. doi: 10.1007/BF02291352
78. Heesen C, Koehler G, Gross R, Tessmer W, Schulz KH, Gold SM. Altered cytokine responses to cognitive stress in multiple sclerosis patients with fatigue. Mult Scler. (2005) 11:51–7. doi: 10.1191/1352458505ms1129oa
79. Sander C, Modes F, Schlake H-P, Eling P, Hildebrandt H. Capturing fatigue parameters: The impact of vagal processing in multiple sclerosis related cognitive fatigue. Mult Scler Relat Disord. (2019) 32:13–8. doi: 10.1016/j.msard.2019.04.013
80. de Rodez Benavent SA, Nygaard GO, Harbo HF, Tønnesen S, Sowa P, Landrø NI, et al. Fatigue and cognition: pupillary responses to problem-solving in early multiple sclerosis patients. Brain Behav. (2017) 7:e00717 doi: 10.1002/brb3.717
81. Rocca MA, Parisi L, Pagani E, Copetti M, Rodegher M, Colombo B, et al. Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology. (2014) 273:511–20. doi: 10.1148/radiol.14140417
82. Hidalgo de la Cruz M, d'Ambrosio A, Valsasina P, Pagani E, Colombo B, Rodegher M, et al. Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler. (2018) 24:1183–95. doi: 10.1177/1352458517717807
83. Sandroni P, Walker C, Starr A. 'Fatigue' in Patients With Multiple Sclerosis: Motor Pathway Conduction and Event-Related Potentials. Arch Neurol. (1992) 49:517–24. doi: 10.1001/archneur.1992.00530290105019
84. Russo M, Calamuneri A, Cacciola A, Bonanno L, Naro A, Dattola V, et al. Neural correlates of fatigue in multiple sclerosis: a combined neurophysiological and neuroimaging approach (R1). Arch Ital Biol. (2017) 155:142–51. doi: 10.12871/00039829201735
85. Pokryszko-Dragan A, Zagrajek M, Slotwinski K, Bilinska M, Gruszka E, Podemski R. Event-related potentials and cognitive performance in multiple sclerosis patients with fatigue. Neurol Sci. (2016) 37:1545–56. doi: 10.1007/s10072-016-2622-x
86. Pokryszko-Dragan A, Dziadkowiak E, Zagrajek M, Slotwinski K, Gruszka E, Bilinska M, et al. Cognitive performance, fatigue and event-related potentials in patients with clinically isolated syndrome. Clin Neurol Neurosurg. (2016) 149:68–74. doi: 10.1016/j.clineuro.2016.07.022
87. Brenner P, Piehl F. Fatigue and depression in multiple sclerosis: pharmacological and non-pharmacological interventions. Acta Neurol Scand. (2016) 134:47–54. doi: 10.1111/ane.12648
88. Scatton B, Cheramy A, Besson MJ, Glowinski J. Increased synthesis and release of dopamine in the striatum of the rat after amantadine treatment. Eur J Pharmacol. (1970) 13:131–3. doi: 10.1016/0014-2999(70)90194-9
89. Rosenberg GA, Appenzeller O. Amantadine, fatigue, and multiple sclerosis. Arch Neurol. (1988) 45:1104–6. doi: 10.1001/archneur.1988.00520340058012
90. Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. (2015) 6:52. doi: 10.3389/fneur.2015.00052
91. Santarnecchi E, Rossi S, Bartalini S, Cincotta M, Giovannelli F, Tatti E, et al. Neurophysiological correlates of central fatigue in healthy, subjects and multiple sclerosis patients before and after treatment with amantadine. Neural Plast. (2015) 2015:616242. doi: 10.1155/2015/616242
92. Reis J, John D, Heimeroth A, Mueller HH, Oertel WH, Arndt T, et al. Modulation of human motor cortex excitability by single doses of amantadine. Neuropsychopharmacology. (2006) 31:2758–66. doi: 10.1038/sj.npp.1301122
93. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. (2009) 256:645–50. doi: 10.1007/s00415-009-0152-7
94. Nagels G, D'hooghe MB, Vleugels L, Kos D, Despontin M, De Deyn PP. P300 and treatment effect of modafinil on fatigue in multiple sclerosis. J Clin Neurosci. (2007) 14:33–40. doi: 10.1016/j.jocn.2005.10.008
95. Targ EF, Kocsis JD. 4-Aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Res. (1985) 328:358–61. doi: 10.1016/0006-8993(85)91049-2
96. Mainero C, Inghilleri M, Pantano P, Conte A, Lenzi D, Frasca V, et al. Enhanced brain motor activity in patients with MS after a single dose of 3,4-diaminopyridine. Neurology. (2004) 62:2044–50. doi: 10.1212/01.WNL.0000129263.14219.A8
97. Sheean GL, Murray NM, Rothwell JC, Miller DH, Thompson AJ. An open-labelled clinical and electrophysiological study of 3,4 diaminopyridine in the treatment of fatigue in multiple sclerosis. Brain. (1998) 121:967–75. doi: 10.1093/brain/121.5.967
98. Marion S, Leonid C, Belinda B, Joanne D, Elise H, Leeanne C, et al. Effects of modified-release fampridine on upper limb impairment in patients with multiple sclerosis. Mult Scler Relat Disord. (2020) 40:101971. doi: 10.1016/j.msard.2020.101971
99. Cogliati Dezza I, Zito G, Tomasevic L, Filippi MM, Ghazaryan A, Porcaro C, et al. Functional and structural balances of homologous sensorimotor regions in multiple sclerosis fatigue. J Neurol. (2015) 262:614–22. doi: 10.1007/s00415-014-7590-6
100. Abdelmoula A, Baudry S, Duchateau J. Anodal transcranial direct current stimulation enhances time to task failure of a submaximal contraction of elbow flexors without changing corticospinal excitability. Neuroscience. (2016) 322:94–103. doi: 10.1016/j.neuroscience.2016.02.025
101. Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci. (2007) 26:242–9. doi: 10.1111/j.1460-9568.2007.05633.x
102. Saiote C, Goldschmidt T, Timaus C, Steenwijk MD, Opitz A, Antal A, et al. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor Neurol Neurosci. (2014) 32:423–36. doi: 10.3233/RNN-130372
103. Ayache SS, Palm U, Chalah MA, Al-Ani T, Brignol A, Abdellaoui M, et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci. (2016) 10:147. doi: 10.3389/fnins.2016.00147
104. Chalah MA, Riachi N, Ahdab R, Mhalla A, Abdellaoui M, Creange A, et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci. (2017) 372:131–7. doi: 10.1016/j.jns.2016.11.015
105. Charvet LE, Dobbs B, Shaw MT, Bikson M, Datta A, Krupp LB. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. (2018) 24:1760–9. doi: 10.1177/1352458517732842
106. Fiene M, Rufener KS, Kuehne M, Matzke M, Heinze HJ, Zaehle T. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J Neurol. (2018) 265:607–17. doi: 10.1007/s00415-018-8754-6
107. Ferrucci R, Vergari M, Cogiamanian F, Bocci T, Ciocca M, Tomasini E, et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabil. (2014) 34:121–7. doi: 10.3233/NRE-131019
108. Hanken K, Bosse M, Mohrke K, Eling P, Kastrup A, Antal A, et al. Counteracting fatigue in multiple sclerosis with right parietal anodal transcranial direct current stimulation. Front Neurol. (2016) 7:154. doi: 10.3389/fneur.2016.00154
109. Cancelli A, Cottone C, Giordani A, Migliore S, Lupoi D, Porcaro C, et al. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult Scler. (2018) 24:1366–74. doi: 10.1177/1352458517720528
110. Porcaro C, Cottone C, Cancelli A, Rossini PM, Zito G, Tecchio F. Cortical neurodynamics changes mediate the efficacy of a personalized neuromodulation against multiple sclerosis fatigue. Sci Rep. (2019) 9:18213. doi: 10.1038/s41598-019-54595-z
111. Tecchio F, Cancelli A, Cottone C, Ferrucci R, Vergari M, Zito G, et al. Brain plasticity effects of neuromodulation against multiple sclerosis fatigue. Front Neurol. (2015) 6:141. doi: 10.3389/fneur.2015.00141
112. Tecchio F, Cancelli A, Cottone C, Zito G, Pasqualetti P, Ghazaryan A, et al. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J Neurol. (2014) 261:1552–8. doi: 10.1007/s00415-014-7377-9
113. Palm U, Chalah MA, Padberg F, Al-Ani T, Abdellaoui M, Sorel M, et al. Effects of transcranial random noise stimulation (tRNS) on affect, pain and attention in multiple sclerosis. Restor Neurol Neurosci. (2016) 34:189–99. doi: 10.3233/RNN-150557
114. Hallett M. Transcranial magnetic stimulation: a primer. Neuron. (2007) 55:187–99. doi: 10.1016/j.neuron.2007.06.026
115. Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. (2006) 5:708–12. doi: 10.1016/S1474-4422(06)70525-7
116. Centonze D, Koch G, Versace V, Mori F, Rossi S, Brusa L, et al. Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology. (2007) 68:1045–50. doi: 10.1212/01.wnl.0000257818.16952.62
117. Gaede G, Tiede M, Lorenz I, Brandt AU, Pfueller C, Dorr J, et al. Safety and preliminary efficacy of deep transcranial magnetic stimulation in MS-related fatigue. Neurol Neuroimmunol Neuroinflamm. (2018) 5:e423. doi: 10.1212/NXI.0000000000000423
118. Korzhova J, Bakulin I, Sinitsyn D, Poydasheva A, Suponeva N, Zakharova M, et al. High-frequency repetitive transcranial magnetic stimulation and intermittent theta-burst stimulation for spasticity management in secondary progressive multiple sclerosis. Eur J Neurol. (2019) 26:680–44. doi: 10.1111/ene.13877
119. Mori F, Codecà C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, et al. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. (2010) 17:295–300. doi: 10.1111/j.1468-1331.2009.02806.x
120. Liu M, Fan S, Xu Y, Cui L. Non-invasive brain stimulation for fatigue in multiple sclerosis patients: A systematic review and meta-analysis. Mult Scler Relat Disord. (2019) 36:101375. doi: 10.1016/j.msard.2019.08.017
121. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. (2011) 69:292–302. doi: 10.1002/ana.22366
Keywords: multiple sclerosis, fatigue, neurophysiology, non-invasive brain stimulation, TMS, tDCS
Citation: Capone F, Motolese F, Falato E, Rossi M and Di Lazzaro V (2020) The Potential Role of Neurophysiology in the Management of Multiple Sclerosis-Related Fatigue. Front. Neurol. 11:251. doi: 10.3389/fneur.2020.00251
Received: 31 January 2020; Accepted: 17 March 2020;
Published: 22 April 2020.
Edited by:
Moussa Antoine Chalah, Hôpitaux Universitaires Henri Mondor, FranceReviewed by:
Massimiliano Valeriani, Bambino Gesù Children Hospital (IRCCS), ItalyMario Habek, University of Zagreb, Croatia
Copyright © 2020 Capone, Motolese, Falato, Rossi and Di Lazzaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fioravante Capone, Zi5jYXBvbmVAdW5pY2FtcHVzLml0
 Fioravante Capone
Fioravante Capone Francesco Motolese
Francesco Motolese Emma Falato
Emma Falato Mariagrazia Rossi
Mariagrazia Rossi Vincenzo Di Lazzaro
Vincenzo Di Lazzaro