
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 07 April 2020
Sec. Sleep Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00198
This article is part of the Research TopicSleep and Circadian Disruption in Critically Ill PatientsView all 8 articles
 Yibing Zhu1,2*†
Yibing Zhu1,2*† Zhiming Jiang3,4,5†
Zhiming Jiang3,4,5† Huibin Huang6,7*
Huibin Huang6,7* Wen Li2
Wen Li2 Chao Ren8,9,10
Chao Ren8,9,10 Renqi Yao9,11
Renqi Yao9,11 Yang Wang1
Yang Wang1 Yongming Yao8,9,10
Yongming Yao8,9,10 Wei Li1*
Wei Li1* Bin Du6
Bin Du6 Xiuming Xi2
Xiuming Xi2Background: Delirium is a commonly found comorbidity in hospitalized patients and is associated with adverse outcomes. Melatonin is an endogenous hormone that exerts multiple biological effects, mainly in regulating diurnal rhythms and in inflammatory process and immune responses. We aimed to assess the efficacy of exogenous melatonergics in the prevention of delirium.
Methods: We conducted a search to identify relevant randomized controlled studies (RCTs) in PubMed, Cochrane Library, and EMBASE databases that had been published up to December 2019. Hospitalized adult patients administered melatonergics were included. The primary outcome measure was the incidence of delirium. The secondary outcome measure was the length of stay in intensive care unit (ICU-LOS). The pooled effects were analyzed as the risk ratio (RR) for delirium incidence, weighted mean difference (WMD) for ICU-LOS, and 95% confidence intervals (CIs).
Results: Nine RCTs with 1,210 patients were included. The forest plots showed that melatonergics were associated with a decreasing incidence of delirium (RR, 0.51; 95% CI, 0.30–0.85; I2 = 70%; p = 0.01). There was no significant difference in ICU-LOS (WMD, −0.08; 95% CI, −0.19–0.03; I2 = 0; p = 0.17).
Conclusion: Administration of exogenous melatonergics to hospitalized patients seems to be associated with a decreasing incidence of delirium.
PROSPERO registration number: CRD42019138863.
Delirium is a complex neuropsychiatric syndrome characterized by cognitive impairment and attentional deficits (1). Delirium in the intensive care unit (ICU) has a high incidence (30–60%) (2–4) and is strongly associated with adverse outcomes, such as increased mortality, prolonged length of stay in ICU (ICU-LOS), increased costs, and long-term cognitive sequelae (5). As increasingly recognized, delirium has become one of the most concerning problems for intensive care physicians. Although delirium appears to have a high incidence and is associated with adverse outcomes, clinical strategies have been very limited (6).
Melatonin is a neurohormone that exerts multiple biological effects, mainly in regulating diurnal rhythms and in modulating inflammatory as well as immune responses (7, 8). Abundant evidence has indicated that decreasing melatonin levels are linked with delirium (9). Melatonergics include melatonin and other melatonin agonists such as ramelteon. Whether supplementation with exogenous melatonin and melatonin agonists could reduce the risk of delirium remains uncertain. Several randomized controlled studies (RCTs) have been conducted related to this issue. Taking Sultan and colleagues' study (10) as an example, 300 elderly patients undergoing hip arthroplasty were randomly assigned to one of four groups; these groups were administered with melatonin, midazolam, clonidine, or no medication, respectively. The results of this study showed that melatonin was associated with a significant reduction in the incidence of delirium compared with the control group, as well as the other two parallel groups. However, a meta-analysis including four RCTs indicated no significant difference (11). Since then, several well-designed RCTs have been conducted (12–16). A recent meta-analysis evaluated delirium as a subset but included only three RCTs for this result (17). With the updated results (12–16), we aimed to conduct a meta-analysis in attention to reevaluate the efficacy of melatonergics in the prevention of delirium.
The protocol has been registered on the PROSPERO (registration no. CRD42019138863) based on the Preferred Reporting Items for Systematic review and Meta-analysis Protocols guidelines (18).
Three electronic databases (PubMed, Cochrane Library, and EMBASE) were searched without language restriction to identify RCTs published from 1960 to December 2019. Multiple keywords including “melatonin,” “melatonergic,” or “ramelteon” combined with “delirium” were developed for the search strategy. The reference lists were searched manually for potentially relevant articles.
Studies meeting the following criteria were included: (1) the study was designed as an RCT; (2) the study subjects consisted of hospitalized adults (aged ≥18 years); (3) melatonin or melatonin agonists were administered; and (4) outcome measures included delirium incidence.
Studies meeting the following criteria were excluded: (1) publications available only in abstract form or as meeting reports or (2) studies of suboptimal quality (modified Jadad score 0–4) (19).
Two reviewers (J.Z.M. and Z.Y.B.) independently extracted data to fill in a predesigned form including the characteristics of the studies, year of publication, demographics and baseline of subjects, intervention, and outcomes. Two reviewers (Z.Y.B. and W.Y.) independently rated the quality of the RCTs and extracted the items for the risk-of-bias assessment (19). Disagreements between reviewers were resolved by two experts (X.X.M. and Y.Y.M.).
The primary outcome measure was the incidence of delirium. The pooled effects were analyzed as risk ratio (RR) using the Mantel–Haenszel technique and 95% confidence intervals (CIs). Sensitivity analyses of the primary outcome were conducted. Subgroup analyses of the primary outcome included (a) differed melatonergics, including melatonin and ramelteon; (b) different age groups, including elderly subjects (mean age, >60 years), middle-aged subjects (mean age, 40–60 years), and younger subjects (mean age, <40 years); (c) different ICU types, including surgical ICU, medical ICU, and mixed ICU; and (d) different delirium assessment methods including the Confusion Assessment Method for the ICU (CAM-ICU), the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), Abbreviated Mental Test (AMT), the Confusion Assessment Method (CAM), the nurse observation, and the methods not reported. The secondary outcome measure was ICU-LOS. The pooled ICU-LOS was measured in days and analyzed using the weighted mean difference (WMD) and 95% CI. If high clinical or statistical heterogeneity was observed, a random-effects model was chosen. Otherwise, a fixed-effects model was used. The I2 statistic was used to estimate statistical heterogeneity (I2 < 30% as low heterogeneity, I2 of 30–70% as medium heterogeneity, and I2 > 70% as high heterogeneity). P < 0.05 was considered to be statistically significant.
A total of 257 potentially relevant articles were identified according to the search strategy. The full text of 31 articles was obtained after screening the titles/abstracts. Twenty-two studies failed to meet the inclusion criteria; therefore, nine studies were included in this meta-analysis. Figure 1 presents the process of study selection.
Melatonin was administered in seven RCTs (10, 12–14, 16, 20, 21) with different doses. Ramelteon was administered in the other two (15, 22). The subjects of six RCTs (10, 13, 15, 20–22) were elderly (mean age, >60 years), whereas they were apparently younger (mean age, <40 years) in the other (12). Table 1 presents the characteristics of the included studies. The nine RCTs were of high quality. Figure 2 presents the risk-of-bias items for each study.
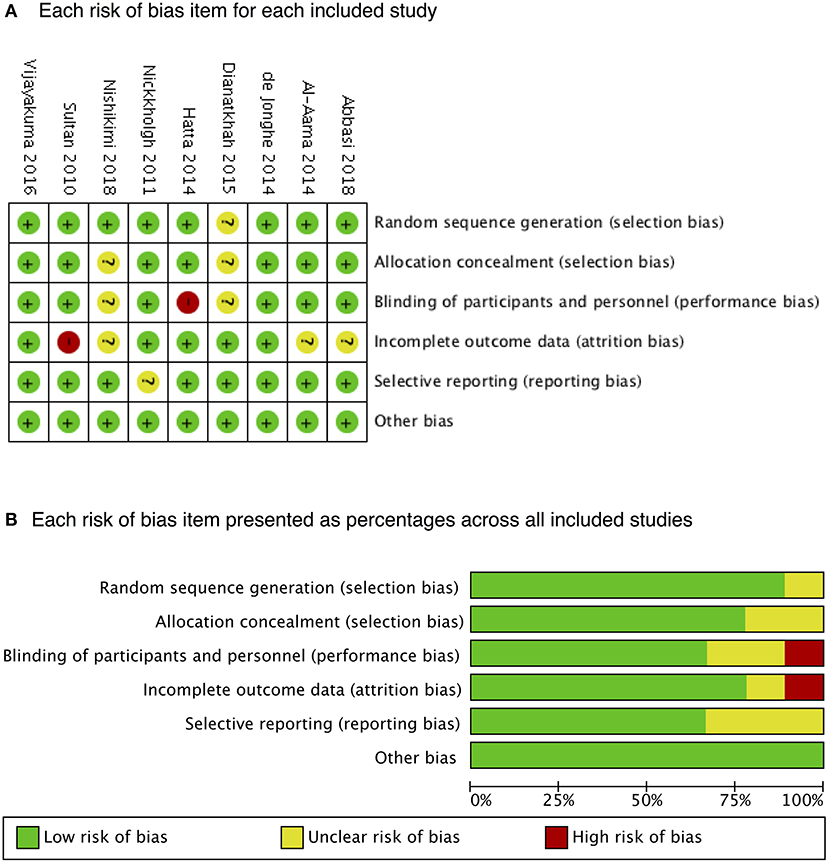
Figure 2. Risk of bias. A summary of (A) each risk of bias item for each included study and (B) each risk-of-bias item presented as percentages across all included studies.
Nine RCTs with 1,210 patients were involved to analyze the incidence of delirium. Forest plots showed that melatonergics were associated with a decreasing incidence of delirium (RR, 0.51; 95% CI, 0.30–0.85; I2 = 70%; p = 0.01; Figure 3). The statistical heterogeneity of the data was high. In the sensitivity analyses, the difference between the melatonergics and the control groups remained significant when we excluded any single RCT (Table 2). In the subgroup analyses, melatonergics showed an association with lower delirium incidence in the subgroup of elderly patients, younger patients, medical ICU patients, delirium screening methods of CAM-ICU, AMT, and CAM. In the melatonin, ramelteon, middle-aged, surgical and mixed ICU, delirium screening methods of DSM-IV, nurse observation, and methods not reported subgroups, there was no significant difference (Table 3).

Figure 3. Forest plot. Administration of melatonergics was associated with a reduction of the incidence of delirium.
For the secondary outcome, the ICU-LOS in the melatonergics group was numerically shorter than that in the control group, with no significant difference (WMD, −0.08; 95% CI, −0.19–0.03; I2 = 0%; p = 0.17; Figure 4).
The results of the present meta-analysis indicated that melatonergics were associated with a decreasing incidence of delirium in critically ill patients. Based on the encouraging results of the primary RCTs (10, 12, 20, 22), suggesting that administration of melatonin and melatonin agonists might be a promising medication for the prevention of delirium in at-risk populations, our meta-analysis provided advanced evidence. These encouraging results were considered to be quite reasonable. First, sleep deprivation is a crucial risk factor for delirium (21). Exogenous melatonergics could be associated with improvement in sleep quality and prolongation of sleep duration in some settings. Meanwhile, a reduction in melatonin levels is associated with the development of delirium. Thus, supplementation with exogenous melatonin can remedy disordered melatonin levels (23). Second, delirium may be precipitated in some patients by inflammation of the central nervous system. Melatonin is an anti-inflammatory drug, and antioxidants function protectively during the inflammatory response (24). Third, delirium occurs commonly as a side effect of benzodiazepines, the most commonly used sedatives in the ICU (25). Administration of melatonergics to hypnosis could reduce the dosage of benzodiazepines accordingly.
Although the ICU-LOS in the melatonergics group was numerically shorter than that in the control group, there was no significant difference. The reasons could be as follows. First, there were only five RCTs with 744 patients included in this outcome measure. The result should be interpreted more cautiously because of the limited sample size. Second, because the context of the ICU is linked to the delirium onset and is bad for delirium recovery, physicians might tend to discharge patients with delirium or a high risk of delirium to general wards more aggressively. This confounder makes the association between delirium and ICU-LOS more obscure and hard to interpret.
There are strengths to our study. First, in comparison to another meta-analysis (11), we excluded previously diagnosed delirium [according to what the authors reported (21)] and analyzed incident delirium to reduce confounding factors and clinical heterogeneity. Second, our sensitivity analyses showed steady and consistent results, which increased the grade of evidence.
There are also limitations to our study. First, clinical heterogeneity was obvious between the RCTs due to varied age, Acute Physiology and Chronic Health Evaluation II score and comorbidity of subjects, varied dosage of melatonergics in intervention strategy, and different methods for delirium assessment. The assessment of delirium has long been challenging for ICU physicians. Without gold standard for diagnosis, different delirium scales have been used (6). The heterogeneity in assessment methods added difficulty in the interpretation of the findings. The 2013 clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the ICU recommended CAM-ICU, the most valid monitoring tool so far (6). After that, the latest RCTs (12, 14, 15) used CAM-ICU as the assessment tool. More experience using CAM-ICU to screen delirium is needed for future studies and clinical practice. Second, although nine RCTs were involved, the overall sample size was not large. In addition, the small number of RCTs (<10) also added difficulty in estimating publication bias. Potential negative unpublished studies may be missing, resulting in overoptimism in our conclusion. Additionally, the effect of melatonergics in the treatment of delirium was not studied in the present meta-analysis, which is of great value and needs to be further studied.
Exogenous melatonergics seem to be associated with a decreased incidence of delirium. No significant difference in ICU-LOS was identified. Additional studies are needed to further evaluate the efficacy and safety of melatonin in preventing delirium.
ZJ, YZ, and HH searched the scientific literature and drafted the manuscript, collect the data, and performed statistical analyses. RY, CR, YW, YY, and WenL polished and revised the manuscript. WeiL, XX, and BD contributed to conception, design, data interpretation, manuscript revision for critical intellectual content, and supervision of the study. All authors read and approved the manuscript.
This work was funded by the Beijing Ronghe Medical Development Foundation (No. 53110000MJ01794845) and the China government grants from CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-2-004) and Construction of Basic Information Technology Support System and Platform for National Prevention and Treatment of Cardiovascular Diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
YZ want to say thank you to Lin Jin (rupxup, Google Inc.).
AMT, Abbreviated Mental Test; CAM, Confusion Assessment Method; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CIs, confidence intervals; ICU, intensive care unit; ICU-LOS, length of stay in intensive care units; OR, odds ratio; RCTs, randomized controlled studies; RR, risk ratio; WMD, weighted mean difference.
1. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: Advances in diagnosis and treatment. JAMA. (2017) 318:1161–74. doi: 10.1001/jama.2017.12067
2. Klein Klouwenberg PM, Zaal IJ, Spitoni C, Ong DSY, van der Kooi AW, Bonten MJM, et al. The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ. (2014) 349:g6652. doi: 10.1136/bmj.g6652
3. van den Boogaard M, Pickkers P, Slooter AJ, Kuiper MA, Spronk PE, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ. (2012) 344:e420. doi: 10.1136/bmj.e420
4. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs. midazolam for sedation of critically ill patients: a randomized trial. JAMA. (2009) 301:489–99. doi: 10.1001/jama.2009.56
5. Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: Systematic review and meta-analysis. BMJ. (2015) 350:h2538. doi: 10.1136/bmj.h2538
6. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. (2013) 41:263–306. doi: 10.1097/CCM.0b013e3182a167d7
7. Brzezinski A. Melatonin in humans. N Engl J Med. (1997) 336:186–95. doi: 10.1056/NEJM199701163360306
8. Di WL, Kadva A, Johnston A, Silman R. Variable bioavailability of oral melatonin. N Engl J Med. (1997) 336:1028–9. doi: 10.1056/NEJM199704033361418
9. Oldham MA, Lee HB, Desan PH. Circadian rhythm disruption in the critically ill: an opportunity for improving outcomes. Crit Care Med. (2016) 44:207–17. doi: 10.1097/CCM.0000000000001282
10. Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. (2010) 4:169–73. doi: 10.4103/1658-354X.71132
11. Chen S, Shi L, Liang F, Xu L, Desislava D, Wu Q, et al. Exogenous melatonin for delirium prevention: a meta-analysis of randomized controlled trials. Mol Neurobiol. (2016) 53:4046–53. doi: 10.1007/s12035-015-9350-8
12. Vijayakumar HN, Ramya K, Duggappa DR, Gowda KV, Sudheesh K, Nethra SS, et al. Effect of melatonin on duration of delirium in organophosphorus compound poisoning patients: a double-blind randomised placebo controlled trial. Indian J Anaesth. (2016) 60:814–20. doi: 10.4103/0019-5049.193664
13. Dianatkhah M, Ghaeli P, Hajhossein Talasaz A, Karimi A, Salehiomran A, Bina P, et al. Evaluating the potential effect of melatonin on the post-cardiac surgery sleep disorder. J Tehran Heart Cent. (2015)10:122–8.
14. Abbasi S, Farsaei S, Ghasemi D, Mansourian M. Potential role of exogenous melatonin supplement in delirium prevention in critically ill patients: a double-blind randomized pilot study. Iran J Pharm Res. (2018)17:1571–80.
15. Nishikimi M, Numaguchi A, Takahashi K, Miyagawa Y, Matsui K, Higashi M, et al. Effect of administration of ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: a single-center randomized placebo-controlled trial. Crit Care Med. (2018) 46:1099–105. doi: 10.1097/CCM.0000000000003132
16. Nickkholgh A, Schneider H, Sobirey M, Venetz WP, Hinz U, Pelzl le H, et al. The use of high-dose melatonin in liver resection is safe: first clinical experience. J Pineal Res. (2011) 50:381–8. doi: 10.1111/j.1600-079X.2011.00854.x
17. Zhang Q, Gao F, Zhang S, Sun W, Li Z. Prophylactic use of exogenous melatonin and melatonin receptor agonists to improve sleep and delirium in the intensive care units: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath. (2019) 23:1059–70. doi: 10.1007/s11325-019-01831-5
18. Zhu Y, Jiang Z, Huang H, Wang Y, Zhang L, Ren C, et al. Assessment of melatonergics in prevention of delirium in critically ill patients: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e18700. doi: 10.1097/MD.0000000000018700
19. Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. (2008) 88:156–75. doi: 10.2522/ptj.20070147
20. Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. (2014) 29:550. doi: 10.1002/gps.4097
21. de Jonghe A, van Munster BC, Goslings JC, Kloen P, van Rees C, Wolvius R, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. (2014) 186:E547–56. doi: 10.1503/cmaj.140495
22. Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, et al. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. (2014) 71:397–403. doi: 10.1001/jamapsychiatry.2013.3320
23. de Rooij SE, van Munster BC. Melatonin deficiency hypothesis in delirium: a synthesis of current evidence. Rejuvenation Res. (2013) 16:273–8. doi: 10.1089/rej.2012.1405
24. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. (2008) 24:789–856. doi: 10.1016/j.ccc.2008.06.004
Keywords: melatonin, delirium, prevention, critical care medicine, systematic review
Citation: Zhu Y, Jiang Z, Huang H, Li W, Ren C, Yao R, Wang Y, Yao Y, Li W, Du B and Xi X (2020) Assessment of Melatonergics in Prevention of Delirium: A Systematic Review and Meta-Analysis. Front. Neurol. 11:198. doi: 10.3389/fneur.2020.00198
Received: 13 December 2019; Accepted: 05 March 2020;
Published: 07 April 2020.
Edited by:
Melissa P. Knauert, Yale University, United StatesReviewed by:
Brian K. Gehlbach, University of Iowa, United StatesCopyright © 2020 Zhu, Jiang, Huang, Li, Ren, Yao, Wang, Yao, Li, Du and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, bGl3ZWlAbXJiYy1uY2NkLmNvbQ==; Huibin Huang, aGhiYTAyOTIyQGJ0Y2guZWR1LmNu; Yibing Zhu, eWl5aV9iaW5nYmluZ0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.