
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 10 January 2020
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.01375
Myofibrillar myopathies (MFM) are a clinically and genetically heterogenous group of inherited myopathies characterized by aggregation of Z-disc proteins. Mutations in desmin account for ~7% of MFM. We report here a Hmong family with an autosomal dominant MFM caused by a novel variant in the desmin gene. The proband presented with lower limb followed by upper limb weakness starting in the 5th decade. On examination, there was distal more than proximal muscle weakness. One sibling was similarly affected, while another had an asymptomatic elevation of creatine kinase. Genetic testing revealed a novel p.Ser13Tyr variant, which was predicted by in silico algorithms to alter protein function. Muscle biopsy revealed a MFM. Muscle MRI demonstrated selective involvement of the tensor fasciae latae, semitendinosus, sartorius, gracilis, gastrocnemius, soleus, and peroneus longus muscles. In this family, the histological and MRI findings assisted in the interpretation of genetic testing results.
Myofibrillar myopathies (MFM) are a clinically and genetically heterogenous group of inherited myopathies histologically characterized by aggregation of Z-disc proteins (1). Mutations in 11 different genes have so far been found to cause MFM (2, 3), but approximately half of patients remain without a molecular diagnosis (4). The increasing availability of genetic testing by next-generation sequencing has also led to the identification of many variants of unknown significance (VUS) in genes known to cause MFM. In light of the paucity of functional assays available for most forms of MFM, it is often challenging to determine the pathogenicity of these variants. Muscle MRI however has shown promise in distinguishing certain forms of MFM from other distal myopathies based on patterns of muscle involvement and may similarly be of assistance in the interpretation of VUS (5). We report here a family with an adult-onset distal myopathy caused by a novel heterozygous missense variant in the desmin gene and highlight the role of muscle MRI in interpreting genetic testing results in this family.
The proband was a 47 year-old man of Hmong descent who presented with a 5-year history of progressive lower limb followed by upper limb weakness. Neurological examination revealed severe distal weakness in the upper and lower limbs, along with a mild to moderate degree of proximal weakness. The patient's 50 year-old sister was more severely affected, requiring assistance walking. She started noticing the weakness at age 37, and had no cardiac involvement. A 52 year-old brother had asymptomatic elevated CK level (493 U/L, normal <171). An 80 year-old paternal aunt has become wheelchair bound from the myopathy, which started around age 50. The proband's father had died at age 53 of lung cancer without displaying any signs of neuromuscular disease. Family members were not available for detailed clinical evaluation.
The proband had a creatine kinase level of 669 U/L (normal < 308 U/L). An electromyogram revealed rapid recruitment of complex short duration motor unit potentials in all muscles examined, mixed with long-duration motor unit potentials in some proximal muscles. Fibrillation potentials were present in most muscles. A biopsy of the left quadriceps showed non-specific changes. An echocardiogram did not reveal evidence of a cardiomyopathy, while an electrocardiogram showed a first-degree atrioventricular block.
A next-generation gene panel targeting 121 known myopathy genes revealed a heterozygous VUS in DES (c.38C>A; p.Ser13Tyr) and a homozygous VUS in RYR1 (c. 12880A>G; p.Thr4294Ala). Neither variant was present in population databases, nor known to be disease-causing. The DES variant was predicted by three in-silico algorithms to adversely affect protein function, while the RYR1 variant was predicted to be benign.
Segregation analysis was subsequently performed. The DES variant was present in the affected sister and in the brother with elevated CK level, but absent in an unaffected sister. By contrast, the RYR1 variant did not segregate with the phenotype. A repeat muscle biopsy of the right quadriceps showed findings typical of MFM (Figure 1). A muscle MRI demonstrated atrophy and fatty replacement most severely affecting the tensor fasciae latae, semitendinosus, sartorius, gracilis, gastrocnemius, soleus and peroneus longus muscles (Figure 2). By contrast, the biceps femoris, semimembranosus and tibialis anterior were relatively spared.

Figure 1. Right quadriceps muscle biopsy findings. Hematoxylin and eosin (A) and modified Gomori trichrome (B) stained sections demonstrating variation in fiber size, increased endomysial and perimysial connective tissue, rimmed vacuoles, and hyaline inclusions with increased immunoreactivity for desmin (C), dystrophin (D), αB-crystallin (E) and myotilin (F), indicating a myofibrillar myopathy. Scale bar = 50 μm.
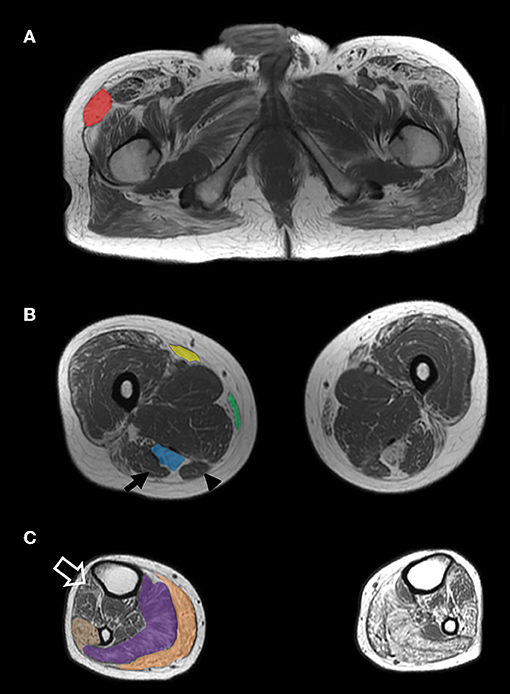
Figure 2. MRI findings. Axiat T1-weighted MRI images of the proximal thigh (A), largest diameter of the mid-thigh (B) and largest diameter of the mid-calf (C) demonstrate grade IV (>50% of the muscle) atrophy and fatty replacement of the tensor fasciae latae (A—red), semitendinosus (B—blue), sartorius (B—yellow), gracilis (B—green), gastrocnemius (C—orange), soleus (C—purple), and peroneus longus (C—tan) muscles. The biceps femoris (B—arrow), semimebranosus (B—arrowhead), and tibialis anterior (C—open arrow) are relatively spared.
Herein, we report a Hmong family with an autosomal dominant distal myopathy and asymptomatic hyperCKemia due to the p.Ser13Tyr missense variant in the desmin gene. The distal-predominant weakness observed in the proband is typical of desminopathy (6). Cardiomyopathy, which is common in desminopathy patients, was absent in this family (6, 7).
The findings observed on muscle biopsy establish the presence of a MFM and thus support the pathogenicity of the mutation in DES. The serine residue at position 13 is highly conserved and serves as a phosphorylation site for protein kinase C (8). Its functional importance is demonstrated by the existence of another pathogenic substitution affecting the same amino acid, p.Ser13Phe. Patients carrying this variant typically present with a severe cardiac phenotype, which may occur in isolation or in association with proximal or distal weakness (8–10). The absence of the c.38C>A variant from population databases and its segregation in the family provide additional supportive evidence of its pathogenicity, as do the proband's MRI findings.
Imaging studies have identified two distinct patterns of muscle involvement in MFM. Selective involvement of the semitendinosus, sartorius, gracilis, and peroneus longus muscles is typical of desminopathies and αB-crystallinopathies (11–13). By contrast, a mirror image is seen in myotilinopathies and filaminopathies, in which there is involvement of the biceps femoris, semimembranosus, tibialis anterior, medial gastrocnemius and soleus. Fischer et al. reported that among a group of 46 MFM patients, MRI involvement of the semitendinosus equalling or exceeding the biceps femoris and involvement of the peroneal muscles equalling or exceeding the tibialis anterior predicted the presence of a desminopathy with 100% sensitivity and 95% specificity (12). It should be noted however that selective involvement of the semitendinosus has also been reported in hereditary myopathy with early respiratory failure caused by titin mutations and is therefore not pathognomonic of desminopathy (14). While muscle MRI was successful in distinguishing myotilinopathy from other distal myopathies (5), its sensitivity and specificity for desminopathy in this context remains to be established. In inherited myopathies, MRI should therefore be interpreted in the context of the clinical picture and results of molecular genetic testing. Muscle MRI can also assist in the interpretation of VUS, particularly in genes such as DES, in which a distinctive imaging pattern of muscle involvement has been described.
The datasets generated for this study are available on request to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SN: study design, data collection, and drafting of a first version of the manuscript. BH: review of the MRI images and critical revision of the manuscript. EN: study design, data collection, study supervision, and critical revision of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Selcen D, Engel AG. Myofibrillar myopathies. Handb Clin Neurol. (2011) 101:143–54. doi: 10.1016/B978-0-08-045031-5.00011-6
2. Milone M, Liewluck T. The unfolding spectrum of inherited distal myopathies. Muscle Nerve. (2019) 59:283–94. doi: 10.1002/mus.26332
3. Palmio J, Udd B. Myofibrillar and distal myopathies. Rev Neurol. (2016) 172:587–93. doi: 10.1016/j.neurol.2016.07.019
4. Claeys KG, Fardeau M. Myofibrillar myopathies. Handb Clin Neurol. (2013) 113:1337–42. doi: 10.1016/B978-0-444-59565-2.00005-8
5. Bugiardini E, Morrow JM, Shah S, Wood CL, Lynch DS, Pitmann AM, et al. The diagnostic value of MRI pattern recognition in distal myopathies. Front Neurol. (2018) 9:456. doi: 10.3389/fneur.2018.00456
6. Behin A, Salort-Campana E, Wahbi K, Richard P, Carlier RY, Carlier P, et al. Myofibrillar myopathies: state of the art, present and future challenges. Rev Neurol. (2015) 171:715–29. doi: 10.1016/j.neurol.2015.06.002
7. van Spaendonck-Zwarts KY, van Hessem L, Jongbloed JD, de Walle HE, Capetanaki Y, van der Kooi AJ, et al. Desmin-related myopathy. Clin Genet. (2011) 80:354–66. doi: 10.1111/j.1399-0004.2010.01512.x
8. Bergman JE, Veenstra-Knol HE, van Essen AJ, van Ravenswaaij CM, den Dunnen WF, van den Wijngaard A, et al. Two related Dutch families with a clinically variable presentation of cardioskeletal myopathy caused by a novel S13F mutation in the desmin gene. Eur J Med Genet. (2007) 50:355–66. doi: 10.1016/j.ejmg.2007.06.003
9. van Spaendonck-Zwarts KY, van der Kooi AJ, van den Berg MP, Ippel EF, Boven LG, Yee WC, et al. Recurrent and founder mutations in the Netherlands: the cardiac phenotype of DES founder mutations p.S13F and p.N342D. Neth Heart J. (2012) 20:219–28. doi: 10.1007/s12471-011-0233-y
10. Pica EC, Kathirvel P, Pramono ZA, Lai PS, Yee WC. Characterization of a novel S13F desmin mutation associated with desmin myopathy and heart block in a Chinese family. Neuromuscul Disord. (2008) 18:178–82. doi: 10.1016/j.nmd.2007.09.011
11. Olive M, Odgerel Z, Martinez A, Poza JJ, Bragado FG, Zabalza RJ, et al. Clinical and myopathological evaluation of early- and late-onset subtypes of myofibrillar myopathy. Neuromuscul Disord. (2011) 21:533–42. doi: 10.1016/j.nmd.2011.05.002
12. Fischer D, Kley RA, Strach K, Meyer C, Sommer T, Eger K, et al. Distinct muscle imaging patterns in myofibrillar myopathies. Neurology. (2008) 71:758–65. doi: 10.1212/01.wnl.0000324927.28817.9b
13. Schramm N, Born C, Weckbach S, Reilich P, Walter MC, Reiser MF. Involvement patterns in myotilinopathy and desminopathy detected by a novel neuromuscular whole-body MRI protocol. Eur Radiol. (2008) 18:2922–36. doi: 10.1007/s00330-008-1071-1
Keywords: desmin, myofibrillar myopathy, distal myopathy, hereditary myopathy, muscle MRI
Citation: Nicolau S, Howe BM and Naddaf E (2020) Novel Desmin Mutation Causing Myofibrillar Myopathy in a Hmong Family. Front. Neurol. 10:1375. doi: 10.3389/fneur.2019.01375
Received: 04 July 2019; Accepted: 12 December 2019;
Published: 10 January 2020.
Edited by:
Edoardo Malfatti, INSERM U1179 Handicap Neuromusculaire: Physiopathologie, Biothérapie et Pharmacologie Appliquées (END-ICAP), FranceReviewed by:
Carmelo Rodolico, University of Messina, ItalyCopyright © 2020 Nicolau, Howe and Naddaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elie Naddaf, bmFkZGFmLmVsaWVAbWF5by5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.