- 1Department of Medicine, The University Sleep Disorders Center, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 2The Strategic Technologies Program of the National Plan for Sciences and Technology and Innovation in the Kingdom of Saudi Arabia, Riyadh, Saudi Arabia
- 3Prince Naif Health Research Center, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
- 4Department of Family and Community Medicine, King Saud University, Riyadh, Saudi Arabia
Obstructive sleep apnea (OSA) can present with or provoke various psychological symptoms. In this article, we critically review studies that have examined dreams, dream recall, and dream content in patients with OSA. Obstructive events induce recurrent sleep fragmentation and intermittent desaturations in patients with OSA, which may trigger different parasomnias, including nightmares. Contradictory results have been reported concerning dreams in patients with OSA; while some investigators have reported less dreams in OSA patients, others have described that patients with OSA have increased dreams with emotional content, mainly violent and hostile content. Although there are reports of respiratory-related dream content in patients with OSA, most studies that have assessed the dream content of patients with OSA revealed that respiratory-related dream content was unusual. A clear association between post-traumatic stress disorders, comorbid OSA, and nightmares has been reported in several studies. Furthermore, an improvement in nightmare frequency with continuous positive airway pressure (CPAP) treatment has been shown. An inverse relationship between the severity of OSA reflected by the apnea-hypopnea index and dream recall has been demonstrated in several studies. Future studies should differentiate between patients with non-stage specific OSA and patients with rapid eye movement (REM) predominant OSA.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder (SRBD). Among the middle-aged population, the prevalence of OSA syndrome ranges from 3 to 18% in men and from 2 to 17% in women. The prevalence of OSA increases with age, and it is projected that 28–67% of older men and 20–54% of older women have OSA syndrome (1–3).
The characteristics feature of OSA is recurrent occurrences of upper airway obstruction during sleep, which ranges from partial upper airway obstruction (hypopnea) or complete upper airway obstruction (apnea). The two major pathophysiological consequences of OSA are sleep fragmentation and arterial oxygen desaturation (4).
OSA is associated with adverse health outcomes, including decreased quality of life, insulin resistance, and increased risk for cardiometabolic disorders and mortality (5). Excessive daytime sleepiness (EDS) is a major consequence of OSA that often constitutes a risk factor for motor vehicle crashes (6). OSA can present with or provoke various psychological symptoms (7). Sleep fragmentation secondary to recurrent obstructive events and recurrent desaturations in patients with OSA may trigger parasomnias (8, 9).
In the nineteenth century, researchers hypothesized that recurrent oxygen desaturation during sleep may prompt the appearance of nightmares (10). In an older study, Boerner reported that blocking the nose and mouth with a cloth induced nightmares (11). Nevertheless, conflicting results have been reported regarding dreams in patients with OSA. Some studies have reported less dreams in OSA patients, and others have described that patients with OSA have increased dreams with emotional content, mainly violent and hostile. In this article, we critically review studies that have examined dreams, dream recall, and dream contents in patients with OSA.
OSA and Emotional Regulation
Human behavior and emotions are regulated by the amygdala, locus coeruleus, and prefrontal cortex (12). Sleep, particularly rapid eye movement (REM) sleep, maintains brain homeostasis, which supports optimal social and emotional functioning (13). Current evidence supports the concept that sleep is critical for the formation of emotionally-valenced information throughout all stages of emotional regulation (12). Moreover, sleep is vital for the regulation of emotions. Hence, sleep disturbances in patients with OSA, such as slow wave sleep (SWS) and REM sleep disturbances, may pre-dispose patients to neurological and psychiatric disorders (12).
Though some researchers have reported less dreams in patients with OSA, others have described that patients with OSA have increased dreams with emotional content, mainly violent and hostile (14–17). It has been postulated that obstructive events during REM sleep may activate the limbic system, which may increase dreams with high emotional contents (15).
Moreover, OSA and psychiatric disorders have a bidirectional relationship, where the presence of one disorder could worsen the other. OSA is prevalent in psychiatric patients (7), especially those with depression (18, 19), anxiety (20), and post-traumatic stress disorder (PTSD). On the other hand, patients with OSA have a higher risk of developing psychiatric symptoms and comorbidities, in comparison to the general population (21). Moreover, patients with OSA have a higher rate of mood disorders, anxiety disorders, PTSD, and psychotic disorders, compared with non-OSA patients (18, 22). The coexistence of comorbid psychiatric disorder may interfere with dream content or nightmares (23, 24).
Dream Recall in Patients With OSA
Studies have reported contradictory results with regards to dream recall in patients with OSA (10, 15, 25). The majority of previous studies that have assessed the relationship between OSA and dream recall has been retrospective. Whereas, some investigators reported less dream recall in patients with OSA and improvement of dream recall after treatment with continuous positive airway pressure (CPAP), others have demonstrated that OSA patients have increased dream recall frequency and increased dreams with emotional content, particularly aggressive and violent content (14–17). Table 1 presents an overview of studies that assessed dream recall in patients with sleep-disordered breathing
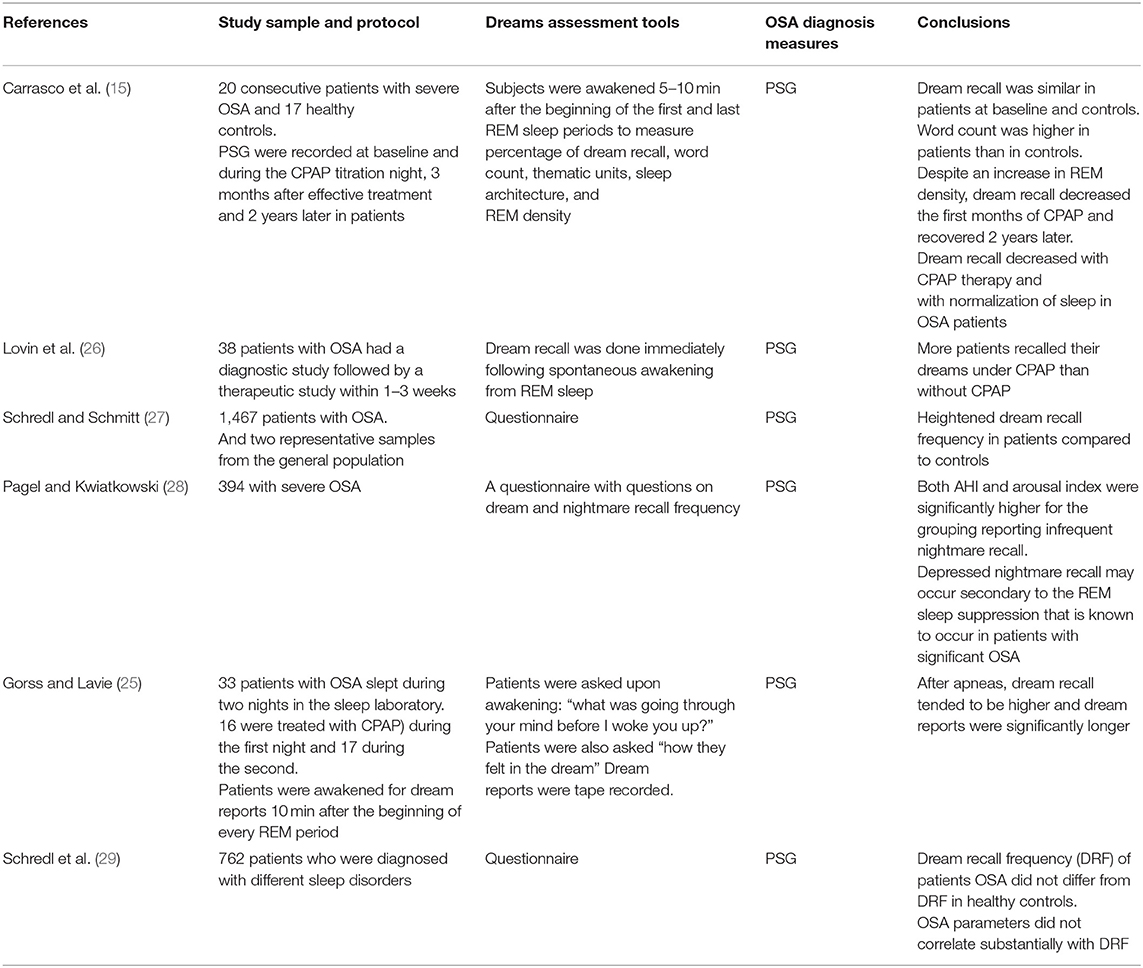
Table 1. A summary of studies that assessed dream recall in patients with sleep-disordered breathing.
Initial studies that assessed dream recall at home by retrospective questionnaire scales demonstrated lesser dream recall in patients with OSA than in healthy controls (14, 30). A subsequent study of 1,243 patients with OSA demonstrated higher dream recall in OSA patients than in a representative sample from the general population (31). A recent study of 1,467 patients with OSA who completed a dream questionnaire reported heightened dream recall frequency in patients compared to that in controls (27). However, several studies reported no correlation between OSA severity parameters [i.e., apnea hypopnea index (AHI) and desaturation index] and dream recall frequency (14, 17, 30, 32, 33).
Studies assessing the effects of CPAP therapy on dream recall have reported conflicting results. In a sample of patients (n = 33) already treated with CPAP for a minimum of 2 months, Gross and Lavie demonstrated that discontinuation of CPAP therapy for one night caused an increased AHI and enhanced dream recall, and dream reports were significantly longer on untreated nights (25). However, the investigators did not assess the recall of dreams before the initiation of CPAP treatment (25). Moreover, dream reports from the untreated nights and after apneas appeared to be more negative (25). However, it is possible that sleeping without CPAP therapy after 2 months of treatment may have caused these patients to worry, thereby enhancing the more negatively toned dreams which were not related to obstructive events.
One more study in patients with severe OSA reported that despite an increase in REM density after CPAP therapy, dream recall diminished after CPAP initiation both acutely and after 3 months (15). However, this finding was independent of REM density and the amount of REM sleep. The authors theorized that the decrease in sleep fragmentation following CPAP therapy could explain this finding (15). Moreover, the recovery increment of SWS proportion induced by CPAP has also been suggested as an explanation for the reduction in dream recall (15).
Gender differences in dream recall in patients with OSA have been reported recently by Schredl and Schmitt (27) with more recall observed in women, which is consistent with the findings of previous studies that reported gender differences in dream recall (34).
Carrasco et al. proposed two theories that may affect dream recall in OSA patients (15). The first theory is the cognitive impairment theory, where impairment of cognitive function in OSA patients may impair dream recall (35). The second contradictory theory is the frequent arousals theory, where frequent arousals and light interrupted sleep may increase dream recall (15).
Possible explanations for the decreased recall of dreams in some patients with OSA include the possibility that time spent awake after respiratory event-induced arousals is too short to allow dream recall (27). Another possibility is that cognitive deficits in patients with OSA may offset the proposed effects of frequent arousals on dream recall (27, 35). Future studies should account for the duration of arousals and obstructive events, cognitive function, and AHI during REM sleep and their impact on dream recall.
Dream Contents
Exposure to stimuli during REM sleep can modify dream contents (36). The effects of external auditory, sensory, and olfactory stimuli during REM sleep have been shown previously (37–39). However, it remains unknown if internal pathological changes (e.g., obstructive apneas and hypopneas) affect dream content (27). Table 2 presents a summary of studies that assessed dream contents and nightmares in patients with sleep-disordered breathing.
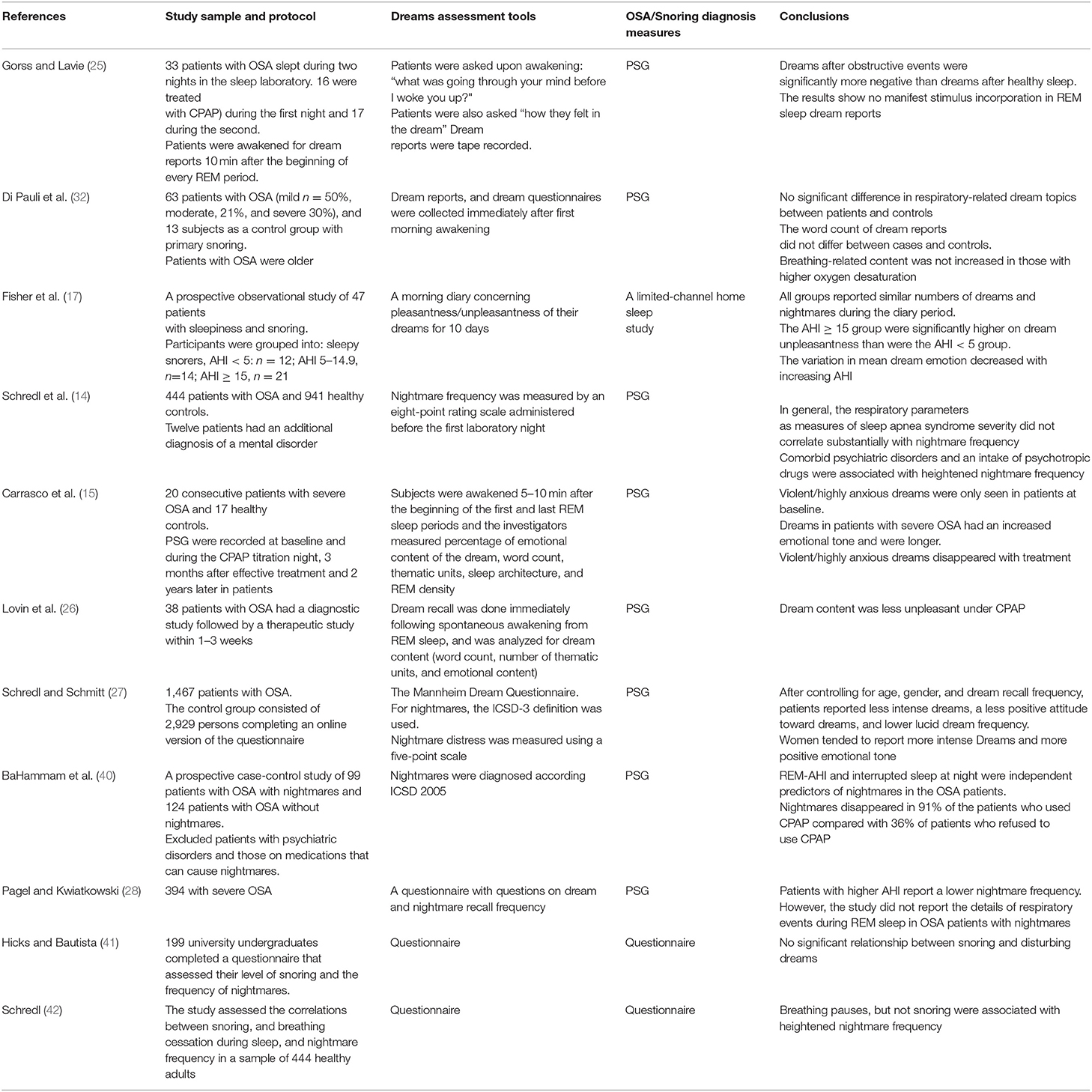
Table 2. A summary of studies that assessed dream contents and nightmares in patients with sleep-disordered breathing.
An earlier study assessed 33 patients with OSA who slept during two nights in the sleep laboratory before and after CPAP therapy (25). Ten minutes after the beginning of every REM period, patients were awakened for dream reports. After awakening from REM sleep secondary to obstructive events, there was a trend toward increased dream recall (60 vs. 72%, p = 0.09) and dream reports were more elaborative (16 vs. 24 words, p = 0.05) than those after awakening from REM sleep without obstructive events (25). However, the apnea stimulus was not incorporated into the dream reports. Nevertheless, after apneas, dreams were significantly more negative than dreams after healthy sleep (p < 0.01) and included significantly more characters, activities, and social interactions (25). The authors proposed that the stress caused by apneas exerted only a global emotional influence on manifest dreaming (25).
Most studies assessing dream contents of patients with OSA revealed that respiratory-related dream contents were unusual (25, 31, 32, 43, 44). Nevertheless, in a prospective case-control study of 99 patients with OSA and nightmares (excluding patients with psychiatric disorders), BaHammam et al. reported that dream contents in most studied patients were related to suffocation (40). Examples of patients' reports included the following: “I dreamt that I fell in a deep well and was gasping for breath” (male, 40 years, AHI 45/h), “that a person was holding my neck preventing me from breathing” (female, 43 years, AHI 49/h), and “I dreamt of drowning in dark water and was gasping for breath” (female, 39 years, AHI 47/h) (40). Similarly, other authors reported respiratory-related dreams including “I am buried under the sand and fighting my way to a surface that I can't seem to reach. I wake gasping for air” (45); “I was lying in a coffin-dead-and cried: My god, please, don't let me die, I am still young and I have small children” (44); “I was diving without oxygen tanks and was gasping for breath. The way to the surface was far and I managed it just in time. After waking up I really gasped for breath” (14).
Differences between studies could be attributed to the dissimilarities in characteristics of the included group in each study (e.g., cognitive function, duration of REM sleep, AHI during REM sleep, and patients' awareness of the severity of their illness) and the adopted exclusion criteria (40), because patients' awareness of an illness and its complications may influence the emotional; contents of their dreams (27).
OSA and Nightmares
The first description we could find suggesting a link between OSA and nightmares was written by Avicenna (980–1,037 C.E.) (46). He stated that people who sleep on the back have an open mouth as the muscles of the mouth and throat get too weak to maintain the normal position (suggesting sleep-disordered breathing), and further, he described that sleeping on the back can lead to stroke, paralysis, and nightmares (46, 47). Subsequently, investigators in the nineteenth century postulated that oxygen desaturation during sleep might cause nightmares (10, 11).
The International Classification of Sleep Disorders-third edition (ICSD-3) defined nightmares as “dreams with strong negative emotions that result in awakening from the dreams. The dream plot can be recalled very vividly upon awakening.” (48). The ICSD-3 has diagnostic criteria for nightmare disorder (nightmares), which includes three conditions that need to be met to fulfill the definition of nightmares (48). These criteria include: (1) repeated incidences of long, extremely dysphoric, and well-remembered dreams that usually involve threats to survival, security, or physical integrity; (2) the individual quickly becomes oriented and alert upon awakening from the dysphoric dreams; (3) the dreams and the resulting sleep disturbances result in clinically significant distress or impairment in social, occupational, or other important areas of functioning (48).
Previous studies have reported significantly lower frequency of nightmares in patients with more severe OSA reflected by a higher AHI (14, 28, 49). However, the majority of previous studies had a relatively small sample size, which affects statistical power and ability to account for confounding factors that may impact both OSA and nightmares.
A recent registry-based cross-sectional study of ~2,800 patients with OSA revealed that the prevalence of nightmares was 40.6% (49); however, the prevalence of nightmares in the controls [individuals without OSA]) was 50.1%, which was much higher than that reported in the general population (19.4%) (50). This reflects the fact that the study did not use a standard definition of nightmares, which may partly explain the discrepancies in the reported prevalence of nightmares in OSA patients. However, the study revealed that the prevalence of nightmares and sleep-related violence were inversely related to OSA severity (49).
“Nightmares” is one of the REM-related parasomnias, which occur during REM sleep. However, REM sleep is usually reduced in patients with severe OSA (40, 51). Therefore, Pagel and Kwiatkowski suggested that the reduction in nightmares in patients with severe OSA and an increased AHI may be related to the suppression of REM sleep in group of patients (28).
Nightmares have been reported in patients with REM-predominant OSA regardless of the degree of oxygen desaturation (40). In a prospective case-control study of 99 patients with OSA with nightmares (defined in accordance with the ICSD), BaHammam et al. demonstrated that although there were no significant differences in REM sleep duration or duration of apneas and hypopneas during REM and NREM sleep between OSA patients with and without nightmares, patients with nightmares had a significantly higher AHI during REM than patients without nightmares (40). However, no relationship was demonstrated between OSA severity reflected by AHI and SPO2 < 90% and the presence of nightmares (40). Logistic regression analysis identified the apnea-hypopnea index during REM sleep and interrupted nocturnal sleep as the independent predictors of nightmares in patients with OSA (40). This suggests that nightmares are possibly associated with the presence of apneas and hypopneas during REM sleep (40). It is possible that the patients who had frequent awakenings due to recurrent apneas/hypopneas during REM sleep recalled more nightmares. Interestingly, one study showed that the patients who are more difficult to arouse report lower nightmare frequencies (52). Nightmares in BaHammam et al. study were mostly related to suffocation (40).
Interestingly, in patients with good adherence to CPAP therapy, nightmares disappeared in 91%, compared with 36% of patients who did not adhere to CPAP (p < 0.001) (40). The authors postulated that recurrent obstruction during REM sleep rather than the duration of REM sleep may increase the vulnerability of patients with OSA to developing nightmares (40). This hypothesis is supported by previous studies, which demonstrated that one night of CPAP therapy caused a reduction in dreams and dream recall, though the amount and duration of REM sleep had increased (15, 25). Moreover, air hunger during wakefulness induced by breathing air that has high concentration of CO2 resulted in stimulation of the limbic/paralimbic brain regions (brain regions activated during REM sleep) (53). This may partly explain the increased nightmares in patients with higher obstructive events during REM sleep. Moreover, Carrasco et al. hypothesized that respiratory events during REM sleep cause stimulation of the limbic system; this, in turn, may generate dreams with high emotional contents (15).
The disturbances caused by sleep fragmentation in individuals with OSA can manifest on the patient emotionally by producing psychical symptoms, particularly, stress (54, 55). CPAP therapy has been shown to reduce stress in patients with OSA (55, 56). Therefore, it is possible that stress associated with OSA can be responsible for nightmares, and the reduction in stress with CPAP therapy results in a reduction in nightmares. This hypothesis needs to be tested.
OSA and Post-traumatic Stress Disorder
According to 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), post-traumatic stress disorder (PTSD) is explained by “the development of a specific cluster of symptoms after exposure to a traumatic event that elicits a response of fear, helplessness, or horror” (57).
Numerous studies have identified a wide range of comorbid sleep disorders in individuals with PTSD such as insomnia, nightmares, OSA, and sleep arousal, and enactment of dreams (58–60). A recent meta-analysis that aimed to identify the pooled prevalence of OSA in patients with PTSD on different AHI criteria showed an OSA prevalence of 76% (95% confidence interval [CI] = 44–93%) (AHI ≥ 5/h) and 44% (95% CI = 21–70%) (AHI ≥ 10/h), respectively (61).
Krakow et al. proposed that PTSD induces sleep fragmentation, which in turn disturbs the human upper airway and increases vulnerability to the consequent development of upper airway collapse (62). This may lead to the development of OSA, which may in turn worsen PTSD symptoms (62, 63). Moreover, it has been shown in some studies that sleep disturbances before traumatic events predict PTSD (64, 65).
A recent study of 59 patients with PTSD and 118 non-PTSD controls reported that weekly nightmare frequency was higher in patients with PTSD compared with the non-PTSD group before starting CPAP (66).
Previous studies have demonstrated a strong association between PTSD, comorbid OSA and nightmares, and an improvement in nightmare frequency with CPAP treatment (67). Germain et al. proposed that REM sleep enhances activation of the amygdala and reduces medial frontal cortex activation in patients with PTSD, which may worsen nightmares (68). As PTSD is associated with disturbances in both sleep and extinction memory, improving sleep quality may improve PTSD symptoms via strengthening naturally acquired extinction. It has been demonstrated that the hippocampus is susceptible to hypoxia, and decline in hippocampal activity correlates with the severity of PTSD (69). Nocturnal hypoxia in untreated OSA has been proposed as a possible trigger for PTSD symptoms including nightmares (66).
Previous studies have reported significant improvements in the frequency of nightmares and the symptoms of PTSD following CPAP therapy (70, 71). However, most of these studies had several limitations, including a retrospective design and small sample size. A recent prospective cohort study recruited 47 combat veterans with PTSD and OSA diagnosed by type one sleep study and reported that adherence to CPAP was associated with a reduction in nightmare distress and frequency (72). Treatment with CPAP resulted in a reduction of nightmare distress at 3 months follow-up in patients with both mild-to-moderate PTSD (p = 0.006) and severe-to-very-severe PTSD (p = 0.02) (72).
However, adherence to CPAP therapy in patients with PTSD and comorbid OSA remains a major problem. A recent meta-analysis revealed that the pooled estimate of regular use was lower in patients with PTSD and OSA than in those with OSA alone (61). The results of the pooled estimate of regular use were inferior in patients with PTSD and comorbid OSA compared with patients with OSA alone, with no significant heterogeneity (I2 = 0%), which points to the unfavorable influence of PTSD symptoms on CPAP compliance in patients with OSA (61).
Newer approaches that use holistic strategies to improve CPAP adherence in PTSD patients with comorbid OSA are needed. Approaches to enhance adherence include group education (73), cognitive-behavioral therapy (74), and motivation (75).
Effects of Continuous Positive Airway Pressure (CPAP) on Dreams
There is a lack of consensus on the effects of CPAP therapy on dream recall and contents in patients with OSA. In a study of 20 patients with OSA (mean AHI of 73/h), Carrasco et al. reported a reduction in dream recall after one night of CPAP therapy accompanied by an increase in dream length and positively-toned dreams (15). Positively toned dreams, especially after long-term CPAP therapy, may be explained by the continuity hypothesis (whereby dreams express waking concerns and emotional preoccupations) because CPAP treatment is often seen as very beneficial in patients with OSA (76). However, another study of 38 patients OSA who underwent a diagnostic study followed by a second night on CPAP therapy reported a rebound of REM sleep accompanied by a quantitative increase in dream recall and a change in dream content (26). CPAP therapy resulted in a change in the dream content with a reduction in unpleasant content (50% without CPAP vs. 37.5% under CPAP) (26). A third study demonstrated that OSA patients with nightmares experienced an increase in sleep interruptions and had more obstructive events in REM sleep than those without nightmares. Notably, CPAP therapy resulted in a significant reduction in nightmares (40).
Conclusion
Although the number of studies investigating dreaming in patients with OSA is relatively small, the literature is intriguing and shows that altered sleep physiology due to recurrent obstructive events, frequent arousals, and repeated desaturations may influence dream recall and dream content in some patients. Nevertheless, studies were inconsistent and reported conflicting results. Future studies addressing dreams and nightmares in patients with OSA should differentiate between REM-predominant OSA and non-stage specific OSA. Additionally, functional brain imaging, particularly of the limbic system during REM sleep in patients with OSA will facilitate understanding of the pathophysiology of dreams in this group of patients.
Moreover, future studies should measure the cognitive function of patients with OSA and dreams before and after CPAP therapy to assess the relationship between cognitive function and dream recall and contents. Similarly, assessment of mental function and stress before and after CPAP therapy is necessary to examine the relationship between improvement in psychical manifestations and dreams.
Author Contributions
AB and AA have contributed equally to review of the literature, analysis, and writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Arnold J, Sunilkumar M, Krishna V, Yoganand S, Kumar MS, Shanmugapriyan D. Obstructive sleep apnea. J Pharm Bioall Sci. (2017) 9(Suppl 1):S26. doi: 10.4103/jpbs.JPBS_155_17
2. Rostanski SK, Westwood AJ, Baboli M, Marshall RS. Sleep apnea and cerebral blood flow: the role of autoregulation. Stroke. (2017) 48:AWP437.
3. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. (2015) 7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11
4. Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thor Soc. (2008) 5:144–53. doi: 10.1513/pats.200707-114MG
5. Redline S. Screening for obstructive sleep apnea: implications for the sleep health of the population. JAMA. (2017) 317:368–70. doi: 10.1001/jama.2016.18630
6. Gupta R, Pandi-Perumal SR, Almeneessier AS, BaHammam AS. Hypersomnolence and Traffic Safety. Sleep Med Clin. (2017) 12:489–99. doi: 10.1016/j.jsmc.2017.03.018
7. Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. (2015) 11:165–75. doi: 10.5664/jcsm.4466
8. Viola-Saltzman M, Kumar S, Undevia N. The frequency of parasomnia symptoms in patients with obstructive sleep apnea. Sleep. (2009) 32:A196.
9. Guilleminault C, Kirisoglu C, Bao G, Arias V, Chan A, Li KK. Adult chronic sleepwalking and its treatment based on polysomnography. Brain. (2005) 128(Pt 5):1062–9. doi: 10.1093/brain/awh481
10. Schredl M. Dreams in patients with sleep disorders. Sleep Med Rev. (2009) 13:215–21. doi: 10.1016/j.smrv.2008.06.002
12. Goldstein AN, Walker MP. The role of sleep in emotional brain function. Ann Rev Clin Psychol. (2014) 10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716
13. Sopp MR, Michael T, Weeß H-G, Mecklinger A. Remembering specific features of emotional events across time: the role of REM sleep and prefrontal theta oscillations. Cogn Affect Behav Neurosci. (2017) 17:1186–209. doi: 10.3758/s13415-017-0542-8
14. Schredl M, Schmitt J, Hein G, Schmoll T, Eller S, Haaf J. Nightmares and oxygen desaturations: is sleep apnea related to heightened nightmare frequency? Sleep Breath. (2006) 10:203–9. doi: 10.1007/s11325-006-0076-8
15. Carrasco E, Santamaria J, Iranzo A, Pintor L, De Pablo J, Solanas A, et al. Changes in dreaming induced by CPAP in severe obstructive sleep apnea syndrome patients. J Sleep Res. (2006) 15:430–6. doi: 10.1111/j.1365-2869.2006.00553.x
16. de Groen JH, Op den Velde W, Hovens JE, Falger PR, Schouten EG, van Duijn H. Snoring and anxiety dreams. Sleep. (1993) 16:35–6.
17. Fisher S, Lewis KE, Bartle I, Ghosal R, Davies L, Blagrove M. Emotional content of dreams in obstructive sleep apnea hypopnea syndrome patients and sleepy snorers attending a sleep-disordered breathing clinic. J Clin Sleep Med. (2011) 7:69–74.
18. BaHammam AS, Kendzerska T, Gupta R, Ramasubramanian C, Neubauer DN, Narasimhan M, et al. Comorbid depression in obstructive sleep apnea: an under-recognized association. Sleep Breath. (2016) 20:447–56. doi: 10.1007/s11325-015-1223-x
19. Kendzerska T, Gershon AS, Hawker GA, Tomlinson GA, Leung RS. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: a historical cohort study. Euro Resp J. (2017) 49:1601361. doi: 10.1183/13993003.01361-2016
20. Rezaeitalab F, Moharrari F, Saberi S, Asadpour H, Rezaeetalab F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci. (2014) 19:205.
21. Lu MK, Tan HP, Tsai IN, Huang LC, Liao XM, Lin SH. Sleep apnea is associated with an increased risk of mood disorders: a population-based cohort study. Sleep Breath. (2017) 21:243–53. doi: 10.1007/s11325-016-1389-x
22. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. (2005) 28:1405–11. doi: 10.1093/sleep/28.11.1405
23. Agargun MY, Kara H, Ozer OA, Selvi Y, Kiran U, Ozer B. Clinical importance of nightmare disorder in patients with dissociative disorders. Psychiatry Clin Neurosci. (2003) 57:575–9. doi: 10.1046/j.1440-1819.2003.01169.x
24. Michels F, Schilling C, Rausch F, Eifler S, Zink M, Meyer-Lindenberg A, et al. Nightmare frequency in schizophrenic patients, healthy relatives of schizophrenic patients, patients at high risk states for psychosis, and healthy controls. Int J Dream Res. (2014) 7:9–13. doi: 10.11588/ijodr.2014.1.11819
25. Gross M, Lavie P. Dreams in sleep apnea patients. Dreaming. (1994) 4:195–204. doi: 10.1037/h0094412
26. Lovin S, Rusu C, Mutica M, Necula A, Georgescu C. Dream content alalysis at the initiation of CPAP for obstructive sleep apnea. Sleep Disord Ther. (2013) 2:127. doi: 10.4172/2167-0277.1000127
27. Schredl M, Schmitt J. Dream recall frequency, nightmare frequency, attitude towards dreams, and other dream variables in patients with sleep-related breathing disorders. Somnologie. (2019) 23:109–15. doi: 10.1007/s11818-019-0199-3
28. Pagel JF, Kwiatkowski C. The nightmares of sleep apnea: nightmare frequency declines with increasing apnea hypopnea index. J Clin Sleep Med. (2010) 6:69–73. Available online at: http://jcsm.aasm.org/ViewAbstract.aspx?pid=27713
29. Schredl M, Bozzer A, Morlock M. Dream recall and sleep disorders. Psychother Psychosom Med Psychol. (1997) 47:108–16.
30. Schredl M, Schmitt J. Dream recall frequency and nightmare frequency in patients with sleep disordered breathing. Somnologie. (2009) 13:12–7. doi: 10.1007/s11818-008-0359-3
31. Schredl M, Binder R, Feldmann S, Göder R, Hoppe J, Schmitt J, et al. Dreaming in patients with sleep disorders: a multicenter study. Somnologie. (2012) 16:32–42. doi: 10.1007/s11818-012-0552-2
32. Di Pauli F, Stefani A, Holzknecht E, Brandauer E, Mitterling T, Holzinger B, et al. Dream content in patients with sleep apnea: a prospective sleep laboratory study. J Clin Sleep Med. (2018) 14:41–6. doi: 10.5664/jcsm.6876
33. Hartmann E, Pavia H. Apnea and dreams: apnea patients dreamless, and have fewer apnea related dreams. Sleep. (2006) 29:A50.
34. Schredl M, Reinhard I. Gender differences in nightmare frequency: a meta-analysis. Sleep Med Rev. (2011) 15:115–21. doi: 10.1016/j.smrv.2010.06.002
35. Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. (2001) 5:423–45. doi: 10.1053/smrv.2001.0157
36. Eiser AS. Physiology and psychology of dreams. Semin Neurol. (2005) 25:97–105. doi: 10.1055/s-2005-867078
37. Schredl M, Atanasova D, Hormann K, Maurer JT, Hummel T, Stuck BA. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. J Sleep Res. (2009) 18:285–90. doi: 10.1111/j.1365-2869.2009.00737.x
38. Wollman MC, Antrobus JS. Sleeping and waking thought: effects of external stimulation. Sleep. (1986) 9:438–48. doi: 10.1093/sleep/9.3.438
39. Trotter K, Dallas K, Verdone P. Olfactory stimuli and their effects on REM dreams. Psychiatr J Univ Ott. (1988) 13:94–6.
40. BaHammam AS, Al-Shimemeri SA, Salama RI, Sharif MM. Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Med. (2013) 14:149–54. doi: 10.1016/j.sleep.2012.07.007
41. Hicks RA, Bautista J. Snoring and nightmares. Percept Mot Skills. (1993) 77:433–4. doi: 10.2466/pms.1993.77.2.433
42. Schredl M. Snoring, breathing pauses, and nightmares. Percept Mot Skills. (2008) 106:690–2. doi: 10.2466/pms.106.3.690-692
43. Macfarlane JG, Wilson TL. A relationship between nightmare content and somatic stimuli in a sleep-disordered population: a preliminary study. Dreaming. (2006) 16:53–9. doi: 10.1037/1053-0797.16.1.53
44. Schredl M, Kraft-Schneider B, Kröger H, Heuser I. Dream content of patients with sleep apnea. Somnologie. (1999) 3:319–23. doi: 10.1007/s11818-999-0042-3
45. Pagel JF. Drugs, dreams, and nightmares. Sleep Med Clin. (2010) 5:277–87. doi: 10.1016/j.jsmc.2010.01.007
47. BaHammam AS, Almeneessier AS, Pandi-Perumal SR. Medieval Islamic scholarship and writings on sleep and dreams. Ann Thorac Med. (2018) 13:72–5. doi: 10.4103/atm.ATM_162_17
48. AASM. American Academy of Sleep Medicine (AASM). International Classification of Sleep Disorders (ICSD). 3rd ed. Darien, IL: AASM (2014).
49. Lundetrae RS, Saxvig IW, Pallesen S, Aurlien H, Lehmann S, Bjorvatn B. Prevalence of parasomnias in patients with obstructive sleep apnea. a registry-based cross-sectional study. Front Psychol. (2018) 9:1140. doi: 10.3389/fpsyg.2018.01140
50. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. (2010) 11:1031–4. doi: 10.1016/j.sleep.2010.07.011
51. Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. (2007) 132:325–37. doi: 10.1378/chest.07-0040
52. Hicks RA, Fortin E, Brassington GS. Arousability and dreaming. Dreaming. (2002) 12:135–9. doi: 10.1023/A:1020114224680
53. Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA. (2001) 98:2035–40. doi: 10.1073/pnas.98.4.2035
54. Santos MA, Nakano TC, Mendes FA, Duarte BB, Marone SA. Emotional stress evaluation of patients with moderate and severe sleep apnea syndrome. Int Arch Otorhinolaryngol. (2017) 21:28–32. doi: 10.1055/s-0036-1586251
55. Trakada G, Chrousos G, Pejovic S, Vgontzas A. Sleep apnea and its association with the stress system, inflammation, insulin resistance and visceral obesity. Sleep Med Clin. (2007) 2:251–61. doi: 10.1016/j.jsmc.2007.04.003
56. Lee MC, Shen YC, Wang JH, Li YY, Li TH, Chang ET, et al. Effects of continuous positive airway pressure on anxiety, depression, and major cardiac and cerebro-vascular events in obstructive sleep apnea patients with and without coronary artery disease. Ci Ji Yi Xue Za Zhi. (2017) 29:218–22. doi: 10.4103/tcmj.tcmj_128_17
57. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (2013).
58. Krakow B, Germain A, Tandberg D, Koss M, Schrader R, Hollifield M, et al. Sleep breathing and sleep movement disorders masquerading as insomnia in sexual-assault survivors. Compr Psychiatry. (2000) 41:49–56. doi: 10.1016/S0010-440X(00)90131-7
59. Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. (2001) 286:537–45. doi: 10.1001/jama.286.5.537
60. Jaoude P, Vermont LN, Porhomayon J, El-Solh AA. Sleep-disordered breathing in patients with post-traumatic stress disorder. Ann Am Thorac Soc. (2015) 12:259–68. doi: 10.1513/AnnalsATS.201407-299FR
61. Zhang Y, Weed JG, Ren R, Tang X, Zhang W. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: a meta-analysis. Sleep Med. (2017) 36:125–32. doi: 10.1016/j.sleep.2017.04.020
62. Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. (2015) 24:37–45. doi: 10.1016/j.smrv.2014.11.001
63. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. (1994) 150:481–5. doi: 10.1164/ajrccm.150.2.8049833
64. Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. (2010) 33:69–74. doi: 10.1093/sleep/33.1.69
65. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. (2002) 159:1696–701. doi: 10.1176/appi.ajp.159.10.1696
66. Ullah MI, Campbell DG, Bhagat R, Lyons JA, Tamanna S. Improving PTSD Symptoms and preventing progression of subclinical PTSD to an overt disorder by treating comorbid OSA with CPAP. J Clin Sleep Med. (2017) 13:1191–8. doi: 10.5664/jcsm.6770
67. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. (2018) 10:409–20. doi: 10.2147/NSS.S166089
68. Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. (2008) 12:185–95. doi: 10.1016/j.smrv.2007.09.003
69. Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. (2011) 183:1419–26. doi: 10.1164/rccm.201005-0693OC
70. Krakow B, Lowry C, Germain A, Gaddy L, Hollifield M, Koss M, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. (2000) 49:291–8. doi: 10.1016/S0022-3999(00)00147-1
71. Youakim JM, Doghramji K, Schutte SL. Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics. (1998) 39:168–71. doi: 10.1016/S0033-3182(98)71365-9
72. El-Solh AA, Vermont L, Homish GG, Kufel T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. (2017) 33:145–50. doi: 10.1016/j.sleep.2016.12.025
73. Delanote I, Borzee P, Belge C, Buyse B, Testelmans D. Adherence to CPAP therapy: comparing the effect of three educational approaches in patients with obstructive sleep apnoea. Clin Respir J. (2018) 12:91–6. doi: 10.1111/crj.12491
74. Bartlett D, Wong K, Richards D, Moy E, Espie CA, Cistulli PA, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. (2013) 36:1647–54. doi: 10.5665/sleep.3118
75. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. (2013) 36:1655–62. doi: 10.5665/sleep.3120
Keywords: sleep-disordered breathing, CPAP, REM sleep, post-traumatic stress disorder, hypopnea, emotion
Citation: BaHammam AS and Almeneessier AS (2019) Dreams and Nightmares in Patients With Obstructive Sleep Apnea: A Review. Front. Neurol. 10:1127. doi: 10.3389/fneur.2019.01127
Received: 24 July 2019; Accepted: 09 October 2019;
Published: 22 October 2019.
Edited by:
Elemer Szabadi, University of Nottingham, United KingdomReviewed by:
Michael Schredl, Central Institute for Mental Health, GermanyMark Blagrove, Swansea University, United Kingdom
Copyright © 2019 BaHammam and Almeneessier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed S. BaHammam, YXNoYW1tYW0yQGdtYWlsLmNvbQ==; YXNoYW1tYW1Aa3N1LmVkdS5zYQ==
†ORCID: Ahmed S. BaHammam orcid.org/0000-0002-1706-6167
Aljohara S. Almeneessier orcid.org/0000-0002-6298-1751
 Ahmed S. BaHammam
Ahmed S. BaHammam Aljohara S. Almeneessier1,4†
Aljohara S. Almeneessier1,4†