
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 15 October 2019
Sec. Stroke
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.01038
 Yu-Wei Chen1,2
Yu-Wei Chen1,2 Sheng-Feng Sung3
Sheng-Feng Sung3 Chih-Hung Chen4
Chih-Hung Chen4 Sung-Chun Tang2
Sung-Chun Tang2 Li-Kai Tsai2
Li-Kai Tsai2 Huey-Juan Lin5
Huey-Juan Lin5 Hung-Yu Huang6
Hung-Yu Huang6 Helen L. Po7
Helen L. Po7 Yu Sun8
Yu Sun8 Po-Lin Chen9
Po-Lin Chen9 Lung Chan10,11,12
Lung Chan10,11,12 Cheng-Yu Wei13
Cheng-Yu Wei13 Jiunn-Tay Lee14
Jiunn-Tay Lee14 Cheng-Yang Hsieh15
Cheng-Yang Hsieh15 Yung-Yang Lin16
Yung-Yang Lin16 Shoou-Jeng Yeh17
Shoou-Jeng Yeh17 Li-Ming Lien12,18*
Li-Ming Lien12,18* Jiann-Shing Jeng2
Jiann-Shing Jeng2Background and Objectives: Intravenous recombinant tissue plasminogen activator (rt-PA) has been approved for acute ischemic stroke (AIS) within 3 h after onset and the treatment was then extended to 4.5 h. However, the Food and Drug Administration did not approve the indication in the expanded time window. This retrospective, matched cohort study aims to investigate the effectiveness and safety of rt-PA in AIS at 3–4.5 h after onset.
Materials and Methods: The treatment group included AIS patients receiving rt-PA at 3–4.5 h after onset, otherwise complying with the regulation, in the stroke registries in 16 hospitals between 2008 and 2017. The control group included age- and sex-matched patients not receiving intravenous thrombolysis from the same registries, excluding those with contraindications. The primary outcome was modified Rankin Scale (mRS) 0–1 at day 90. The safety outcomes were any intracerebral hemorrhage (ICH), early neurological deterioration and 3-month mortality.
Results: Each group had 374 patients. There were 34.0% of patients with 3-month mRS 0-1 in the treatment group vs. 22.7% in the control group with an odds ratio of 1.75 (95% confidence intervals, 1.27 to 2.42, P = 0.001). There was no difference in symptomatic ICH, early neurological deterioration and 3-month mortality rates between two groups. The 3-month mRS and symptomatic ICH did not differ significantly in patients receiving standard dose or low dose of rt-PA.
Conclusions: Our results support the prescription of rt-PA in AIS patients 3–4.5 h after onset as an effective and tolerable treatment in their functional recovery.
Stroke is the second most common cause of death, accounting for 11% of total deaths (~ 6.3 million deaths) in 2015 (1). More than 80% of strokes are ischemic strokes (2), and the emergency management of patients with acute ischemic stroke (AIS) has been emphasized to improve the prognosis (3). After the efficacy of intravenous recombinant tissue plasminogen activator (rt-PA) was proved in 1995 (4), it was first approved for AIS treatment within 3 h of stroke onset in the United States of America (USA) in 1996 and has been then approved in the European Union and other countries subsequently, including Taiwan. In 2008, the European Cooperative Acute Stroke Study (ECASS) III demonstrated an increased likelihood of having a favorable outcome in patients treated with rt-PA within 3–4.5 h of the stroke onset compared to the placebo group, along with an increased risk of symptomatic intracranial hemorrhage (5). The third International Stroke Trial (IST-3) found that rt-PA treatment within 6 h of AIS could improve the functional outcome of patients assessed and did not increase the incidence of death at 6- and 18-month follow-ups (6, 7). Studies in China and Japan comparing the outcome and safety between patients using rt-PA within 3 and 3–4.5 h of stroke onset showed no difference in functional outcome, rates of symptomatic intracerebral hemorrhage (SICH) and mortality rates in two time window groups (8, 9).
To date, guidelines for the early management of AIS patients from professional organizations in the USA, Europe, Japan, China, and Taiwan strongly recommend the extension of the intervention time span to 4.5 h after the onset of AIS (3, 10–13). Although evidence from randomized control trials and related meta-analysis supports the efficacy of rt-PA within 3–4.5 h of AIS onset, little information is available from the studies in Asian populations in the literature. The Food and Drug Administration (FDA) in the USA and in Taiwan have not approved the indication of rt-PA at 3–4.5 h after AIS onset. Therefore, we planned a multicenter, retrospective, matched cohort study in Taiwan to collect clinical real-world data aiming to evaluate the effectiveness and safety for AIS patients receiving rt-PA between 3 and 4.5 h after onset.
The study was a multicenter, retrospective, matched cohort study in Taiwan to investigate the effectiveness and safety of patients who had experienced AIS with or without the administration of rt-PA therapy at 3–4.5 h after the stroke onset. Patients with other additional reperfusion or interventional therapy were excluded, and data were extracted from databases of 16 participating hospitals in Taiwan Stroke Registry (TSR). The TSR is a multicenter, pre-specified prospective registry, recruiting patients from more than 50 centers in Taiwan (14). This current study planned to enroll at least 500 eligible patients in the treatment and control groups at a ratio of 1:1 according to whether they received rt-PA therapy or not in the emergency room (ER). In general, information on the basic characteristics of eligible patients (e.g., demographics and medical history), the dates of admission and discharge, the results of laboratory examinations, clinical medications and evaluation for the stroke, and the follow-up outcome were prospectively collected. The Full Analysis Set (FAS) population was utilized for all the analyses in each group to evaluate functional outcomes assessed by a modified Rankin Scale (mRS), the neurological status, mortality, and the incidence of SICH. The TSR was approved by Institutional Review Board of each participating hospital, and written informed consent for participation in the registry program was obtained from each patient or his/her legal representatives with the approval of attending physicians. A numeric code was assigned to each patient whose clinical data were in the registry and the database contained no individual identification numbers or any privacy data. The whole data set as a large database has been approved for big data analytics for research topics, such as the one in this study.
The inclusion criteria were based on the local license and reimbursement regulation in Taiwan, except the rt-PA treatment windows of 3–4.5 h for otherwise eligible patients. The treatment and control groups included patients with an age ≥18 years and were diagnosed with an AIS. The diagnosis was based on clinical evaluation and was documented by appropriate diagnostic procedures of brain computed tomography and/or magnetic resonance imaging, confirmed by in-charge neurologists, and radiologists. AIS were further classified into 5 subtypes by the Trial of Org 10172 in the Acute Stroke Treatment (TOAST) classification (15), including large artery atherosclerosis, small artery lacunes, cardioembolism, other specific etiology and undetermined etiology. Stroke of undetermined etiology includes those with negative or incomplete study, and two or more identified causes (15). AIS patients with pre-morbid functional status of mRS >1 and thrombolyzed patients with final diagnosis other than AIS (stroke mimics) were excluded. The presenting scores of NIH Stroke Scale (NIHSS) of both groups were between 4 and 25, and none of the patients received any other reperfusion therapy (i.e., intra-arterial thrombolysis or endovascular thrombectomy). Patients in both groups did not participate in any clinical trials regarding the treatment of AIS.
In the treatment group, patients received rt-PA within 3–4.5 h after stroke onset, otherwise in accordance with the regulations of Taiwan FDA and of the reimbursement of the National Health Insurance in Taiwan (16). In the control group, age- and sex-matched patients arriving at the ER within 2–4.5 h after stroke onset, but not receiving rt-PA, at the same hospital if possible, were included. The lower limit of 2 h onset-to-door time was defined because they were probably not able to receive rt-PA treatment within 3 h. Patients in the control group were without any obvious contraindication to rt-PA treatment, including an abnormal hemogram profile of an international normalized ratio ≥1.7 and platelet counts <105/μL, experiencing a previous hemorrhagic stroke, or were not receiving rt-PA treatment based on the investigator's discretion.
The standard dose of 0.9 mg/Kg of rt-PA in AIS has been approved by FDA in Taiwan and it was regularly used in the daily practice. In 2016, the contraindication of rt-PA for AIS patients older than 80 years was removed in the label, with adding the warning that rt-PA should be cautiously used in patients older than 80 years. The label also provided that low dose of intravenous rt-PA was associated with lower risk of SICH in Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke (ENCHANTED) study (17) and Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study (18). The TTT-AIS study also demonstrated that patients older than 70 years treated by standard dose were less likely to have 3-month mRS 0–2 than those treated by lower dose. We had a subgroup analysis for the baseline characteristics and outcomes of patients receiving standard dose (0.9 mg/Kg) and low dose (0.6 mg/Kg) in the treatment group.
The primary outcome was the percentage of patients with modified Rankin Scale (mRS) scores of 0–1 at 90 days after AIS. The secondary outcomes were: (1) the percentage of patients with mRS scores of 0–2 at 90 days after AIS; (2) the percentage of patients with early neurological deterioration, defined as deterioration with NIHSS scores ≧2 within 72 h after AIS (19, 20), and (3) the mortality rates within 90 days after the onset of AIS. The safety assessments included the percentages of patients with any intracerebral hemorrhage within 36 h after the administration of rt-PA, and of patients with SICH determined by three well-accepted definitions. Safe Implementation of Thrombolysis in Stroke-Monitoring study (SITS-MOST) was defined as a local or remote parenchymal hematoma type 2 on the imaging scan obtained 22 to 36 h after treatment, plus neurologic deterioration, as indicated by a score on the NIHSS that was higher by 4 or more points than the baseline value or the lowest value between baseline and 24 h, or hemorrhage leading to death (21). European Cooperative Acute Stroke Study III (ECASS III) was defined as any hemorrhage with neurologic deterioration, as indicated by an NIHSS score that was higher by 4 points or more than the value at baseline or the lowest value in the first 7 days, or any hemorrhage leading to death. In addition, the hemorrhage must have been identified as the predominant cause of the neurologic deterioration (5). National Institute of Neurological Disorders and Stroke (NINDS) was defined as an intracranial hematoma not been seen on a previous CT scan but was subsequently either a suspicious of hemorrhage or any decline in neurologic status (4). To detect intracranial hemorrhage, brain CT or MRI scans were required at 24 h or 7 to 10 days after the onset of stroke when clinical findings suggested hemorrhage.
To ensure data accuracy, completeness, and reliability, the following procedures were enforced. Before collecting data for the study, the principal investigator (J. S. Jeng) conducted a Site Initiation Visit meeting to instruct the investigators and study coordinators on the protocol, the guideline for the completion of the Case Report Form (CRF), and for procedures of the study. The clinical data of patients in the participating hospitals were recorded based on the instructional materials, were audited regularly and were reported to the Data Monitoring Board. A visual review and standard computer editing monitored the completeness and accuracy of data on the CRFs.
To protect the safety of the subjects and to ensure data accuracy, completeness, and reliability, the research staff preserved documented data from all sources on CRFs, including lab test results, chart records, treatment conditions, physical examination, concomitant medications, and any adverse events.
A sample size of at least 250 patients in each of the treatment and control groups was required to provide an α error and power of 0.05 and 80%, respectively.
All data extracted from the eligible patients were evaluated. No withdrawal criteria were applied for the retrospective study. Eligible patients not receiving rt-PA therapy were categorized into the control group and patients treated with rt-PA into the treatment group at a ratio of 1:1. The demographic and clinical characteristics between groups were compared by Student's t-test or chi-square test. Since this study was a retrospective study, the study did not interfere with the patients' medical procedure and complied with routine practice in Taiwan.
As a retrospective study, the FAS population consisting of all eligible patients used for the assessment of effectiveness and safety. We assessed mRS scores and mortality rates of patients by the chi-square test, or other non-parametric methods (Mann-Whitney U-test). The variables significantly associated to primary outcome with mRS 0–1 at 90 days in the univariate analysis were further analyzed by a logistic regression model. Safety outcomes of the percentages of patients with intracerebral hemorrhage (ICH) were assessed by a chi-square test or other non-parametric methods (Mann-Whitney U-test). The mRS shift analysis by Mann-Whitney U-test was performed between the treatment and control groups and between the standard dose and low dose sub-groups in the treatment group.
From January 2008 to December 2017, a total of 748 eligible patients, of whom 502 (67.1%) were males, treated with/without rt-PA (in the treatment and control groups, respectively) within 3–4.5 h after the onset of an AIS were enrolled in our study from 16 participating hospitals at the ratio of 1:1, with 374 patients in each group. All institutes in the study were either medical centers or qualified stroke centers and contributed patients in both treatment and control groups. The characteristics of patients with AIS in the treatment and control groups at baseline are presented in Table 1. The mean age of patients in the treatment and control groups was 66.1 ± 13.2 and 67.8 ± 12.5 years (P = 0.290), respectively. The mean NIHSS score was insignificantly higher in the treatment group than in the control group (12.2 ± 6.0 vs. 10.7 ± 6.4, P = 0.197). Regarding other variables, including laboratory data and medical histories, there was no statistically significant difference between the two groups (all P > 0.05).
No statistically significant difference was found between two groups regarding stroke subtypes or type of large artery atherosclerosis in the TOAST classification, except that the percentage of undetermined causes of stroke was higher in the treatment group (27.5 vs. 20.1%) (Supplementary Table 1).
A summary of medications taken prior to the onset of strokes was illustrated in Supplementary Table 2. In general, records of prior medications were not significantly different between the treatment and control groups, except higher percentage of prior aspirin use in the control group.
A shorter average stroke onset-to-door time was observed in the treatment group (128.6 ± 42.8 min vs. 196.0 ± 58.8 min in the control group, P < 0.001). But the onset-to-needle time in the treatment group (206.9 ± 25.9 min) was longer than the onset-to-door time in the control group (196.0 ± 58.8 min, P = 0.001). The averages of dose in mg per patient and of dose per Kg of administered rt-PA in AIS patients were 48.4 ± 13.4 mg and 0.74 ± 0.14 mg/Kg, respectively. Among these patients, 48.7% (n = 182) received the standard dose (0.9 mg/Kg) and 51.3% (n = 192) received a low dose (0.6 mg/Kg).
The percentage of patients with a mRS score of 0–1 at 90 days after the stroke onset was 34.0% in the treatment group, as compared with 22.7% in the control group, with an odds ratio (OR) of 1.75 (95% Confidence Intervals (CI) 1.27 to 2.42, P = 0.001). In terms of the distribution of patients with mRS scores at 90 days after the stroke onset, the shift analysis by Mann-Whitney U-test showed P = 0.04 between the treatment and control groups (Figure 1).
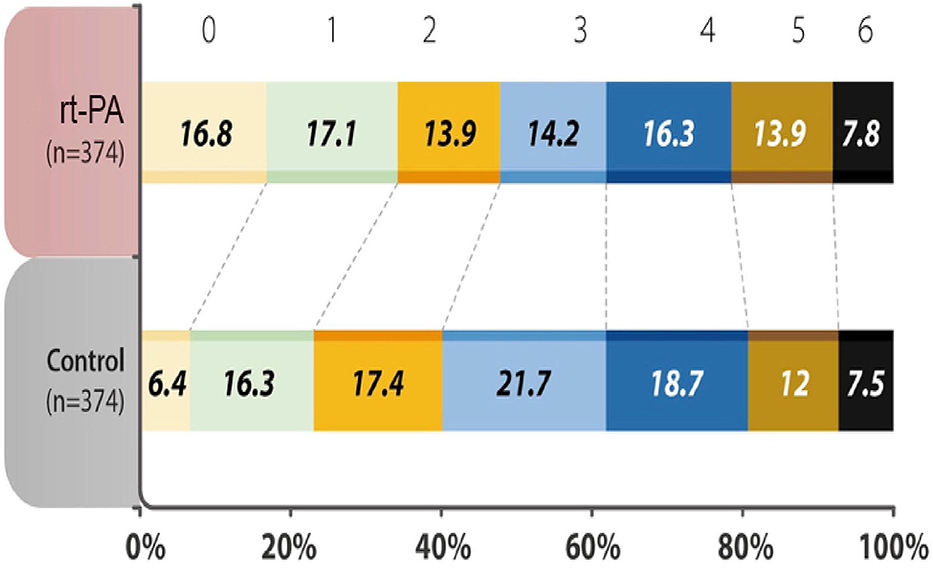
Figure 1. Distribution of acute ischemic stroke patients in each group was evaluated by modified Rankin Scale (mRS) scores at 90 days after the onset of the index stroke. The analysis of primary outcome showed a significantly higher percentages (mRS 0-1) in the recombinant tissue plasminogen activator (rt-PA)-treated group (P < 0.001). mRS shift analysis by Mann-Whitney U-test showed P = 0.04 for 3-month mRS between the treatment and control groups.
The proportion of rt-PA-treated patients with a mRS score of 0–2 was 47.9%, which was also significantly higher than patients without rt-PA treatment (40.1%, OR, 1.37, 95% CI, 1.03 to 1.83, P = 0.033) (Table 2). Fifteen percent of patients in the treatment group suffered from early neurological deterioration, insignificantly lower than 19.5% in the control group (OR, 0.73, 95% CI 0.61 to 1.06, P = 0.101). The incidences of death within 90 days after the onset of AIS were 7.8 and 7.5% in the treatment and control groups, respectively (Table 2).
Follow-up neuroimaging study by MRI was performed in 195 (52.1%), by CT 170 (45.5%), and by none 9 (2.4%) in the treatment group, as compared with by MRI 237 (63.4%), by CT 105 (28.1%), and by none 32 (8.6%) in the control group. Concerning safety assessment, a significantly more percentages (17.4%) in the treatment group experienced ICH (OR, 2.25, 95% CI 1.43 to 3.53, P < 0.001) than 8.6% in the control group. However, the percentage of patients with SICH was not significantly different between the two groups (treatment vs. control: 3.5 vs. 2.4%, 3.5 vs. 2.4% and 5.6 vs. 2.9%, based on three definitions of SICH by SITS-MOST, ECASS III and NINDS) (Table 3).
The study used a logistic regression model to identify factors affecting the good neurological function of a mRS score of 0–1 at 90 days, and results are shown in Table 4. Being younger, having a lower NIHSS score, receiving rt-PA (adjusted OR 2.38, 95% CI 1.655 to 3.427, P < 0.001), and not with any ICH were independent predictors of having better neurological function at 90 days after the onset of an ischemic stroke (P < 0.05).
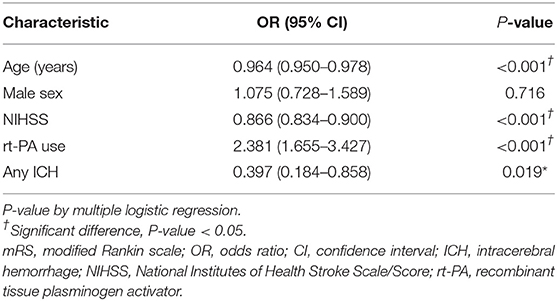
Table 4. Multiple logistic regression analysis for the good neurological function (mRS 0-1) at 90 days.
The baseline characteristics of the standard dose and low dose subgroups were summarized in Supplementary Table 3. Except the patients receiving low dose were older, there were no significant differences between the two groups. The analysis of mRS 0–1 at 90 days, early neurological deterioration, any ICH, SICH and mortality within 90 days showed no difference between subgroups receiving different doses of rt-PA at 3–4.5 h after the stroke onset (Table 5). No variable was considered affecting effectiveness or safety outcomes in patients treated with rt-PA, regardless of whether they received the standard dose or a low dose (all P > 0.05) (Figure 2). The mRS shift analysis by Mann-Whitney U-test showed P = 0.12 between standard dose and low dose groups.
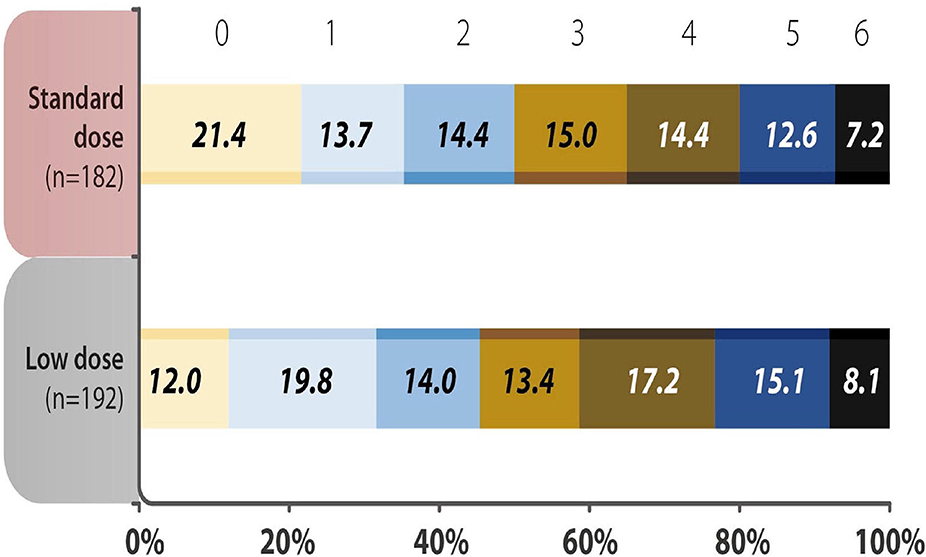
Figure 2. Distribution of acute ischemic stroke patients using different doses of recombinant tissue plasminogen activator (rt-PA) were evaluated by modified Rankin Scale (mRS) scores 90 days after the onset of stroke. The analysis showed no difference in the 3-month mRS scores in the standard vs. low dose rt-PA-treated groups (P = 0.346). mRS shift analysis by Mann-Whitney U-test showed P = 0.12 for 3-month mRS between the standard-dose and low-dose groups.
The study showed effectiveness and safety of rt-PA administered at 3–4.5 h after the onset of AIS when compared with age and sex-matched patients in this multicenter retrospective cohort study. Although evidence from the randomized control trials and meta-analysis support the efficacy of rt-PA in the 3–4.5 h window after AIS, little information is obtained from the investigation in Asian populations in the literature. Our study might be the first case-matched cohort study for the indication in Asian populations. Studies conducted in China and Japan showed comparable outcomes assessed by a mRS score of 0–1 and by mortality rates between the groups of patients using rt-PA within 3 and at 3–4.5 h after the stroke onset. A subgroup analysis of the study comparing the reimbursement (treated <3 h after the onset of AIS) and non-reimbursement (treated 3–4.5 h after the onset) groups for insurance coverage of intravenous rt-PA in Taiwan showed comparable results in functional outcome, in-hospital mortality and SICH (16).
The percentage of rt-PA-treated patients with a 90-day mRS score of 0–1 was 34.0%, significantly higher than 22.7% in the control group. Similar effects were observed in the secondary outcome analysis of a 90-day mRS score of 0–2. Our results are comparable to the results in the meta-analysis including 9 randomized control trials showing 35.3% with a 3-month mRS score of 0–1 in the treatment group in the 3–4.5 h window (22) and to 35.3% (standard dose) and 32.4% (low dose) in a study in Korea (23). However, the value was lower than 52.4% treated in 3–4.5 h window in the ECASS III study (5), which may be explained by that we did not exclude age older than 80 years as in the ECASS III study, reflecting by the older mean age in our study. Besides, there were possible differences between randomized control studies and real-world practice, and among race-ethnicities.
Regarding safety outcomes, the administration of rt-PA had no difference on the risk of SICH by different definitions, early neurological deterioration and the incidence of death at 90 days after the stroke onset, but it was significantly associated with any ICH (17.4%) compared with the control group (8.6%). This observation was consistent with previous studies, in which the incidence rates of ICH were 2.5–27.0% for AIS patients treated with rt-PA beyond 3 h after the onset, higher than patients receiving placebo (all ≤1.0%) (5, 6, 22, 24). Comparable results were also observed in Asian countries with a range in rates of 1.9–12.1%, compared with the placebo group (<1.5%) (8, 25, 26). Extension of infarct volume was the most frequent cause of early neurological deterioration after AIS (27), which could be decreased by thrombolysis (28). A hypothetical analysis showed that the approval of rt-PA treatment within 4.5-h may increase the chance of having a mRS score of 0–1 at 3–6 months (OR 1.37, 95% CI 1.17–1.59) without increasing mortality and SICH rates (29). Although fatal intracranial hemorrhage in 7 days increased in patients treated at 3–4.5 h after the stroke onset in the meta-analysis, adverse effect of the complication was offset by increasing disability-free survival (22).
Intravenous rt-PA may benefit more AIS patients in the extended 3–4.5 h window since only 27% AIS patients were admitted to ER in 3 h (30). The treatment guidelines in the USA, Europe, Japan, and China now all advise to extend the treatment window time to 3–4.5 h after the stroke onset (3, 10–12, 31). Taiwan Stroke Society issued a guideline in 2013 and its update in 2019 to advocate administering rt-PA for AIS patients within 3–4.5 h of the stroke onset (32). However, it still has not been approved by the FDA in the USA and in Taiwan.
Our study supported that patients receiving low dose of rt-PA had similar effectiveness and safety profile as compared with those receiving standard dose in the treatment group in the 3–4.5 h window. The subgroup analysis showed the patients receiving low dose were older than those having standard dose of rt-PA. The practice was supported by the TTT-AIS study in Taiwan demonstrating the lower-than-standard dose administration was associated with less SICH by ECASS III definition and mortality, and with more independence rates in the population older than 70 years (18). Although the randomized control study conducted in Asia didn't support the non-inferiority hypothesis of low dose rt-PA (0.6 mg/Kg) within 4.5 h of AIS (17), the real-world prospective registry in Korea supported the strategy of low dose intravenous thrombolysis (23).
The logistic regression model in our study showed rt-PA treatment is one of the independent factors predicting favorable neurological outcome in patients at 3–4.5 h after AIS onset, as well as younger age, lower NIHSS scores, and absence of ICH. More stroke severity is associated with more risk of SICH (22) and is a strong predictor of functional outcome (5). SICH following thrombolysis was related to early neurological deterioration and poor outcomes (20, 33, 34).
There are limitations of the current study. First, missing data exists since some information were retrospectively collected from the hospital databases. Second, the data were only collected in a short follow-up period after the onset of AIS. Third, it was a non-randomized retrospective study, and we did not apply any population matching methods between the study groups and the characteristics of enrolled patients might be imbalanced. That could have had an influence on the study results. Finally, the regimen of rt-PA was decided at the discretion of different investigators, which might affect the assessment of endpoints. However, the present study demonstrated the effectiveness and safety of rt-PA for patients at 3–4.5 h after the onset of AIS in a real-world setting.
In conclusion, our results support the use of rt-PA for ischemic stroke patients within 3–4.5 h after the stroke onset and under clinical observation as an effective and tolerable measure for the functional recovery after stroke.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Institute Review Board of National Taiwan University Hospital. The patients/participants provided their written informed consent to participate in this study.
Y-WC, S-FS, C-HC, S-JY, J-SJ, and L-ML contributed conception and design of the study. S-JY, J-SJ, and L-ML organized the database. J-SJ and L-ML performed the statistical analysis. Y-WC wrote the first draft of the manuscript. All authors contributed to acquisition, analysis or interpretation of patients' data, critical manuscript revision, read, and approved the submitted version. The corresponding author L-ML takes primary responsibility for communication with the journal and editorial office during the submission process, throughout peer review and during publication. The corresponding author is also responsible for ensuring that the submission adheres to all journal requirements including, but not exclusive to, details of authorship, study ethics and ethics approval, clinical trial registration documents, and conflict of interest declaration. The corresponding author should also be available post-publication to respond to any queries or critiques.
This study was supported by the Taiwan Stroke Society, Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.01038/full#supplementary-material
1. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1459–544. doi: 10.1016/S0140-6736(16)31012-1
2. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. (2008) 371:1612–23. doi: 10.1016/S0140-6736(08)60694-7
3. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–110. doi: 10.1161/STR.0000000000000172
4. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
5. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
6. Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third International Stroke Trial (IST-3)): a randomised controlled trial. Lancet. (2012) 379:2352–63. doi: 10.1016/S0140-6736(12)60768-5
7. IST-3 Collaborative Group. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third International Stroke Trial (IST-3)): 18-month follow-up of a randomised controlled trial. Lancet Neurol. (2013) 12:768–76. doi: 10.1016/S1474-4422(13)70130-3
8. Liao XL, Wang CX, Wang YL, Wang CJ, Zhao XQ, Zhang LQ, et al. Implementation and outcome of thrombolysis with alteplase 3 to 4.5 h after acute stroke in Chinese patients. CNS Neurosci Ther. (2013) 19:43–7. doi: 10.1111/cns.12031
9. Morihara R, Kono S, Sato K, Hishikawa N, Ohta Y, Yamashita T, et al. Thrombolysis with low-dose tissue plasminogen activator 3-4.5 h after acute ischemic stroke in five hospital groups in Japan. Transl Stroke Res. (2016) 7:111–9. doi: 10.1007/s12975-016-0448-8
10. Karolinska stroke update. Consensus Statements 2012: Update on Intravenous Thrombolysis. Available online at: www.strokeupdate.org/Cons_ Reperf_IVT_2012.aspx (accessed September 9, 2019).
11. Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, et al. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis. (2013) 22:571–600. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001
12. Dong Q, Dong Y, Liu L, Xu A, Zhang Y, Zheng H, et al. The Chinese Stroke Association scientific statement: intravenous thrombolysis in acute ischaemic stroke. Stroke Vasc Neurol. (2017) 2:147–59. doi: 10.1136/svn-2017-000074
13. Hu HH. Taiwan Guidelines for the Management of Stroke 2008. Taiwan Stroke Society (2008). Available online at: http://www.stroke.org.tw/GoWeb2/include/pdf/08%20%E5%8F%B0%E7%81%A3%E8%85%A6%E4%B8%AD%E9%A2%A8%E9%98%B2%E6%B2%BB%E6%8C%87%E5%BC%952008.pdf (accessed September 9, 2019).
14. Hsieh FI, Lien LM, Chen ST, Bai CH, Sun MC, Tseng HP, et al. Get With the Guidelines-Stroke performance indicators: surveillance of stroke care in the Taiwan Stroke Registry: Get With the Guidelines-Stroke in Taiwan. Circulation. (2010) 122:1116–23. doi: 10.1161/CIRCULATIONAHA.110.936526
15. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
16. Su YH, Chen CH, Lin HJ, Chen YW, Tseng MC, Hsieh HC, et al. Safety and effectiveness of intravenous thrombolysis for acute ischemic stroke outside the coverage of National Health Insurance in Taiwan. Acta Neurol Taiwan. (2017) 26:3–12
17. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. (2016) 374:2313–23. doi: 10.1056/NEJMoa1515510
18. Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MH, et al. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study. Stroke. (2010) 41:885–90. doi: 10.1161/STROKEAHA.109.575605
19. Ferrari J, Knoflach M, Kiechl S, Willeit J, Schnabl S, Seyfang L, et al. Early clinical worsening in patients with TIA or minor stroke: the Austrian Stroke Unit Registry. Neurology. (2010) 74:136–41. doi: 10.1212/WNL.0b013e3181c9188b
20. Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. (2006) 99:625–33. doi: 10.1093/qjmed/hcl082
21. Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
22. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Stroke Thrombolysis Trialists' Collaborative, Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
23. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Low-versus standard-dose alteplase for ischemic strokes within 4.5 hours: a comparative effectiveness and safety study. Stroke. (2015) 46:2541–8. doi: 10.1161/STROKEAHA.115.010180
24. Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. (2008) 7:299–309. doi: 10.1016/S1474-4422(08)70044-9
25. Navarro JC, Jose MCS, Collantes E, Macrohon-Valdez MC, Roxas A, Hiyadan J, et al. Stroke thrombolysis in the Philippines. Neurology Asia. (2018) 23:115–20.
26. Rha JH, Shrivastava VP, Wang Y, Lee KE, Ahmed N, Bluhmki E, et al. Thrombolysis for acute ischaemic stroke with alteplase in an Asian population: results of the multicenter, multinational Safe Implementation of Thrombolysis in Stroke-Non-European Union World (SITS-NEW). Int J Stroke. (2014) 9 (Suppl. A100):93–101. doi: 10.1111/j.1747-4949.2012.00895.x
27. Simonsen CZ, Schmitz ML, Madsen MH, Mikkelsen IK, Chandra RV, Leslie-Mazwi T, et al. Early neurological deterioration after thrombolysis: clinical and imaging predictors. Int J Stroke. (2016) 11:776–82. doi: 10.1177/1747493016650454
28. Hansen CK, Christensen A, Havsteen I, Ovesen C, Christensen H. Prevalence of early neurological deterioration after IV - thrombolysis in acute ischaemic stroke patients - A hospital-based cohort study. Clin Neurol Neurosurg. (2018) 171:58–62. doi: 10.1016/j.clineuro.2018.05.003
29. Hacke W, Lyden P, Emberson J, Baigent C, Blackwell L, Albers G, et al. Effects of alteplase for acute stroke according to criteria defining the European Union and United States marketing authorizations: Individual-patient-data meta-analysis of randomized trials. Int J Stroke. (2018) 13:175–89. doi: 10.1177/1747493017744464
30. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. (2001) 56:1015–20. doi: 10.1212/WNL.56.8.1015
31. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:870–947. doi: 10.1161/STR.0b013e318284056a
32. Chen CH, Hsieh HC, Sung SF, Hsieh CY, Chen PL, Tsai LK, et al. 2019 Taiwan Stroke Society guideline for intravenous thrombolysis in acute ischemic stroke patients. Formos J Stroke. (2019) 1:1–22. doi: 10.6318/FJS.201906_1(1).0001
33. Demchuk AM, Tanne D, Hill MD, Kasner SE, Hanson S, Grond M, et al. Predictors of good outcome after intravenous tPA for acute ischemic stroke. Neurology. (2001) 57:474–80. doi: 10.1212/WNL.57.3.474
Keywords: acute ischemic stroke, tissue plasminogen activator, intravenous thrombolysis, 3–4.5 h after stroke onset, functional recovery
Citation: Chen Y-W, Sung S-F, Chen C-H, Tang S-C, Tsai L-K, Lin H-J, Huang H-Y, Po HL, Sun Y, Chen P-L, Chan L, Wei C-Y, Lee J-T, Hsieh C-Y, Lin Y-Y, Yeh S-J, Lien L-M and Jeng J-S (2019) Intravenous Thrombolysis Administration 3–4.5 h After Acute Ischemic Stroke: A Retrospective, Multicenter Study. Front. Neurol. 10:1038. doi: 10.3389/fneur.2019.01038
Received: 01 July 2019; Accepted: 13 September 2019;
Published: 15 October 2019.
Edited by:
Bruce Campbell, The University of Melbourne, AustraliaReviewed by:
Manabu Inoue, National Cerebral and Cardiovascular Center, JapanCopyright © 2019 Chen, Sung, Chen, Tang, Tsai, Lin, Huang, Po, Sun, Chen, Chan, Wei, Lee, Hsieh, Lin, Yeh, Lien and Jeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Ming Lien, bGllbjIxNzdAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.