
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 21 August 2019
Sec. Stroke
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00907
 Sophie Broussy1,2*
Sophie Broussy1,2* Florence Saillour-Glenisson1,2,3
Florence Saillour-Glenisson1,2,3 B. García-Lorenzo4
B. García-Lorenzo4 Francois Rouanet5
Francois Rouanet5 Emilie Lesaine1,2
Emilie Lesaine1,2 Melanie Maugeais1
Melanie Maugeais1 Florence Aly6
Florence Aly6 Bertrand Glize6
Bertrand Glize6 Roger Salamon1,2
Roger Salamon1,2 Igor Sibon5
Igor Sibon5Introduction: Knowledge about residual deficiencies and their consequences on daily life activities among stroke patients living at home 1-year after the initial event managed in stroke units is poor. This multi-dimensional study assessed the types of deficiencies, their frequency and the consequences that the specific stroke had upon the daily life of patients.
Methods: A cross-sectional survey, assessing, using standardized scales, 1 year post-stroke disabilities, limitations of activities, participation and quality of life, was carried out by telephone interview and by mail in a sample of stroke patients who returned home after having been initially managed in a stroke unit.
Results: A total of 161 patients were included (142 able to answer the interview on their own; 19 needing a care-giver). Amongst a sub-group of the patients interviewed, 55.4% (95% Confidence Interval [47.1–63.7]) complained about pain and 60.0% (95% CI [51.4–68.6]) complained of fatigue; about 25% presented neuropsychological or neuropsychiatric disability. Whilst 87.3% (95% CI [81.7–92.9]) were independent for daily life activities, participation in every domains and quality of life scores, mainly in daily activity, pain, and anxiety subscales, were low.
Conclusion: Despite a good 1-year post-stroke functional outcome, non-motor disabling symptoms are frequent amongst patients returned home and able to be interviewed, contributing to a low level of participation and a poor quality of life. Rehabilitation strategies focused on participation should be developed to break the vicious circle of social isolation and improve quality of life.
Stroke is the leading cause of acquired physical disability in adults worldwide. Although stroke units have dramatically improved post-stroke functional outcome and reduce post-stroke mortality (1) by focusing on acute stroke management, they often failed to consider stroke as a chronic disorder with potential delayed neuropsychological and emotional consequences. Indeed, while motor impairment, spasticity or aphasia are easily recognized complications, other deficiencies, such as cognitive impairment (2), depression (3), or fatigue (4) are also frequently reported but under-evaluated and poorly managed amongst stroke survivors. These so-called “invisible” deficiencies are thought to contribute to reduced ability to participate in daily life activities and also impaired quality of life (5). Furthermore, while most of patients able to return at home following stroke managed in stroke units are supposed to have a good outcome, they may suffer from a lack of monitoring.
Residual deficiencies and their consequences upon the daily life activities have mainly been assessed in large and representative populations of stroke patients (6). However, little information is available about post-stroke sequelae in the population of patients living at home and having been managed in stroke units.
Moreover, most of studies assessing post-stroke sequelae are focused on a small spectrum of sequelae; and present a high variability of results due to heterogeneity of populations, study designs, diagnostic scores and stroke types (4, 7, 8).
To address this lack of information, we adopted a multi-dimensional approach to assess the frequency and type of deficiencies with a focus on their daily-life consequences in a cohort of patients who are living at home 1 year after stroke having managed in stroke units.
A cross-sectional survey of 1-year post-stroke deficiencies, limitations in activities, participation and quality of life was conducted in a population of patients, included in the Aquitaine Observatory of Stroke (ObA2), from October 1st to December 4th 2013 with a stroke managed in one of the stroke units participating to ObA2.
ObA2 is a regional cohort of patients with a recent stroke, diagnosed and managed in the short-stay services of the hospitals in the Aquitaine region, Southwestern France. ObA2 objectives are to (1) describe the management of stroke (practices, delays, referral of patients, etc.) prior to hospitalization, during hospitalization and in the post-hospital phase, (2) describe the population of stroke patients in socio-demographic and clinical terms, (3) monitor the population of these patients in terms of: occurrence of complications during the stay, mortality at 1 year, disability at 1 year. ObA2 is the only stroke cohort in France covering a whole region, allowing the inclusion of a large and heterogenic panel of stroke patients.
The Oba2 inclusion criteria are: (i) aged over 18 years (ii) living in metropolitan France; (iii) suffering from a recent stroke whose diagnosis is confirmed by a neuro-vascular physician; (iv) stroke managed in one of the participating hospitals of the Aquitaine region with more than 30 strokes per year; (v) giving consent to participate. The study sample included patients of the study population alive at 1-year after a stroke managed in a stroke unit, living at home at 1 year post-stroke, with an available phone number at ObA2 admission and willing to participate. According to French regulations applicable at the time of research (9), this study was exempted from examination by an Ethics Committee in France and did not require written or verbal informed consent to participate. Oba2 cohort had received authorization from the French Commission nationale de l'informatique et des libertés (authorization n°911201). According to regulations, patients received oral and written information at Oba2 inclusion about the project, its objectives and data management process and about their refusal rights and process. Moreover, the study has been presented to the local institutional review board of the Bordeaux University Hospital.
Demographic data, stroke characteristics at admission (type of stroke, gravity of stroke assessed by the National Institute of Health Stroke Score-NIHSS), modified Rankin scale (mRS) at discharge, complication during acute stay, rehabilitation during acute stay, length of stay and admission to rehabilitation service after discharge or during follow-up were recorded at Oba2 inclusion using medical records. A selection of scales measuring each of the sequelae explored were selected, based on elements of feasibility, frequency of use in the exploration of the dimension considered and metrological performance (validity and reproducibility). The mode of collection of sequelae has been adapted according to the validated mode of administration for each of the scales.
At 1-year, two types of evaluations were performed, an initial interview conducted by telephone followed by self-assessment questionnaires which were sent by mail. Patients were asked to return their responses to the questionnaires using a prepaid envelope.
After 4 attempts, patients that could not be reached by phone were qualified as non-responders. The non-responders vital status was carried out in the local death registry. Those for whom vital status could not be retrieved 1 year post-stroke were considered as lost-to-follow-up. When patients were unable to answer questions on their own due to aphasia or severe cognitive impairment, caregivers were asked to answer the phone interview and complete the questionnaires on the patients' behalf.
Data recorded during the phone interview were: socio-economic characteristics (living alone, ability to drive), cognition and pain using the French Telephone Interview for Cognitive Status Modified (F-TICS-m) (10) and the simple verbal scale (EVS) (11) questionnaires, dysphagia, sphincteric and genito-sexual disorders using closed-ended question “yes/no,” and functional disability using the mRS (12). Data recorded using self-assessment questionnaires were: depression and anxiety using the hospital anxiety and depression scale (HADS) (13), fatigue using the Fatigue Severity Scale (FSS) (14), functional outcome using the Barthel Index (BI) (15). Moreover, we explored several categories of participation according to the ICF model (16) and focused on Domestic Life, Economic/social productivity, Interpersonal Interactions and Relationships using the Community Integration Questionnaire (CIQ) which is composed by a global score and three sub-scores (integration at home, social integration, productivity) (17). The higher these sub-scores are, the higher the participation in specific categories is. Finally, because limitations in activities or participations do not necessarily impact the quality of life, we explored the latter using the EUROQOL (EQ5D-3L) (18). An EQ-5D utility score for each patient 1-year post-stroke was calculated using information from the EQ5D-3L and a validated algorithm to calculate index values for the EQ-5D instrument (19). The mean value of the EQ-5D utility score and the standard error associated was calculated for the patients' sample and subgroups (19).
Three groups were considered: the entire study sample and 2 sub-groups of patients (interviewed and unable to answer the phone interview). Analyses presented in the article are focused in the sub-group of interviewed patients. Wilcoxon Mann-Whitney test was used to compare continuous variables. Chi-square test with or without Yates correction and Fisher's exact were used to compare categorical variables. P < 0.05 was considered statistically significant. Spearman correlation tests were performed to study the relationship between F-TICS-m, EVS, HADS, FSS, CIQ, or EQ5D-3L scores and either mRS or BI scores.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Of the 215 patients of the study population, 161 were included in the final study sample, amongst whom 142 were able to answer on their own and 19 needed a caregiver to answer the phone interview (Figure 1, Table 1). A total of 134 patients returned the self-assessment questionnaires.
Mean age of the subgroup of the 142 interviewed patients was of 69 years (Standard Deviation-SD: ± 14.0), the male/female ratio was of 1.6 and 86.0% of strokes were ischemic. The mean NIHSS score at admission was of 4.7 (SD: ± 5.5). Fifty six percent of the patients presented complication during acute in-hospital stay (neurological complication—7%, infectious complication-−11%, cardiovascular complication—4%). At hospital discharge, 74.6% of study sample patients had a mRS ≤ 2 and 62.7% returned directly to home. A total of 55% of patients received early rehabilitation during the acute stay, 34% were admitted directly to rehabilitation at discharge and 7% during follow-up.
The comparison of the socio-demographic characteristics and the clinical history between the sub-sample of interviewed patients and the group of patients lost-to-follow-up showed no statistical differences. However, a higher proportion of patients lost-to-follow-up had a length of stay >8 days (p = 0.049), a severe NIHSS score at admission (>13), (p = 0.0006) and a lower proportion of these patients had a mRS ≤ 2 (p = 0.01). The group of patient lost-to-follow-up seemed to have presented a more severe stroke with a less positive prognostic than the interviewed patients.
Overall, 21.8% (95% Confidence Interval - CI [15.0–28.6]) of the subgroup of interviewed patients had a cognitive impairment and 60.0% (95% CI [51.4–68.6]) reported high fatigue (FSS≥ 5.5/7) (Table 2). About one quarter of this group of patients reported anxiety and depressive disorders (HADS≥11/14) and 55.4% (95% CI [47.1–63.7]) complained about pain. More than two-thirds of the interviewed patients showed good functional outcome: 70.4% (95% CI [62.9–77.9]) reported a mRS ≤ 2 and 87.3% (95% CI [81.7–92.9]) were assessed as independent enough to live at home (BI≥60). Evaluation of participation demonstrated low levels of participation (CIQ global score: mean 13.5/29; SD ± 6.6; 95% CI [12.3–14.6]), mainly in the domains of home integration (CIQ-Home integration: mean 3.8/12; SD ± 2.9) and productivity (CIQ-Productivity: mean 3.07; SD ± 2.3). Quality of life measured as the mean EQ-5D utility score is 0.67 (SD ± 0.03; Table 2).
The correlation analyses showed that the higher the limitations of activities were, the lower participation (CIQ-Home integration: r = −0.50. p < 0.001; CIQ-Social integration: r = −0.57, p < 0.001; CIQ-Productivity: r = −0.49, p < 0.0001), and the higher the limitations of activities were, the lower the quality of life (mRS: r = −0.67; BI: r = 0.72; Table 3).
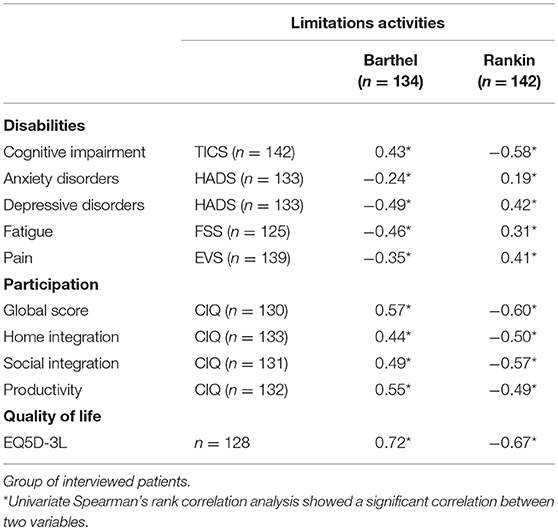
Table 3. Spearman correlation coefficients between F-TICS-m, EVS, HADS, FSS, or EQ5D-3L scores and either mRS or BI scores.
This study providing original data on the outcome of a specific population of stroke patients living at home after stroke managed in a stroke unit suggests that (i) pain and fatigue represents the main persistent symptoms while cognitive and neuropsychiatric disabilities are observed in about 20% of the population, (ii) despite an overall good functional outcome, patients who are independent in their daily-life activities have multidomain impairment in participation and a lower level of quality of life scores compared to the general population at the same age range in France (0.81) (20).
The frequency of pain and fatigue as well as cognitive and neuropsychiatric disabilities observed in our selected population with a good functional outcome is very similar to what is usually reported in the global stroke population (21). This result suggests that the rate of “invisible handicap” is persistent across patients independently from stroke severity. Interestingly, the level of home and social integration as well as productivity and quality of life remains very low in this population suggesting strong participation restrictions and daily life consequences despite a good functional outcome. The predominance of restriction participation in the field of home integration might reflect the difficulties of stroke patients to face their new physical condition in their own environment with overprotective relatives. Moreover, the strong correlation between objective and subjective measurements of deficiencies and limitations of activities shows the critical consequences of these sequelae on post-stroke quality of life.
Specific rehabilitation and occupational therapy in the patients' living place might improve the home integration of these patients. Impaired cognition, and the well-known difficulty to perform dual-task in stroke patients (22) could also contribute to this restriction of participation suggesting that cognitive rehabilitation is mandatory to improve the probability for patients to reach a level of participation similar that which they had prior to the stroke. The difficulties of reintegration at home and in society could in turn increase anxiety and depression, fatigue, and lead to a vicious circle of social isolation with its impact upon patients' quality of life which must be broken (23).
Interestingly the high rate of invisible deficiencies observed in this study was observed despite an initial acute management of these patients in a stroke unit that should have ensured optimal outcomes. Despite real improvement in the access to stroke units in Europe, about 4% to 50% of stroke patients are not admitted to stroke units; the rate of invisible handicap in this population might be even higher than in the present cohort, and highlight the need to build dedicated workflow for all stroke patients to improve their outcome.
However, the results have to be interpreted cautiously due to several limitations represented by the specificity of the French healthcare system and cultural factors, the low number of patients included and their clinical characteristics. Indeed, the low level of severity at admission (62–65% with the NIHSS score at admission ≤5) and good functional outcome at hospital discharge (74.6% had mRS ≤2) suggest that the present results cannot be generalized to the whole stroke population admitted in stroke units. Moreover, only 66% of the patients responded on their own to the interview which might represent a significant bias to interpret the results. Similarly to those who were lost to follow-up and those who denied to participate we can assume that those who were unable to answer on their own had an even worse functional outcome, suggesting that the present study underestimate the true daily-life consequences of stroke in patients with minor to mild stroke.
Because the improvement of acute stroke treatments now offers considerable reduction in the severity of physical disability, there is an urgent need to develop more systematic detection and management of the remaining invisible deficiencies that contribute to persistent restriction of participation in daily-life.
The datasets generated for this study are available on request to the corresponding author.
The study was approved by the local ethics committee and the ObA2 cohort had received CNIL authorization (n°911201). All patients received individual written information.
SB, FS-G, BG-L, FR, EL, MM, FA, BG, RS and IS contributed to the study design, data collection and manuscript writing. SB and BG-L performed statistical analyses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140–6736(14)60584–5
2. Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford vascular study. Lancet Neurol. (2019) 18:248–58. doi: 10.1016/S1474–4422(18)30442–3
3. Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2017) 48:e30–43. doi: 10.1161/STR.0000000000000113
4. Cumming TB, Packer M, Kramer SF, English C. The prevalence of fatigue after stroke: a systematic review and meta-analysis. Int J Stroke J Int Stroke Soc. (2016) 11:968–77. doi: 10.1177/1747493016669861
5. Gadidi V, Katz-Leurer M, Carmeli E, Bornstein NM. Long-Term Outcome Poststroke: predictors of activity limitation and participation restriction. Arch Phys Med Rehabil. (2011) 92:1802–8. doi: 10.1016/j.apmr.2011.06.014
6. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. (2016) 15:913–24. doi: 10.1016/S1474–4422(16)30073–4
7. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. (2009) 8:1006–18. doi: 10.1016/S1474–4422(09)70236–4
8. Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. (2005) 36:1330–40. doi: 10.1161/01.STR.0000165928.19135.35
9. Ministère de l'Enseignement Supérieur et de la Recherche- Article R1123–21 du Code de la santé publique relatif à la procédure d'avis des comités de protection des personnes - Modifié par Décret n°2007–1220 du 10 août 2007 - art. 5 JORF 14 août 2007 [Internet].
10. Lacoste L, Trivalle C. Adaptation française d'un outil d'évaluation par téléphone des troubles mnésiques: le French Telephone Interview for Cognitive Status Modified (F-TICS-m). NPG Neurologie-Psychiatrie-Gériatrie. (2009) 9:17–22. doi: 10.1016/j.npg.2008.06.009
11. Tyson SF, Brown P. How to measure pain in neurological conditions? A systematic review of psychometric properties and clinical utility of measurement tools. Clin Rehabil. (2013) 28:669–86. doi: 10.1177/0269215513514231
12. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
13. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–370. doi: 10.1111/j.1600–0447.1983.tb09716.x
14. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. doi: 10.1001/archneur.1989.00520460115022
15. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md State Med J. (1965) 14:61–5.
16. Organisation Mondiale de la Santé. Classification Internationale du Fonctionnement, du Handicap et de la Santé. (2001). Available online at: http://www.who.int/classifications/icf/wha-fr.pdf
17. Gerrard P, Kazis LE, Ryan CM, Shie VL, Holavanahalli R, Lee A, et al. Validation of the community integration questionnaire in the adult burn injury population. Qual Life Res. (2015) 24:2651–5. doi: 10.1007/s11136-015-0997-4
18. Pinto ÉB, Maso I, Vilela RNR, Santos LC, Oliveira-Filho J. Validation of the EuroQol quality of life questionnaire on stroke victims. Arq Neuropsiquiatr. (2011) 69:320–3. doi: 10.1590/S0004–282X2011000300010
19. Chevalier J, de Pouvourville G. Valuing EQ-5D using time trade-off in France. Eur J Health Econ. (2013) 14:57–66. doi: 10.1007/s10198–011-0351-x
20. Szende A, Janssen B, Cabases J. Self-Reported Population Health: an International Perspective Based on EQ-5D. Dordrecht: Springer (2014)
21. Kapoor A, Lanctôt KL, Bayley M, Kiss A, Herrmann N, Murray BJ, et al. “Good Outcome” Isn't Good Enough. Stroke. (2017) 48:1688–90. doi: 10.1161/STROKEAHA.117.016728
22. Lees R, Fearon P, Harrison JK, Broomfield NM, Quinn TJ. Cognitive and mood assessment in stroke research. Stroke. (2012) 43:1678–80. doi: 10.1161/STROKEAHA.112.653303
23. Tse T, Binte Yusoff SZ, Churilov L, Ma H, Davis S, Donnan GA, et al. Increased work and social engagement is associated with increased stroke specific quality of life in stroke survivors at 3 months and 12 months post-stroke: a longitudinal study of an Australian stroke cohort. Top Stroke Rehabil. (2017) 24:405–14. doi: 10.1080/10749357.2017.1318339
Keywords: stroke, cognition, depressive disorder, fatigue, activities of daily living, social participation
Citation: Broussy S, Saillour-Glenisson F, García-Lorenzo B, Rouanet F, Lesaine E, Maugeais M, Aly F, Glize B, Salamon R and Sibon I (2019) Sequelae and Quality of Life in Patients Living at Home 1 Year After a Stroke Managed in Stroke Units. Front. Neurol. 10:907. doi: 10.3389/fneur.2019.00907
Received: 13 May 2019; Accepted: 05 August 2019;
Published: 21 August 2019.
Edited by:
Margit Alt Murphy, University of Gothenburg, SwedenReviewed by:
Majanka H. Heijenbrok-Kal, Rijndam Revalidatiecentrum, NetherlandsCopyright © 2019 Broussy, Saillour-Glenisson, García-Lorenzo, Rouanet, Lesaine, Maugeais, Aly, Glize, Salamon and Sibon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Broussy, c29waGllLmJyb3Vzc3lAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.