- Department of Neurosurgery & Brain and Nerve Research Laboratory, The First Affiliated Hospital of Soochow University, Suzhou, China
Background: The role of intrathecal fibrinolysis for the treatment of patients with aneurysmal subarachnoid hemorrhage (aSAH) has been widely investigated; however, the results have been contradictory. In our study, we conducted a meta-analysis to evaluate the safety and efficacy of intrathecal (intracisternal or intraventricular) fibrinolysis for aSAH.
Methods: PubMed, Web of Science, Embase, Medline, and the Cochrane library databases were searched up to February 1, 2019. The outcomes analyzed were neurologic recovery, delayed ischemic neurologic deficit (DIND), mortality, and the incidence of chronic hydrocephalus and hemorrhage.
Results: A total of 21 studies comprising 1,373 patients were analyzed, including nine randomized controlled trials (RCTs) and 12 non-RCTs. The results showed that intracisternal fibrinolysis significantly decreased poor neurologic outcomes (RR = 0.62, 95% CI = 0.50–0.76, P < 0.001) and reduced the incidence of DIND (RR = 0.52, 95% CI = 0.41–0.65, P <0.001), chronic hydrocephalus (RR = 0.59, 95% CI = 0.42–0.82, P = 0.002) and mortality (RR = 0.58, 95% CI = 0.37, 0.93, P = 0.02). There was no significant difference in the occurrence of hemorrhage. Moreover, the results of the Egger test and Begg's funnel plot showed no evidence of publication bias.
Conclusions: Current evidence suggests that intracisternal fibrinolysis has beneficial effects on the clinical outcomes of patients with aSAH. However, further well-designed randomized trials are needed to confirm the efficacy and safety of intracisternal fibrinolysis for the treatment of aSAH.
Introduction
Despite significant progress in the reduction of mortality, survivors of aneurysmal subarachnoid hemorrhage (aSAH) still experience long-term cognitive and functional limitations (1, 2). Cerebral vasospasm and delayed ischemic neurologic deficit (DIND) are the two major contributors to secondary morbidity and mortality following severe aSAH (3–6). Moreover, hydrocephalus, another common cause of neurologic deterioration, is associated with worse outcomes (7). Previous studies indicate that the volume of SAH and intraventricular hemorrhage (IVH), as well as the clearance rate of clots, were independent predictors of poor neurologic outcomes (8, 9). In this respect, intrathecal fibrinolysis using tissue plasminogen activator (tPA) or urokinase (UK) to reduce subarachnoid or intraventricular blood was reported to be beneficial, reducing poor neurologic outcomes in patients with aSAH (10–13). However, the results of published randomized and observational trials failed to consistently substantiate the efficacy of thrombolytic agents in preventing DIND and poor clinical outcomes (14–16). A recent meta-analysis investigated the effect of intrathecal fibrinolysis in patients with aSAH (17), however, several recent RCTs were not included (15–18). Moreover, one study described as “randomized” in a previous meta-analysis was not classified as a RCT in our meta-analysis because the authors did not offer details regarding their methodology (19). Because of these shortcomings, we performed a systematic review and meta-analysis to assess the effect of intrathecal fibrinolysis on neurologic outcomes, DIND, and mortality as well as the resulting complications, including hemorrhage and chronic hydrocephalus.
Methods
Search Strategy
This systematic review and meta-analysis were conducted in accordance with the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (20). Our comprehensive electronic search of PubMed, Web of Science, Embase, Medline, and the Cochrane library databases was performed using the following search terms: thrombolytic therapy, tPA, fibrinolytic agents, urokinase, SAH, subarachnoid hemorrhage, DIND, delayed cerebral ischemia, clinical vasospasm, and cerebral aneurysm (the last search update was made on February 1, 2019). In addition, the references of all retrieved articles were checked for possible additional studies.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) publications comparing intrathecal (intracisternal or intraventricular) thrombolytic therapy with a control treatment in patients with aSAH and (2) papers assessing outcomes in terms of the development of DIND, the Glasgow Outcome Scale (GOS) score, modified Rankin Scale (mRS) score, mortality, chronic hydrocephalus, hemorrhage, and/or a rebleeding complication.
Articles were excluded if (1) a control group was unavailable or (2) no information regarding outcomes was provided. We also excluded case reports, review articles, articles comprised of abstracts only, meta-analyses, and duplicate reports.
Data Extraction
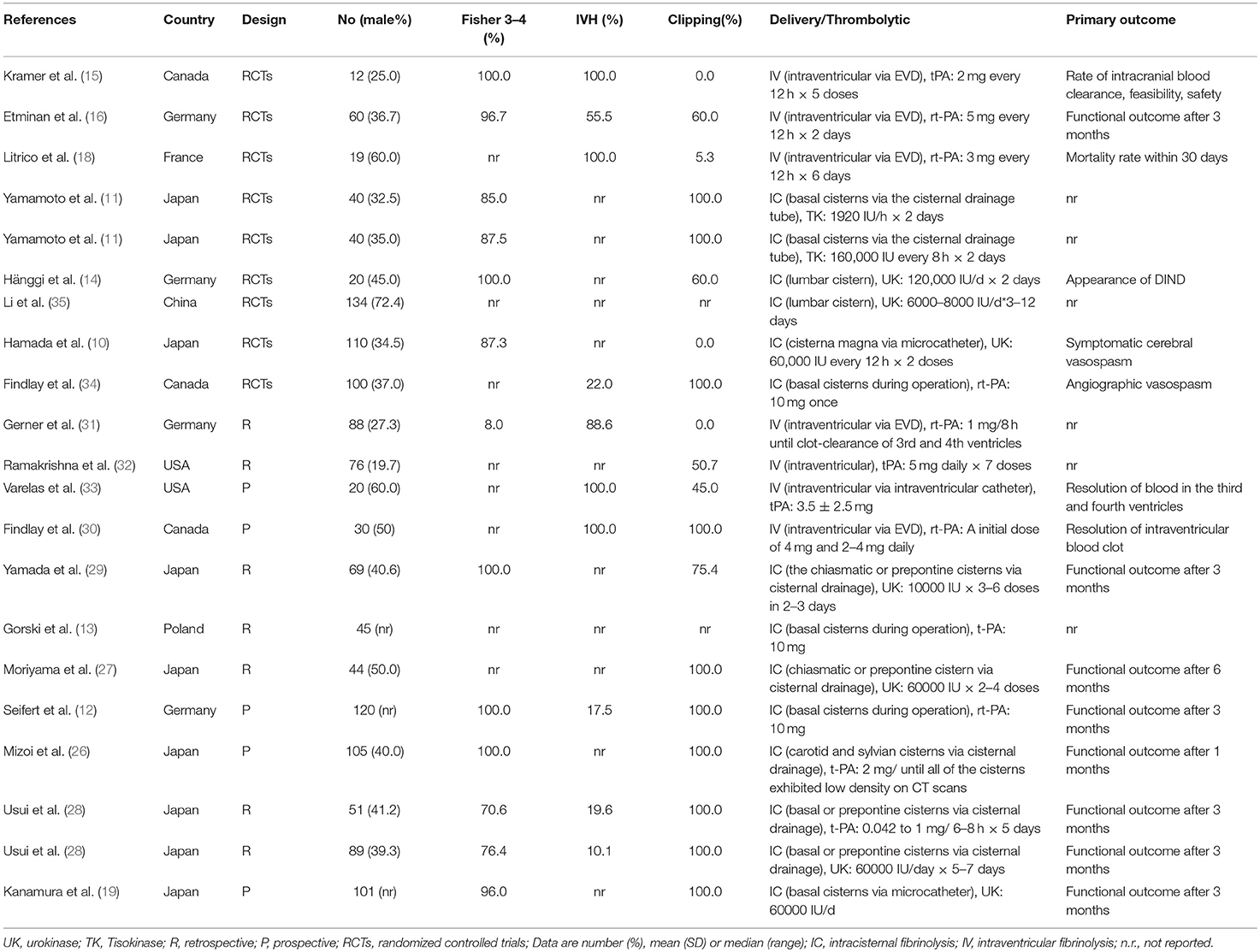
For each eligible study, the following information was abstracted by two independent authors using a standardized data extraction form comprising study design, sample size, patient eligibility criteria, duration of follow-up, treatment group criteria, patient characteristics, sex and age of patients, severity of hemorrhage (indicated by Fisher grade), neurologic grade (indicated by World Federation of Neurosurgical Societies [WFNS]) criteria or Glasgow Coma Scale [GCS] score), interventions (type, timing, dose, and method of delivery of the fibrinolytic agents and concomitant therapies), and outcomes (development of DIND, GCS score, mRS score, mortality, chronic hydrocephalus, hemorrhage and/or rebleeding complications). Disagreements were resolved by discussion with a third author.
Quality Assessment
The quality of all eligible studies was assessed by two independent authors. For RCTs, the Cochrane Collaboration's tool was used to assess risk of bias according to the following domains: selection bias (random sequence generation and allocation concealment), attrition bias (incomplete outcomes data), performance and detection bias (blinding of participants, personnel, and outcome assessment), reporting bias (selective reporting), and other sources of bias (21). In addition, the Newcastle–Ottawa Scale (NOS) was used to assess the risk of bias in the non-RCTs (22).
Data Analysis and Statistical Methods
The following outcomes analyses were included: association of intrathecal (intracisternal or intraventricular) fibrinolysis following aSAH with (1) neurologic recovery (defined as “poor” if patients with Glasgow Outcome Scale (GOS) score between 1 and 3 and/or a mRS score between 4 and 6); (2) DIND, which was defined according to the criteria of individual papers; (3) mortality; (4) chronic hydrocephalus, defined as the need for a permanent shunt; and (5) hemorrhage and/or rebleeding complications.
Between-study heterogeneity was assessed by the χ2-based Q test and I2 test. Heterogeneity was considered significant when the P-value was less than 0.1 or I2 was >50%, then pooled risk estimates were calculated using the random-effects model (DerSimonian-Laird); otherwise, a fixed-effects model (Mantel-Haenszel) was used (23, 24).
Assessment of publication bias was performed using a graphic evaluation of funnel plots and the Egger regression test. A P-value of less than 0.05 from the Egger test was considered statistically significant (25). All statistical analyses were performed using Review Manager (RevMan) (version 5.2, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2012) and Stata (version 12 software, StataCorp LP, College Station, TX, USA).
Results
Characteristics of Included Studies
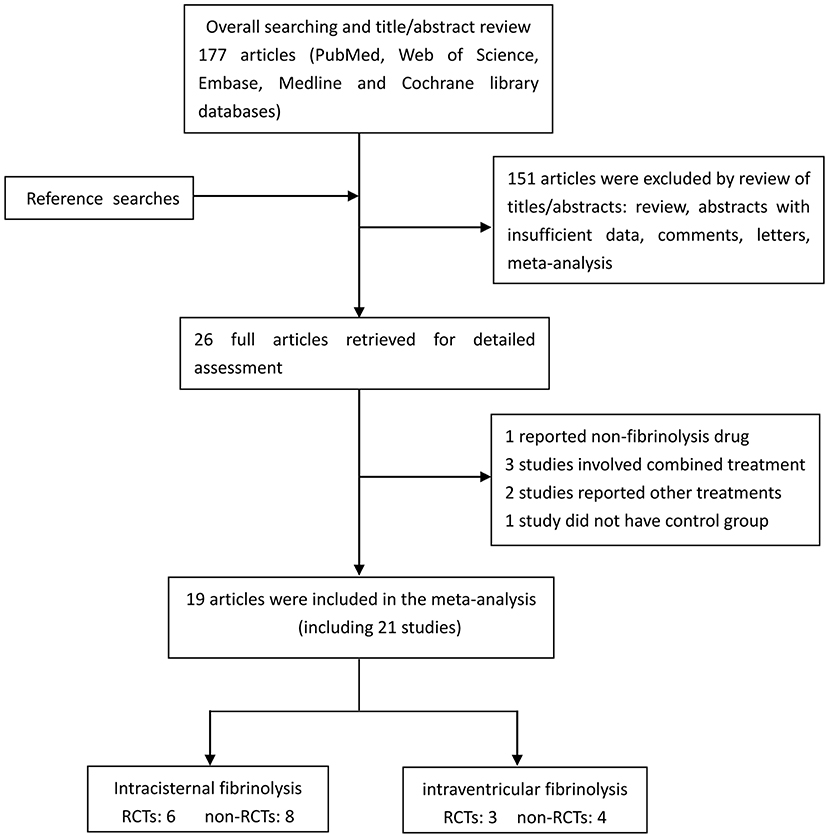
A flow diagram detailing the study selection is shown in Figure 1. Briefly, the literature search produced 177 citations, of which 151 were excluded by review of the abstracts. Thereafter, full texts of the remaining 26 articles were analyzed and reviewed in detail. Finally, a total of 19 studies comprising 1,373 aSAH patients met our inclusion criteria (10–16, 18, 19, 26–35). Among these studies, one reported data on intermittent and continuous fibrinolysis, and another allocated patients to therapy with tPA or UK; that is, we treated them independently (11, 28). In total, nine RCTs and 12 non-RCTs were analyzed. The main characteristics of the studies included in this meta-analysis are summarized in Table 1.
Risk of Bias
The results of quality assessments for RCTs are summarized in Figure 2. Briefly, randomization methods were not proper in one study and not described in another. The allocation concealments were not adequate in five studies. Moreover, five studies did not describe the blinding of outcomes assessment and participants. For cohort studies, quality assessments were made by using NOS, and the results showed that five studies had a low risk of bias (8 out of 9 points) and seven studies had a moderate risk of bias (6–7 out of 9 points) (Supplementary Tables 1, 2).
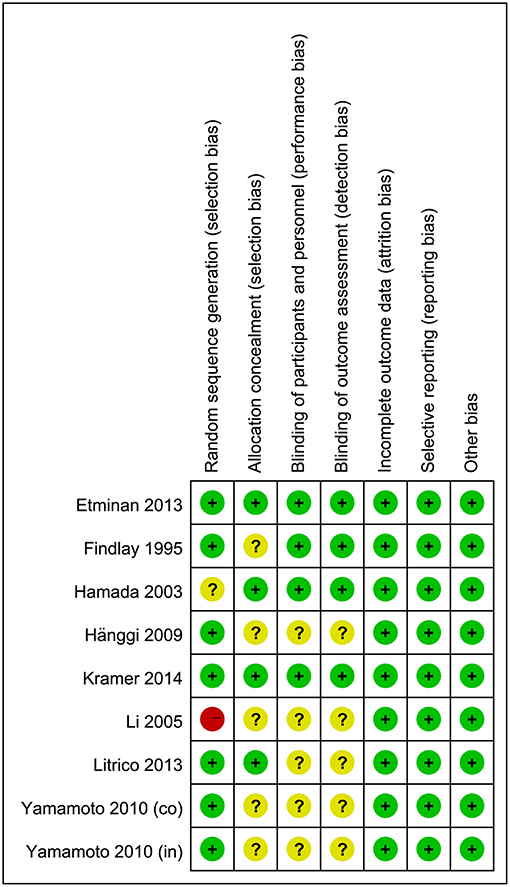
Figure 2. Risk-of-bias assessment for randomized controlled trials. A plus sign (+) indicates a low risk of bias, a minus sign (–) indicates a high risk of bias, and a question mark (?) indicates an unclear risk of bias.
Neurologic Recovery and DIND
Combining the results of all 14 studies (6 RCTs and 8 non-RCTs), treatment with intracisternal fibrinolysis significantly reduced the proportion of patients with poor neurologic recovery (RR = 0.62, 95% CI = 0.50–0.76, P < 0.001 for overall analysis; RR = 0.62, 95% CI = 0.47–0.81, P < 0.001 for the RCT group; RR = 0.61, 95% CI = 0.45–0.84, P < 0.001 for the non-RCT group; Figure 3A). The overall incidence of DIND was 17.0% in the intracisternal fibrinolysis group as compared with 30.3% in the control group. The pooled relative risk of DIND was 0.52 (95% CI = 0.41–0.65) for the overall analysis (in the subgroup analysis, RR = 0.58, 95% CI = 0.42–0.80, P < 0.001 for the RCT group; RR = 0.46, 95% CI = 0.33–0.65, P < 0.001 for the non-RCT group; Figure 3B). For intraventricular fibrinolysis, no significant improvements in poor neurologic outcome and DIND were observed in either RCTs (RR = 0.86, 95% CI = 0.55–1.34, P = 0.50 and RR = 0.86, 95% CI = 0.49–1.51, P = 0.59, respectively) or non-RCTs (RR = 1.07, 95% CI = 0.70–1.62, P = 0.77 and RR = 0.55, 95% CI = 0.24–1.24, P = 0.15, respectively; Figures 4A,B).
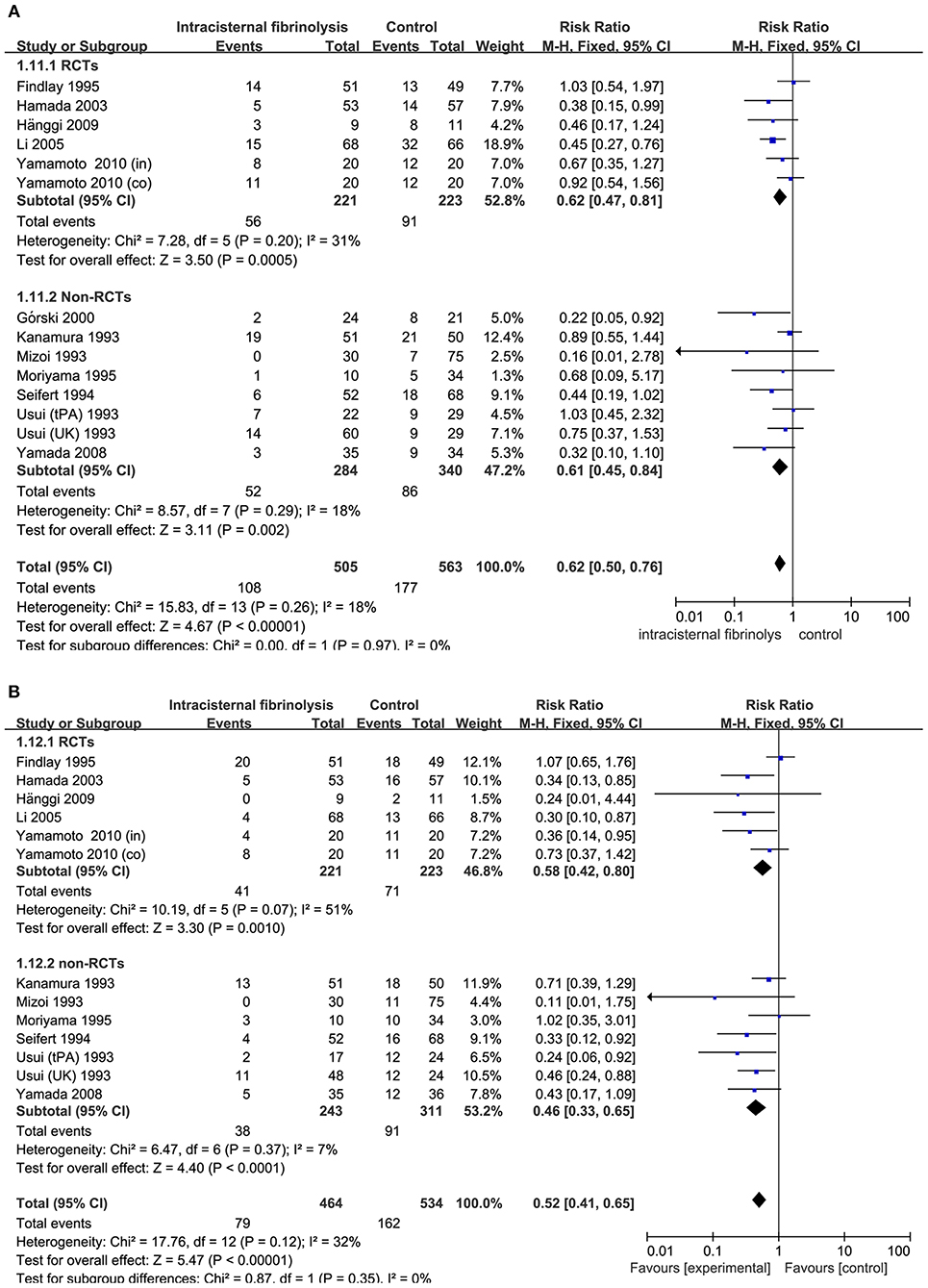
Figure 3. Meta-analysis of associations between intracisternal fibrinolysis and the risk of poor neurologic recovery (A) or the incidence of DIND (B) in patients with aSAH. aSAH, aneurysmal subarachnoid hemorrhage; CI, confidence interval; DIND, delayed ischemic neurologic deficit; M-H, Mantel-Haenszel method; RCTs, randomized controlled trials.
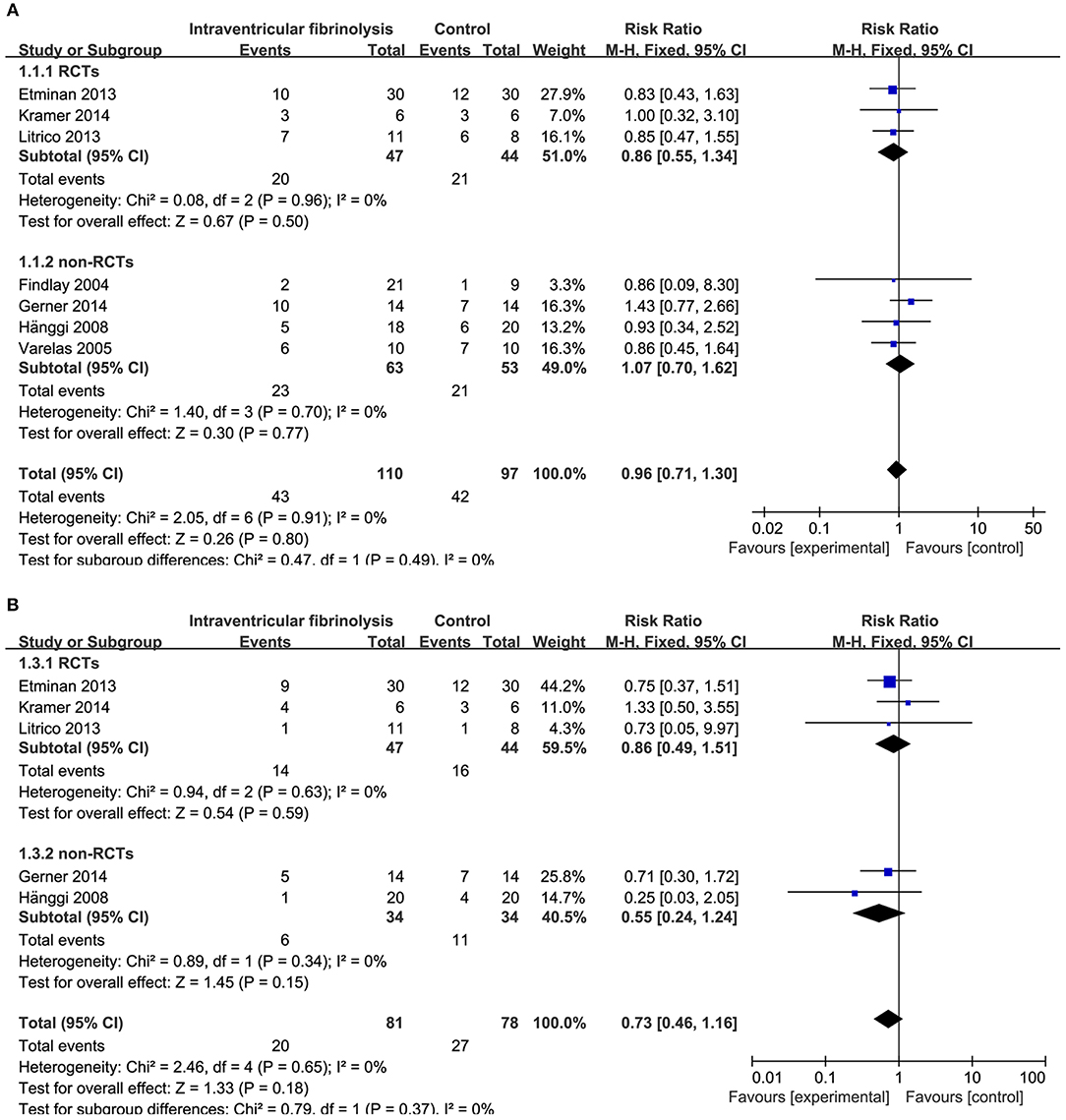
Figure 4. Meta-analysis of associations between intraventricular fibrinolysis and the risk of poor neurologic recovery (A) or the risk of DIND (B) in aSAH patients. aSAH, aneurysmal subarachnoid hemorrhage; CI, confidence interval; DIND, delayed ischemic neurologic deficit; M–H, Mantel–Haenszel method; RCTs, randomized controlled trials.
In the subgroup analysis stratified by types of thrombolytic agents, the results showed that either intracisternal tPA or UK infusion significantly decreased the risk of pool neurologic outcomes (RR = 0.68, 95% CI = 0.51–0.92, P = 0.01 and RR = 0.48, 95%CI = 0.34–0.67, P < 0.0001, respectively; Figure 5A) and DIND (RR = 0.56, 95%CI = 0.36–0.85, P = 0.005 and RR = 0.42, 95% CI = 0.28–0.63, P < 0.001, respectively; Figure 5B). Moreover, the subgroup analysis by the methods of aneurysm treatment (clipping or coiling) showed intracisternal fibrinolysis significantly reduced the proportion of patients with poor neurologic recovery in the clipping subgroup (RR = 0.77, 95% CI = 0.61–0.98, P = 0.04). However, there was only one study reporting the patients with aSAH treated with coiling (RR = 0.38, 95% CI = 0.15–0.99, P = 0.05) (10). For intraventricular fibrinolysis, there were insufficient data to perform a subgroup analysis by types of thrombolytic agents and the methods of aneurysm treatment.
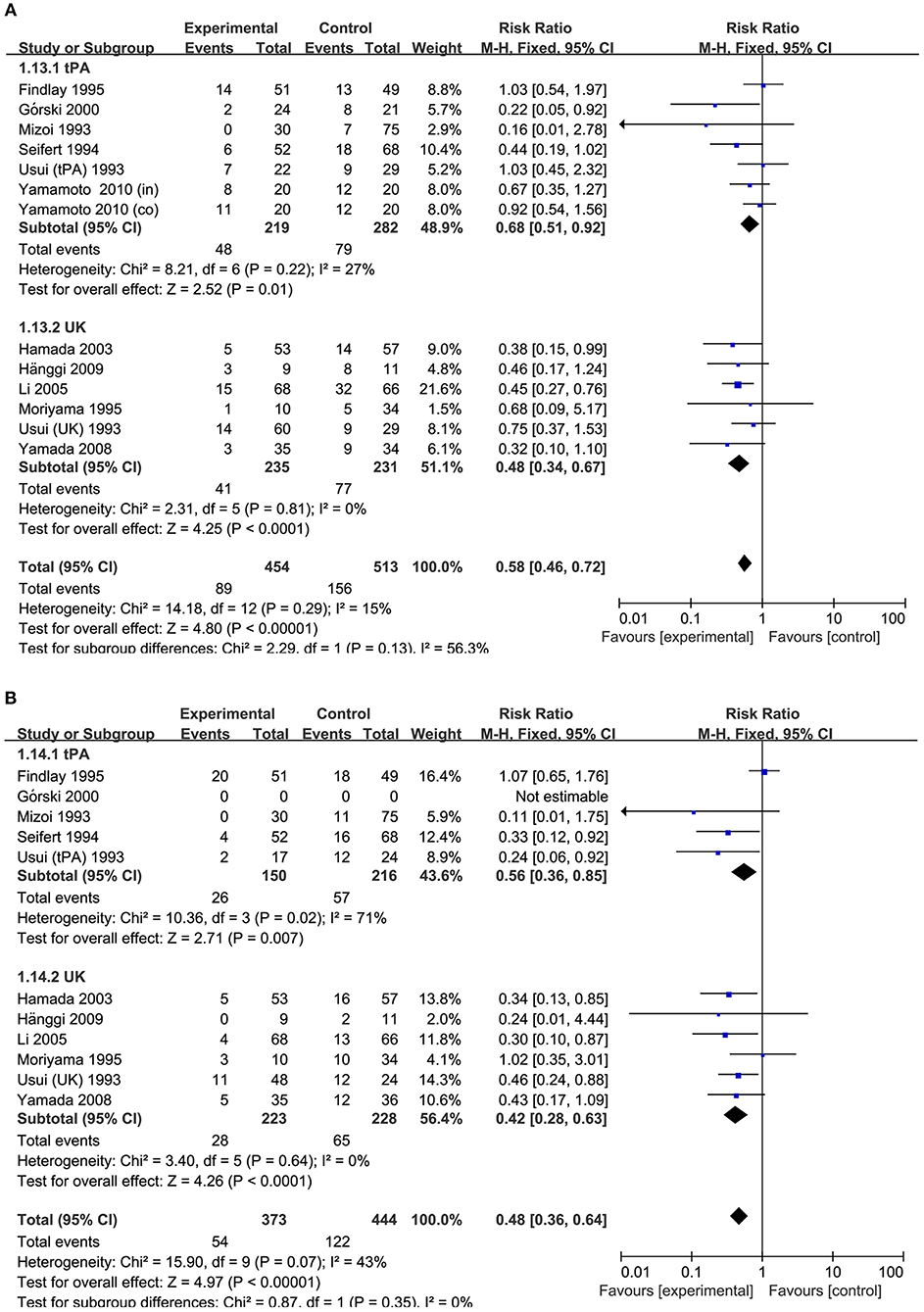
Figure 5. Meta-analysis of associations between intracisternal fibrinolysis and the risk of poor neurologic recovery (A) or the incidence of DIND (B) in patients with aSAH (stratified by types of thrombolytic agents). aSAH, aneurysmal subarachnoid hemorrhage; CI, confidence interval; M–H, Mantel–Haenszel method; UK, urokinase; tPA, tissue plasminogen activator.
Mortality and Chronic Hydrocephalus
For intracisternal fibrinolysis, a meta-analysis of 11 studies (including five RCTs and six non-RCTs) showed that intracisternal fibrinolysis significantly decreased mortality among patients with severe SAH (RR = 0.58, 95% CI = 0.37, 0.93, P = 0.02) compared with patients receiving conventional treatment. However, the results of a pooled analysis did not show any difference in mortality between the intraventricular fibrinolysis group and the control group (RR = 0.76, 95% CI = 0.46, 1.26, P = 0.28).
The risk of chronic hydrocephalus, defined as the need for a permanent shunt, was significantly decreased in patients treated with intracisternal fibrinolysis (RR = 0.59, 95% CI = 0.42–0.82, P = 0.002; Figure 6A). However, for intraventricular fibrinolysis-treated patients, no significant association was observed between fibrinolytic therapy and chronic hydrocephalus in overall estimation (RR = 1.00, 95% CI = 0.71–1.40, P = 0.98), as well as in RCTs (RR = 1.05, 95% CI = 0.69–1.59, P = 0.82) or non-RCT subgroups (RR = 0.93, 95% CI = 0.53–1.62, P = 0.80; Figure 7A).
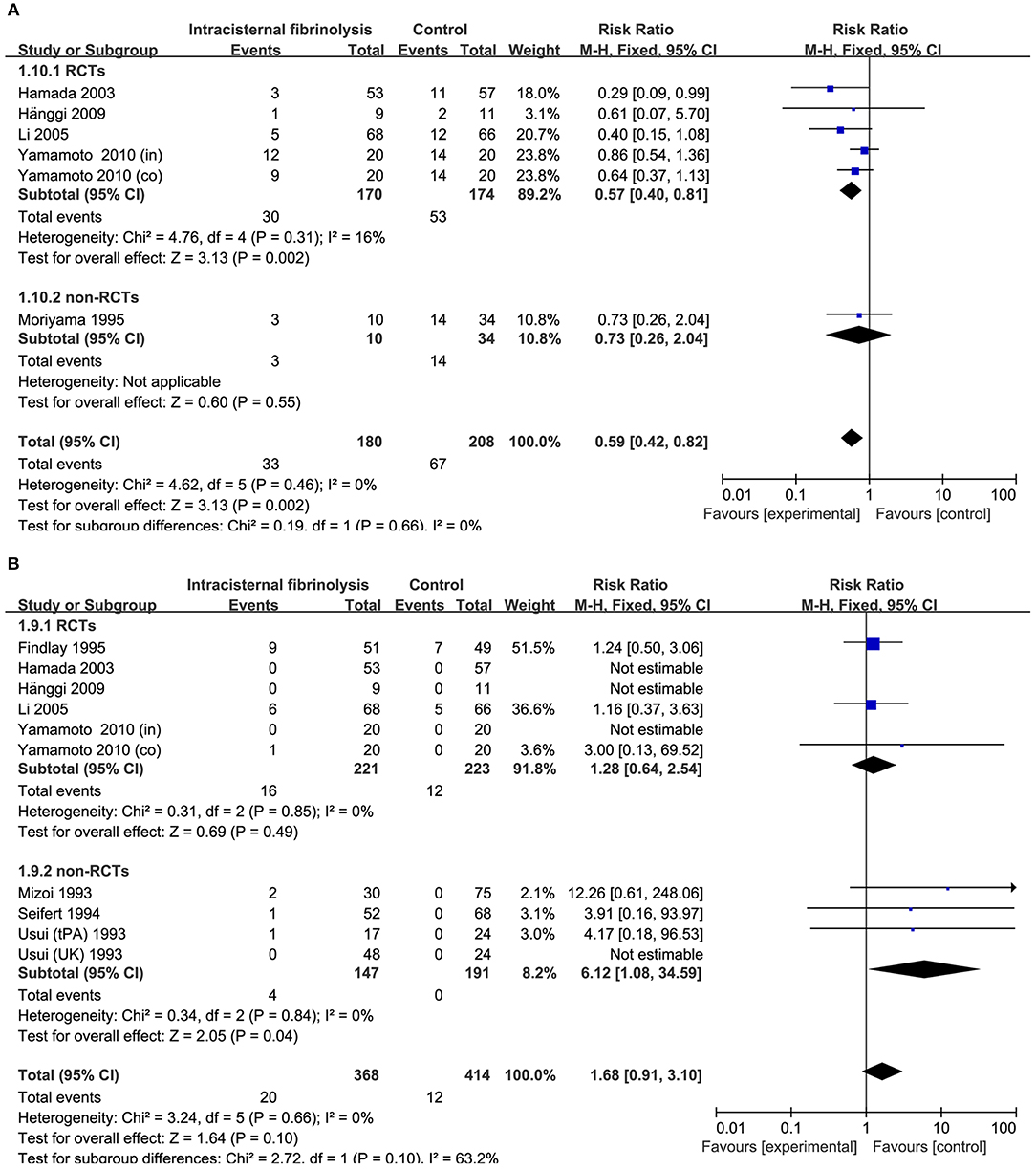
Figure 6. Meta-analysis of associations between intracisternal fibrinolysis and the risk of chronic hydrocephalus (A) or the risk of hemorrhagic complications (B) in patients with aSAH. aSAH, aneurysmal subarachnoid hemorrhage; CI, confidence interval; M–H, Mantel–Haenszel method; RCTs, randomized controlled trials.
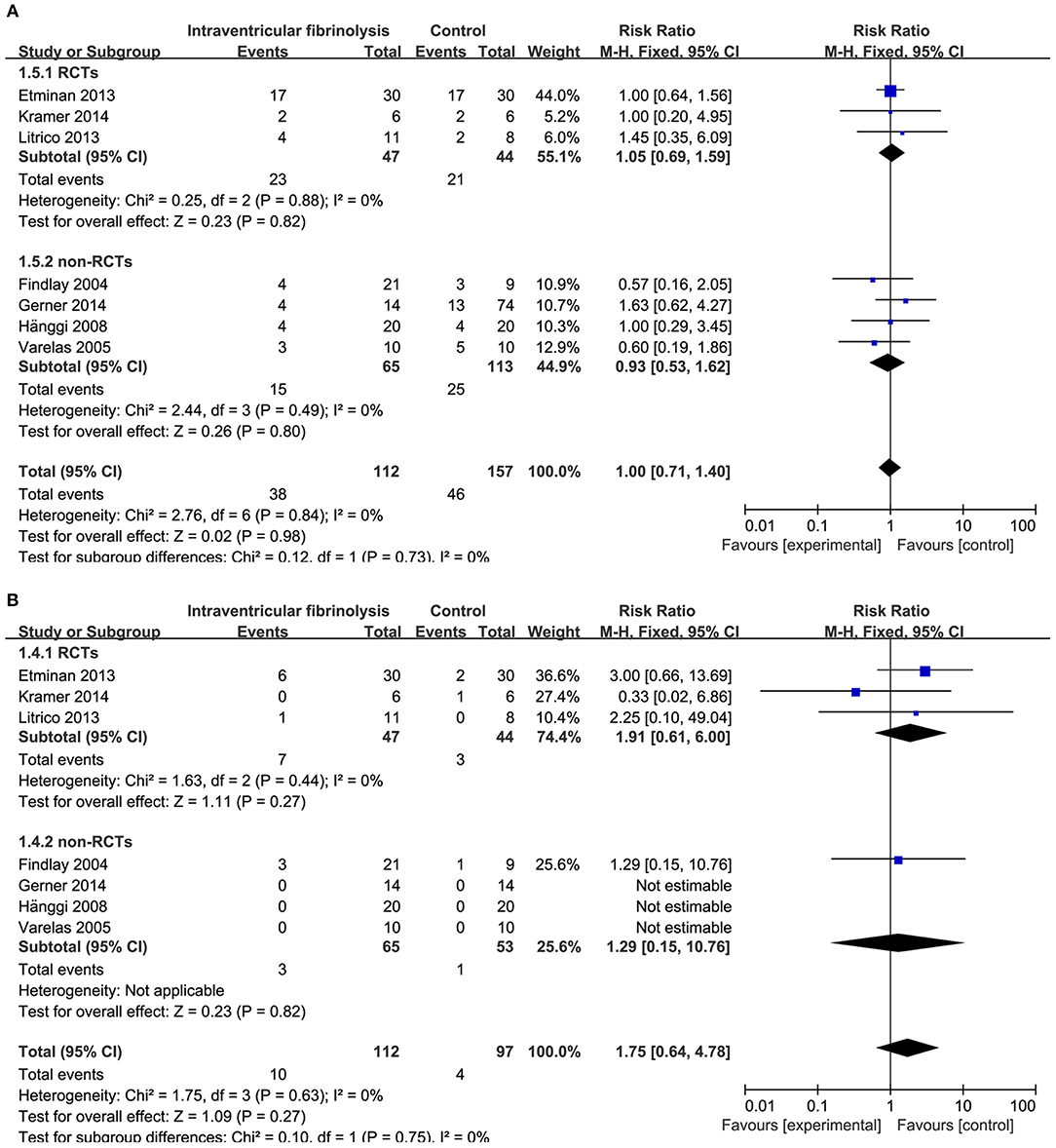
Figure 7. Meta-analysis of associations between intraventricular fibrinolysis and the risk of chronic hydrocephalus (A) or the risk of hemorrhagic complications (B) in patients with aSAH. aSAH, aneurysmal subarachnoid hemorrhage; CI, confidence interval; M–H, Mantel–Haenszel method; RCTs, randomized controlled trials.
Hemorrhagic Complications
Hemorrhagic complications were described in 10 studies for intracisternal fibrinolysis. The results of a pooled analysis demonstrated that hemorrhagic complications were not increased overall (RR = 1.68, 95% CI = 0.91–3.10, P = 0.10) or in RCT subgroups (RR = 1.28, 95% CI = 0.64–2.54, P = 0.49), whereas which was significantly increased in non-RCTs subgroups (RR = 6.12, 95% CI = 1.08-34.59, P = 0.04; Figure 6B). For intraventricular fibrinolysis, seven studies reported the incidence of hemorrhagic complications. After combining the results of these studies, a difference in the rate of hemorrhage between intraventricular fibrinolysis and the control groups was not shown (RR = 1.75, 95% CI = 0.64–4.78, P = 0.27; Figure 7B).
Test of Heterogeneity and Publication Bias
Between-study heterogeneity was assessed by the χ2-based Q and I2 tests. The results revealed non-significant heterogeneity in all analyses (P > 0.2 for all analyses). For publication bias, the shapes of the funnel plots did not show evidence of obvious asymmetry in any of the studies included in the pooled analysis (Supplementary Figure 1). In addition, we performed an Egger test to provide statistical evidence of funnel plot symmetry, which supported the results of the funnel plots.
Discussion
Although the subarachnoid clot volume and clot clearance rate were important risk factors for the development of DIND and hydrocephalus following a ruptured cerebral aneurysm, it remained uncertain whether therapies aimed to the clearance of blood improved outcomes in aSAH patients (7, 8). By combining the results of nine RCTs and 12 retrospective or prospective studies involving a total of 1,373 patients, we found that intracisternal fibrinolysis reduced the incidence of DIND, chronic hydrocephalus, and mortality and also improved functional recovery in patients with aSAH. There was no significant difference in rebleeding complications between the fibrinolysis and control groups.
DIND is a distinctive syndrome of cerebral ischemia after SAH; it is a major cause of mortality and disability and is difficult to treat (36, 37). One reason for this difficulty is that the exact pathophysiology of DIND is unclear. Recent research has shown that vasospasm is not the only cause of DIND (38). Regional hypoperfusion often occurs in territories without angiographic vasospasm, and other factors—such as early brain injury, intravascular inflammation, and microthrombosis—have been reported to cause DIND (39, 40). Previously published studies reported a rate of 33% to 38% for DIND after SAH (41). In our study, we found that intracisternal fibrinolysis significantly decreased the occurrence of DIND (by 17.0 and 30.3% in the intracisternal fibrinolysis and placebo-treated patients, respectively), whereas no significant difference was observed in the intraventricular fibrinolysis group.
Chronic hydrocephalus is a well-known post-aSAH complication (42). According to recent studies, the rate of chronic hydrocephalus following aSAH requiring shunt placement has ranged from 17.2 to 31.2% (43, 44). Moreover, patients who developed hydrocephalus following aSAH had a worse prognosis than those who did not (45). We evaluated the association between intrathecal fibrinolysis and chronic hydrocephalus as reported in 13 studies (seven focusing on intraventricular fibrinolysis and six focusing on intracisternal fibrinolysis). The results showed a significant decrease in the risk of chronic hydrocephalus in aSAH patients treated with intracisternal fibrinolysis. One theory explaining the pathogenesis of hydrocephalus after aSAH is that subarachnoid blood interferes with the circulation of cerebrospinal fluid (CSF) at the basal cisterns, the foramen of Monro, or throughout the subarachnoid space (46, 47). Intracisternal fibrinolysis reduced the hematoma in the basal cisterns at 48 h after aSAH, which might have lowered the risk of hydrocephalus (10).
Another consideration is the safety of intrathecal fibrinolysis. We therefore investigated whether fibrinolysis led to a rebleeding complication after aSAH. In the overall analysis, the pooled results showed a non-significant impact of intrathecal fibrinolysis on rebleeding complications (intracisternal fibrinolysis group vs. control group: 5.4 vs. 2.9%), whereas in the non-RCTs subgroup of intracisternal fibrinolysis, a significantly increased risk for rebleeding was observed (intracisternal fibrinolysis non-RCT group vs. control non-RCT group: 2.7 vs. 0.0%). We also found that the ratio of patients with epidural hemorrhage was 50% (two of four rebleeding patients) in the fibrinolysis group in the non-RCTs studies. It is possible that intrathecally injected tPA leaks extradurally and leads to epidural hematoma formation, which might have occurred if the dural closure was not completely watertight (12, 26, 28).
To date, the association between the type of fibrinolytic agent and outcome remains incompletely understood. Most of the thrombolytic agents used in these studies were tPA, rt-PA, and UK. Only one study allocated patients to therapy with tPA or UK; it showed that there were non-significant differences in the effect of fibrinolysis on poor neurologic outcomes or DINDs between patients treated either agent (28). We performed subgroup analyses stratified by thrombolytic agents, which showed that the rate of patients with poor neurologic outcomes or DINDs was significantly decreased in the tPA and UK groups as compared with the control groups. This result was consistent with a randomized primate study, indicating that both drugs (t-PA and UK) had similar effects on the elimination of subarachnoid clot (48).
Several potential limitations to this meta-analysis should be considered. First, the methodology of different studies varied, including the type and dosage of thrombolytic agents, whereas there were insufficient data to perform a subgroup analysis. Second, this meta-analysis included only published studies, which might introduce publication bias, although the Egger tests and funnel plot indicated no bias. Third, a recent study showed that intraventricular tPA administration might produce a transient local inflammatory response (49). However, intracranial infections were not evaluated in this meta-analysis owing to insufficient data. Finally, a language bias might be possible because only studies published in English and Chinese were included in this study.
Conclusion
Despite these limitations, the present meta-analysis provides evidence that intracisternal fibrinolysis was effective in improving aSAH patients' functional recovery, as well as in lowering the risk of DIND, chronic hydrocephalus, and mortality. A recent prospective RCT including 440 patients is running, which aimed to investigate the effect of intraventricular fibrinolysis for aSAH (50). Moreover, further multicenter RCTs are needed to better evaluate the safety and efficacy of intrathecal fibrinolysis, including the type and dosage of thrombolytic agents and the incidence of intracranial infective complications.
Author Contributions
ZW and GC contributed to study design, data extraction, data analysis, and manuscript drafting. XL and CJ were responsible for data extraction and data analysis. WY and JW involved in the literature search. WW was responsible for the data extraction and manuscript drafting.
Funding
This work is supported by the National Natural Science Foundation of China (No. 81601064).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.00885/full#supplementary-material
References
1. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. (2009) 8:355–69. doi: 10.1016/S1474-4422(09)70025-0
2. Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology. (2010) 74:1494–501. doi: 10.1212/WNL.0b013e3181dd42b3
3. Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. (2014) 85:1343–53. doi: 10.1136/jnnp-2014-307711
4. Bauer AM, Rasmussen PA. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front Neurol. (2014) 5:72. doi: 10.3389/fneur.2014.00072
5. Kamp MA, Lieshout JHV, Dibue-Adjei M, Weber JK, Schneider T, Restin T, et al. A systematic and meta-analysis of mortality in experimental mouse models analyzing delayed cerebral ischemia after subarachnoid hemorrhage. Transl Stroke Res. (2017) 8:206–19. doi: 10.1007/s12975-016-0513-3
6. Macdonald RL, Rosengart A, Huo D, Karrison T. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg. (2003) 99:644–52. doi: 10.3171/jns.2003.99.4.0644
7. Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. (2007) 38:2315–21. doi: 10.1161/STROKEAHA.107.484360
8. Reilly C, Amidei C, Tolentino J, Jahromi BS, Macdonald RL. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. (2004) 101:255–61. doi: 10.3171/jns.2004.101.2.0255
9. Kramer AH, Mikolaenko I, Deis N, Dumont AS, Kassell NF, Bleck TP, et al. Intraventricular hemorrhage volume predicts poor outcomes but not delayed ischemic neurological deficits among patients with ruptured cerebral aneurysms. Neurosurgery. (2010) 67:1044–52; discussion 52–3. doi: 10.1227/NEU.0b013e3181ed1379
10. Hamada J, Kai Y, Morioka M, Yano S, Mizuno T, Hirano T, et al. Effect on cerebral vasospasm of coil embolization followed by microcatheter intrathecal urokinase infusion into the cisterna magna: a prospective randomized study. Stroke. (2003) 34:2549–54. doi: 10.1161/01.STR.0000094731.63690.FF
11. Yamamoto T, Esaki T, Nakao Y, Mori K. Efficacy of low-dose tissue-plasminogen activator intracisternal administration for the prevention of cerebral vasospasm after subarachnoid hemorrhage. World Neurosurg. (2010) 73:675–82. doi: 10.1016/j.wneu.2010.04.002
12. Seifert V, Stolke D, Zimmermann M, Feldges A. Prevention of delayed ischaemic deficits after aneurysmal subarachnoid haemorrhage by intrathecal bolus injection of tissue plasminogen activator (rTPA). A prospective study. Acta Neurochir (Wien). (1994) 128:137–43. doi: 10.1007/BF01400664
13. Gorski R, Zabek M, Jarmuzek P. Influence of intraoperative using of recombinant tissue plasminogen activator on the development of cerebral angiospasm after subarachnoid haemorrhage in patients with ruptured intracranial aneurysms. Neurol Neurochir Pol. (2000) 34:41–7.
14. Hanggi D, Eicker S, Beseoglu K, Behr J, Turowski B, Steiger HJ. A multimodal concept in patients after severe aneurysmal subarachnoid hemorrhage: results of a controlled single centre prospective randomized multimodal phase I/II trial on cerebral vasospasm. Cent Eur Neurosurg. (2009) 70:61–7. doi: 10.1055/s-0028-1087214
15. Kramer AH, Roberts DJ, Holodinsky J, Todd S, Hill MD, Zygun DA, et al. Intraventricular tissue plasminogen activator in subarachnoid hemorrhage patients: a prospective, randomized, placebo-controlled pilot trial. Neurocrit Care. (2014) 21:275–84. doi: 10.1007/s12028-014-9965-z
16. Etminan N, Beseoglu K, Eicker SO, Turowski B, Steiger HJ, Hanggi D. Prospective, randomized, open-label phase ii trial on concomitant intraventricular fibrinolysis and low-frequency rotation after severe subarachnoid hemorrhage. Stroke. (2013) 44:2162–8. doi: 10.1161/STROKEAHA.113.001790
17. Kramer AH, Fletcher JJ. Locally-administered intrathecal thrombolytics following aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. (2011) 14:489–99. doi: 10.1007/s12028-010-9429-z
18. Litrico S, Almairac F, Gaberel T, Ramakrishna R, Fontaine D, Sedat J, et al. Intraventricular fibrinolysis for severe aneurysmal intraventricular hemorrhage: a randomized controlled trial and meta-analysis. Neurosurg Rev. (2013) 36:523–30; discussion 30–1. doi: 10.1007/s10143-013-0469-7
19. Kanamura K, Waga S, Sakakura M, Morikawa A, Yamamoto Y, Morooka Y, et al. Comparative study of cisternal lavage methods for the treatment of cerebral vasospasm. In: Findlay JM, editor. Cerebral Vasospasm Proceedings of the 5th international conference. Amsterdam: Elsevier (1993), p. 471–3.
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. (2011). Available online at: http://handbookcochraneorg/ (accessed December 20, 2017).
22. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of Non-randomized studies in meta-analysis. (2014). Available online at: www.ohrica/programs/clinical_epidemiology/oxfordasp (accessed December 10, 2017).
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
24. Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP. Meta-analysis methods. Adv Genet. (2008) 60:311–34. doi: 10.1016/S0065-2660(07)00413-0
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Mizoi K, Yoshimoto T, Takahashi A, Fujiwara S, Koshu K, Sugawara T. Prospective study on the prevention of cerebral vasospasm by intrathecal fibrinolytic therapy with tissue-type plasminogen activator. J Neurosurg. (1993) 78:430–7. doi: 10.3171/jns.1993.78.3.0430
27. Moriyama E, Matsumoto Y, Meguro T, Kawada S, Mandai S, Gohda Y, et al. Combined cisternal drainage and intrathecal urokinase injection therapy for prevention of vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo). (1995) 35:732–6. doi: 10.2176/nmc.35.732
28. Usui M, Saito N, Hoya K, Todo T. Vasospasm prevention with postoperative intrathecal thrombolytic therapy: a retrospective comparison of urokinase, tissue plasminogen activator, and cisternal drainage alone. Neurosurgery. (1994) 34:235–44; discussion 44–5. doi: 10.1097/00006123-199402000-00005
29. Yamada K, Yoshimura S, Enomoto Y, Yamakawa H, Iwama T. Effectiveness of combining continuous cerebrospinal drainage and intermittent intrathecal urokinase injection therapy in preventing symptomatic vasospasm following aneurysmal subarachnoid haemorrhage. Br J Neurosurg. (2008) 22:649–53. doi: 10.1080/02688690802256373
30. Findlay JM, Jacka MJ. Cohort study of intraventricular thrombolysis with recombinant tissue plasminogen activator for aneurysmal intraventricular hemorrhage. Neurosurgery. (2004) 55:532–7; discussion 7–8. doi: 10.1227/01.NEU.0000134473.98192.B1
31. Gerner ST, Kuramatsu JB, Abel H, Kloska SP, Lucking H, Eyupoglu IY, et al. Intraventricular fibrinolysis has no effects on shunt dependency and functional outcome in endovascular-treated aneurysmal SAH. Neurocrit Care. (2014) 21:435–43. doi: 10.1007/s12028-014-9961-3
32. Ramakrishna R, Sekhar LN, Ramanathan D, Temkin N, Hallam D, Ghodke BV, et al. Intraventricular tissue plasminogen activator for the prevention of vasospasm and hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. (2010) 67:110–7; discussion 7. doi: 10.1227/01.NEU.0000370920.44359.91
33. Varelas PN, Rickert KL, Cusick J, Hacein-Bey L, Sinson G, Torbey M, et al. Intraventricular hemorrhage after aneurysmal subarachnoid hemorrhage: pilot study of treatment with intraventricular tissue plasminogen activator. Neurosurgery. (2005) 56:205–13; discussion 205–13. doi: 10.1227/01.NEU.0000147973.83688.D8
34. Findlay JM, Kassell NF, Weir BK, Haley EC Jr, Kongable G, Germanson T, et al. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery. (1995) 37:168–76; discussion 77–8. doi: 10.1097/00006123-199507000-00041
35. Li Y, Guo K, Zi X, Song Z. Combining exchange of cerebrospinal fluid with small dose of urokinase injection for subarachnoid hemorrhage. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2005) 30:217–20.
36. Simard JM, Schreibman D, Aldrich EF, Stallmeyer B, Le B, James RF, et al. Unfractionated heparin: multitargeted therapy for delayed neurological deficits induced by subarachnoid hemorrhage. Neurocrit Care. (2010) 13:439–49. doi: 10.1007/s12028-010-9435-1
37. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. (2010) 41:2391–5. doi: 10.1161/STROKEAHA.110.589275
38. Munakata A, Naraoka M, Katagai T, Shimamura N, Ohkuma H. Role of cyclooxygenase-2 in relation to nitric oxide and endothelin-1 on pathogenesis of cerebral vasospasm after subarachnoid hemorrhage in rabbit. Transl Stroke Res. (2016) 7:220–7. doi: 10.1007/s12975-016-0466-6
39. Caner B, Hou J, Altay O, Fujii M, Zhang JH. Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem. (2012) 123:12–21. doi: 10.1111/j.1471-4159.2012.07939.x
40. Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. (2006) 129:3224–37. doi: 10.1093/brain/awl297
41. Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P, et al. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I. A cooperative study in Europe, Australia, New Zealand, and South Africa. J Neurosurg. (1999) 90:1011–7. doi: 10.3171/jns.1999.90.6.1011
42. Walcott BP, Iorgulescu JB, Stapleton CJ, Kamel H. Incidence, timing, and predictors of delayed shunting for hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2015) 23:54–8. doi: 10.1007/s12028-014-0072-y
43. Zaidi HA, Montoure A, Elhadi A, Nakaji P, McDougall CG, Albuquerque FC, et al. Long-term functional outcomes and predictors of shunt-dependent hydrocephalus after treatment of ruptured intracranial aneurysms in the brat trial: revisiting the clip vs coil debate. Neurosurgery. (2015) 76:608–13; discussion 13–4; quiz 14. doi: 10.1227/NEU.0000000000000677
44. Jabbarli R, Bohrer AM, Pierscianek D, Muller D, Wrede KH, Dammann P, et al. The CHESS score: a simple tool for early prediction of shunt dependency after aneurysmal subarachnoid hemorrhage. Eur J Neurol. (2016) 23:912–8. doi: 10.1111/ene.12962
45. Graff-Radford NR, Torner J, Adams HP Jr, Kassell NF. Factors associated with hydrocephalus after subarachnoid hemorrhage. a report of the cooperative aneurysm study. Arch Neurol. (1989) 46:744–52. doi: 10.1001/archneur.1989.00520430038014
46. Black PM, Tzouras A, Foley L. Cerebrospinal fluid dynamics and hydrocephalus after experimental subarachnoid hemorrhage. Neurosurgery. (1985) 17:57–62. doi: 10.1227/00006123-198507000-00009
47. Gjerris F, Borgesen SE, Sorensen PS, Boesen F, Schmidt K, Harmsen A, et al. Resistance to cerebrospinal fluid outflow and intracranial pressure in patients with hydrocephalus after subarachnoid haemorrhage. Acta Neurochir (Wien). (1987) 88:79–86. doi: 10.1007/BF01404142
48. Hariton GB, Findlay JM, Weir BK, Kasuya H, Grace MG, Mielke BW. Comparison of intrathecal administration of urokinase and tissue plasminogen activator on subarachnoid clot and chronic vasospasm in a primate model. Neurosurgery. (1993) 33:691–6; discussion 6–7. doi: 10.1227/00006123-199310000-00020
49. Kramer AH, Jenne CN, Zygun DA, Roberts DJ, Hill MD, Holodinsky JK, et al. Intraventricular fibrinolysis with tissue plasminogen activator is associated with transient cerebrospinal fluid inflammation: a randomized controlled trial. J Cereb Blood Flow Metab. (2015) 35:1241–8. doi: 10.1038/jcbfm.2015.47
50. Gaberel T. Intraventricular Fibrinolysis for Aneurysmal Subarachnoid Hemorrhage. (FIVHeMA). Available online at: https://clinicaltrials.gov/ct2/show/NCT03187405 (accessed February 01, 2019).
Keywords: aneurysmal subarachnoid hemorrhage, intracisternal fibrinolysis, intraventricular fibrinolysis, delayed ischemic neurological deficit, meta-analysis
Citation: Lu X, Ji C, Wu J, You W, Wang W, Wang Z and Chen G (2019) Intrathecal Fibrinolysis for Aneurysmal Subarachnoid Hemorrhage: Evidence From Randomized Controlled Trials and Cohort Studies. Front. Neurol. 10:885. doi: 10.3389/fneur.2019.00885
Received: 10 April 2019; Accepted: 30 July 2019;
Published: 19 August 2019.
Edited by:
Raimund Helbok, Innsbruck Medical University, AustriaReviewed by:
Emanuela Keller, University of Zurich, SwitzerlandDimitre Staykov, Independent Researcher, Vienna, Austria
Copyright © 2019 Lu, Ji, Wu, You, Wang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Wang, ZHJfemhvbmd3YW5nQDEyNi5jb20=; Gang Chen, bmp1X251ZXJvc3VyZ2VyeUAxNjMuY29t
†These authors have contributed equally to this work
 Xiaocheng Lu
Xiaocheng Lu Chengyuan Ji†
Chengyuan Ji†