- 1Department of Neurology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
- 2Ph.D. Program for Neural Regenerative Medicine, College of Medical Science and Technology, Taipei Medical University and National Health Research Institutes, Taipei, Taiwan
- 3Taipei Neuroscience Institute, Taipei Medical University, Taipei, Taiwan
- 4Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 5Graduate Institute of Neural Regenerative Medicine, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan
- 6Faculty of Medicine and Health, Brain and Mind Centre, Central Clinical School, The University of Sydney, Sydney, NSW, Australia
Introduction: Abuse of nitrous oxide (N2O) has an unusually high lifetime prevalence in developed countries and represents a serious concern worldwide. Myeloneuropathy following the inhalant abuse is commonly attributed to the disturbance of vitamin B12 metabolism, with severe motor deficits are often noted. The present study aims to elucidate its underlying pathophysiology.
Methods: Eighteen patients with N2O abuse or vitamin B12 deficiency were recruited. Comprehensive central and peripheral neuro-diagnostic tests were performed, including whole spine MRI, and thermal quantitative sensory testing (QST). Specifically, paired motor and sensory nerve excitability tests were performed in order to obtain a complete picture of the sensorimotor axonal damage.
Results: The mean duration of N2O exposure for the N2O abuse patients was 17.13 ± 7.23 months. MRI revealed T2 hyperintensity in 87.5% of the N2O abuse patients and 50% of the vitamin B12 deficiency patients. In N2O abuse patients, the motor nerve excitability test showed decreased in peak response (7.08 ± 0.87 mV, P = 0.05), increased latency (7.09 ± 0.28 ms, P < 0.01), increased superexcitability (−32.95 ± 1.74%, P < 0.05), and decreased accommodation to depolarizing current [TEd (40–60 ms) 56.53 ± 0.70%, P < 0.05]; the sensory test showed only decreased peak response (30.54 ± 5.98 μV, P < 0.05). Meanwhile, motor test in vitamin B12 deficiency patients showed only decreased accommodation to depolarizing current [TEd (40–60 ms) 55.72 ± 1.60%, P < 0.01]; the sensory test showed decreased peak response (25.86 ± 3.44 μV, P < 0.05) increased superexcitability (−28.58 ± 3.71%, P < 0.001), increased subexcitability (8.31 ± 1.64%, P < 0.05), and decreased accommodation to depolarizing current [TEd (peak) 67.31 ± 3.35%, P < 0.001].
Conclusion: Compared to vitamin B12 deficiency, N2O abuse patients showed prominent motor superexcitability changes and less prominent sensory superexcitability changes, hinting a unique pathological process different from that of vitamin B12 deficiency. N2O abuse might cause axonal dysfunction not only by blocking methionine metabolism but also by toxicity affecting the paranodal region.
Introduction
Recreational use of nitrous oxide (N2O) is escalating in many countries, including the United States, the United Kingdom, and Taiwan (1, 2). With an unusually high lifetime prevalence in developed countries (38.6% in the UK and 29.4% in the US), N2O consistently ranked the seventh most popular drug in the world in the Global Drug Survey (GDS) from 2016 to 2018 (3–5). Abuse of N2O frequently leads to overexposure, and subsequently causing myeloneuropathy, subacute combined degeneration, psychosis, megaloblastic bone marrow changes, and pernicious anemia (6). As N2O can inactivate methionine synthase that converts homocysteine to methionine via a methylation process, many medical consequences associated with N2O overexposure, including peripheral neuropathy, are often attributed to vitamin B12 deficiency (7–9).
Although myeloneuropathy following N2O abuse and vitamin B12 deficiency can both result in motor and sensory symptoms, experienced clinicians have often noted somewhat different clinical presentations between the two conditions, where N2O abuse might cause severe motor deficits, especially in the lower limbs (10, 11). Johnson et al. have reported such a typical case of nitrous oxide abuse leading to significant distal limbs weakness, a clinical feature less likely to be observed in vitamin B12 deficiency from other causes (12).
Certain reports have suggested that N2O neurotoxicity might cause neural injury on top of vitamin B12 deficiency, but the exact pathophysiology of N2O-induced myeloneuropathy is still unclear (6, 13). The present study is the first that uses comprehensive neurodiagnostic tests covering central and peripheral nervous systems, including whole spine MRI, conventional nerve conduction study (NCS), thermal quantitative sensory test (QST), and the nerve excitability tests to study the myeloneuropathy. In particular, the nerve excitability test has been shown to be able to provide valuable data on the nodal, paranodal, and internodal regions of axons in various peripheral nerve diseases (14, 15), and reveal changes in axonal properties that cannot be detected using conventional NCS. It is hoped that paired motor and sensory nerve excitability test utilized in the study would provide a complete picture of the sensorimotor axonal damage, and further elucidate the pathophysiology underlying the inhalant abuse that could cause catastrophic consequences.
Methods
A total of 18 patients with either N2O abuse or vitamin B12 deficiency due to other causes were recruited for the study. Each patient received clinical evaluation (including history taking, complete physical examination, and neurologic examination); laboratory examinations for levels of vitamin B12, folic acid, and homocysteine; conventional NCS; and paired motor and sensory nerve excitability tests; certain patients received a thermal QST. All patients received whole spine MRI, except one vitamin B12 deficiency patient.
All N2O abuse patients had multiple exposures to N2O inhalation (1). On the other hand, a patient was considered to have vitamin B12 deficiency if the measured serum vitamin B12 level was <150 pg/ml (9, 16–18). Patients with carpal tunnel syndrome, hyperkalemia/hypokalemia, or with other potential causes for sensory polyneuropathy such as diabetes mellitus, alcohol abuse, and uremia were excluded based on clinical assessments and NCS results. All patients enrolled in the study were recruited from Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan. Control nerve excitability data were obtained from 39 healthy control (HC) subjects that were divided into two age cohorts, HC1 with a mean age of 22.67 ± 0.21 years (n = 6) and HC2 with a mean age of 62.11 ± 7.51 years (n = 33). HC1 was age-matched to the N2O abuse patients, and HC2 was age-matched to the vitamin B12 deficiency patients.
This study was carried out in accordance with the recommendations of the Joint Institutional Review Board of Taipei Medical University. The protocol was approved by the Joint Institutional Review Board of Taipei Medical University. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Clinical Evaluation, NCS, and QST
The Medical Research Council (MRC) strength score combined for 12 specified muscle groups (the MRC sum score; ranging from 0 [paralysis] to 60 [normal strength]) (19) and Neuropathy Impairment Score in the Lower Limbs (NIS-LL; ranging from 0 [normal] to 88 [total impairment]) (20) were obtained during neurological examination. Conventional NCS assessing the median, ulnar, peroneal, tibial, and sural nerves were performed in all subjects using standard clinical neurophysiology equipment. Cold and warm thermal QSTs were performed in the upper and lower limbs in certain patients.
Nerve Excitability Testing
Nerve excitability studies were performed by stimulating the nerve median at the wrist according to previously described protocols, with skin temperature over the wrist maintained at a minimum of 32.0°C (14, 15). Paired recordings of the motor and sensory nerve excitability indices were obtained for each subject. Compound muscle action potentials (CMAPs) were recorded from the abductor pollicis brevis muscle while sensory nerve action potentials (SNAPs) were recorded from the index finger, Stimulation and recording were manipulated by software (QTRAC version 8/11/2014; Institute of Neurology, London, U.K.), and stimulus current was administered using an isolated linear bipolar constant-current stimulator (DS5; Digitimer, Welwyn Garden City, U.K.). The changes in current required to produce a target potential corresponding to predetermined target CMAP or SNAP (e.g., 40% of maximum) were tracked. Latency was defined as the time delay (ms) between stimulus onset and the peak of the CMAP or SNAP response. Stimulus threshold was defined as the current (mA) that is required to produce amplitudes of CMAP or SNAP response of the target amplitude.
The nerve excitability protocol incorporated the following recordings: (1) a stimulus-response (SR) curve; (2) strength-duration (SD) relationship, which determined strength-duration time constant (SDTC); (3) threshold electrotonus (TE) utilizing subthreshold 100-ms polarizing currents in both depolarizing (TEd; +40%) and hyperpolarizing (TEh; −40%) directions to change the potential difference across the internodal membrane; and (4) recovery cycle (RC) using a paired-pulse paradigm with a supramaximal conditioning stimulus followed by a test stimulus at interstimulus intervals from 2 to 200 ms. Superexcitability was measured as the maximal threshold reduction and subexcitability as the maximal threshold increase after an interstimulus interval of 10 ms.
Statistical Analysis
Nerve excitability recording data of N2O abuse patients, vitamin B12 deficiency patients, and healthy controls were analyzed by using unpaired T-tests or Mann-Whitney U-tests, depending on normality. Equality of variances was calculated with Levene's test. Correlation studies were performed with Pearson's R. Data analysis was performed using Statistical Package for the Social Sciences (SPSS) for Windows version 21 (SPSS Inc., Chicago, U.S.A.) or QTRAC software. All data are presented as the mean ± standard error of the mean. P-values were considered significant if ≤ 0.05.
Results
Patient Clinical Profiles
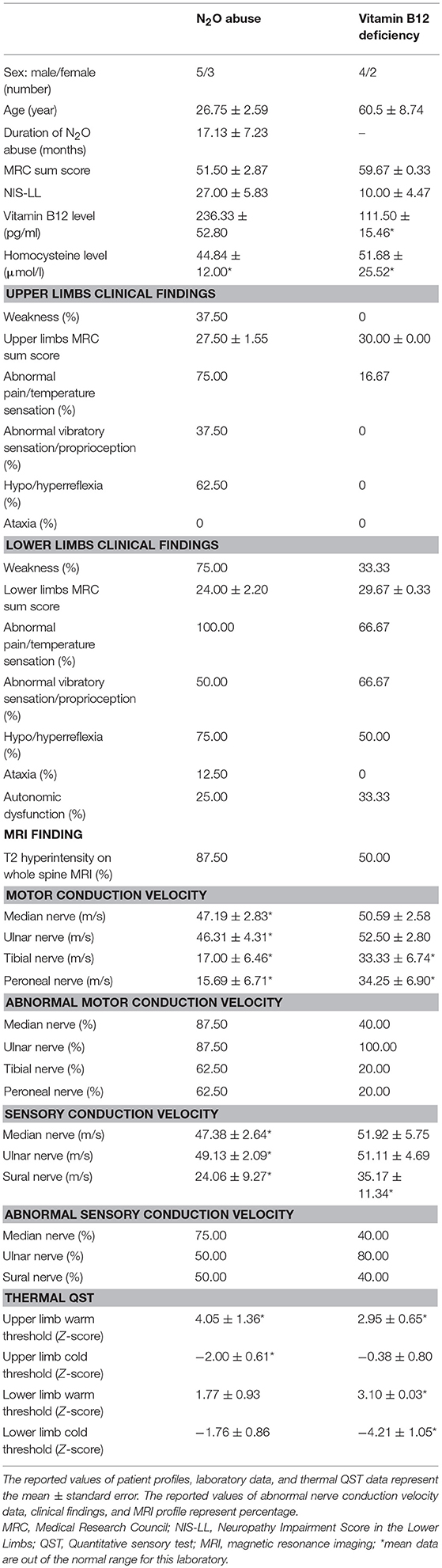
Eighteen patients were recruited and categorized into N2O abuse patients (n = 8) and vitamin B12 deficiency patients (n = 6). Two patients were excluded due to comorbid diabetes, and two for carpal tunnel syndrome (Figure 1). The clinical, MRI, and laboratory profiles for the patients were listed in Table 1. The average duration of N2O exposure for the N2O abuse group was 17.13 ± 7.23 months. The MRC sum score was 51.50 ± 2.87 for the N2O abuse group, and 59.67 ± 0.33 for the vitamin B12 deficiency group; NIS-LL was 27.00 ± 5.83 for the N2O abuse group, and 10.00 ± 4.48 for the vitamin B12 deficiency group.
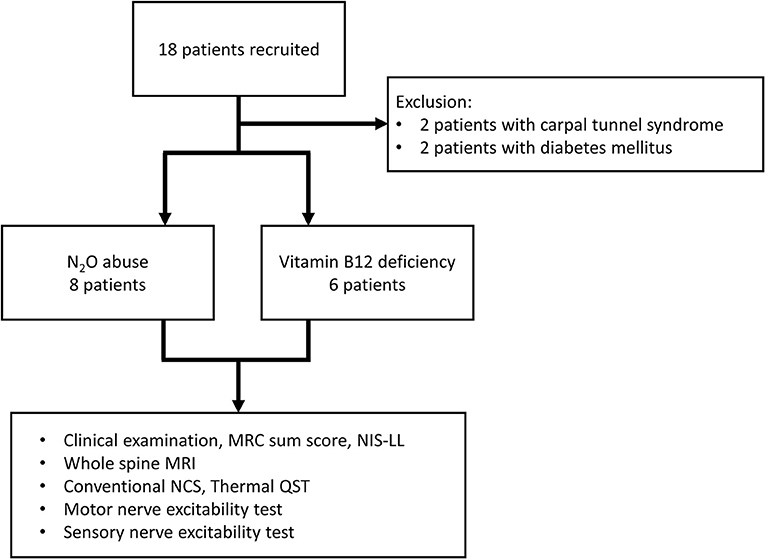
Figure 1. This flowchart depicts the recruitment and the subjects involved in the final data analysis. Eighteen patients were categorized into the N2O abuse group and vitamin B12 deficiency group. MRC, Medical Research Council; NIS-LL, Neuropathy Impairment Score in the Lower Limbs; MRI, magnetic resonance imaging; NCS, nerve conduction study; QST, quantitative sensory test.
The vitamin B12 level was 236.33 ± 52.80 pg/ml in the vitamin B12 deficiency group and 111.50 ± 15.46 pg/ml in the N2O abuse group. The homocysteine level was elevated in both the N2O abuse group (44.84 ± 12.00 μmol/l) and vitamin B12 deficiency group (51.68 ± 25.52 pg/ml).
A summary of the patients' neurological signs and symptoms are shown in Table 1. Weakness was more prevalent in the N2O abuse group than in the vitamin B12 group, both in the upper (37.50 vs. 0.00%) and lower (75.00 vs. 33.33%) limbs. While sensory abnormalities are more prevalent in N2O abuse group than in the vitamin B12 group in the upper limbs, the prevalence of sensory abnormalities of both conditions are similar in the lower limbs.
The whole spine MRI revealed T2 hyperintense lesions in the posterior columns in 87.5% of the N2O abuse patients (100% were cervical spine lesions), and in 50% of the vitamin B12 deficiency patients (66.6% were cervical spine lesions and 33.3% were thoracolumbar lesions). Figures 2A,B shows MRI of cervical cord hyperintense changes in T2-weighted images from a case with N2O abuse. Furthermore, Figure 2C showed a hyperpigmented maculopapular rash, a feature that could be observed in N2O abuse patients, on one of the patients in the nitrous oxide abuse group.
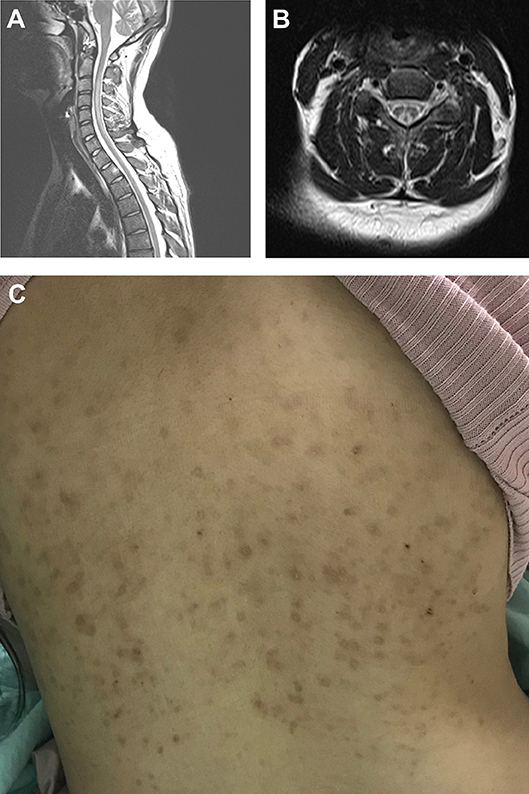
Figure 2. Typical MRI showing cervical spine T2 hyperintense lesion in an N+O abuse patient. (A) Sagittal view and (B) axial view. (C) Hyperpigmented maculopapular rash observed in an N2O abuse patient.
NCS revealed motor and sensory slowing in conduction velocity in both N2O abuse and vitamin B12 deficiency patients. Thermal QST in the upper limb (N2O abuse warm threshold Z-score: 4.05 ± 1.36, cold threshold Z-score: −2.00 ± 0.61; Vitamin B12 deficiency warm threshold Z-score: 2.95 ± 0.65, cold threshold Z-score: −0.38 ± 0.80) and lower limb (N2O abuse warm threshold Z-score: 1.77 ± 0.93, cold threshold Z-score: −1.76 ± 0.86; Vitamin B12 deficiency warm threshold Z-score: 3.10 ± 0.03, cold threshold Z-score: −4.21 ± 1.05) confirmed abnormal temperature sensation in the patients.
Motor Axonal Dysfunction in N2O Abuse and Vitamin B12 Deficiency Patients
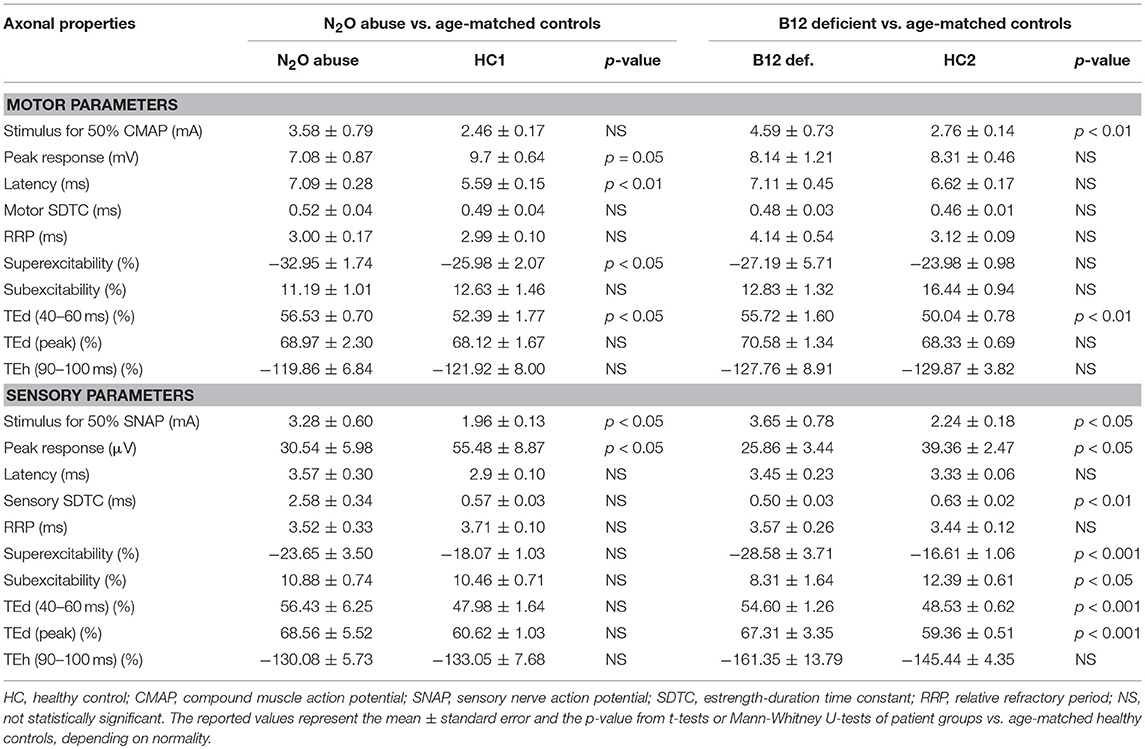
A comparison of the motor and sensory nerve excitability parameters between groups are shown in Table 2. Figure 3 shows the motor and sensory recovery cycles and threshold electrotonus in N2O abuse and vitamin B12 deficiency patients.
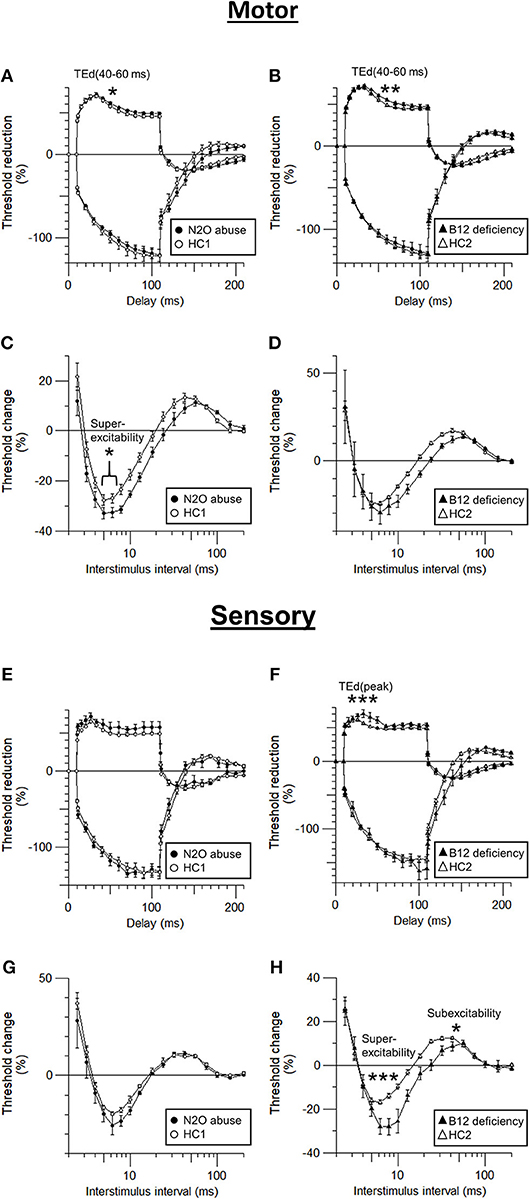
Figure 3. Comparison of sensory and motor axonal dysfunction parameters in N2O abuse and vitamin B12 deficiency patients. (A,B) motor threshold electrotonus, (C,D) motor recovery cycle, (E,F) sensory threshold electrotonus, and (G,H) sensory recovery cycle. *p < 0.05, **p < 0.01, ***p < 0.001.
Compared to those of HC, the motor nerve excitability of the patients with N2O abuse showed a trend of decreased peak response (7.08 ± 0.87 mV, p = 0.05), increased latency (7.09 ± 0.28 ms, p < 0.01), increased superexcitability (−32.95 ± 1.74%, p < 0.05), and decreased TE accommodation to depolarizing current [TEd [40–60 ms] 56.53 ± 0.70%, p < 0.05].
On the other hand, the motor nerve excitability test of vitamin B12 deficiency patients showed only increased stimulus for 50% CMAP (4.59 ± 0.73 mA, p < 0.01) and decreased TE accommodation toward depolarization in TEd (40–60 ms) (55.72 ±1.60%, p < 0.01).
More prominent motor superexcitability changes in N2O abuse patients compared to those seen in vitamin B12 deficiency patients clearly suggested motor axonal dysfunction is more severe in N2O abuse, possibly affecting the paranodal region.
Sensory Axonal Dysfunction in N2O Abuse and Vitamin B12 Deficiency Patients
The sensory nerve excitability test of the patients with N2O abuse showed increased stimulus for 50% SNAP (3.28 ± 0.60 mA, p < 0.05) and decreased peak response (30.54 ± 5.98 μV, p < 0.05).
Sensory nerve excitability test of the vitamin B12 deficiency patients also showed increased stimulus for 50% SNAP (3.65 ± 0.78 mA, p < 0.05) and decreased peak response (25.86 ± 3.44 μV, p < 0.05). Moreover, it revealed additional evidence of sensory axonal dysfunction including decreased SDTC (0.50 ± 0.03, p < 0.01), increased superexcitability (−28.58 ± 3.71%, p < 0.001), decreased subexcitability (8.31 ± 1.64%, p < 0.05), and decreased TE accommodation toward depolarizing current (TEd[peak] 67.31 ± 3.35, p < 0.001).
The fact that superexcitability and other TE & RC parameters showed significant changes in vitamin B12 deficiency but not in N2O abuse suggested that vitamin B12 deficiency patients suffered from more severe sensory axonal dysfunction, compared to N2O abuse patients.
Correlational study between peak response and latency for motor (R = −0.65, p = 0.08) and sensory (R = −0.69, p = 0.06) axon in N2O abuse patients, as well as for sensory axon in vitamin B12 deficiency patients (R = −0.11, p = 0.80), suggested that decrease in peak response observed in the present study might be related to axonal loss instead of temporal dispersion.
Correlation Studies Between Clinical Parameters and Excitability Parameters
In order to clarify the cumulative effect of prolonged intermittent exposure of N2O on the axon, the present study performed correlation analysis between the duration of intermittent N2O exposure and motor and sensory excitability parameters in the N2O abuse group. The analysis demonstrated that the duration of intermittent N2O exposure is significantly correlated to both motor and sensory excitability parameters, specifically N2O exposure is correlated to subexcitability (R = 0.85, p < 0.01) for motor and TEd (peak) (R = 0.76, p < 0.05) for sensory axons.
Also, vitamin B12 level was correlated with stimulus for 50% SNAP response (R = −0.81, p < 0.05) and sensory rheobase (R = −0.90, p < 0.05) of vitamin B12 deficiency patients. Interestingly, MRC strength score of tested muscle is correlated with TEh(overshoot) (R = −0.82, p < 0.05) in motor axonal study of N2O abuse patients.
In the sensory axonal study of N2O abuse patients, the present study found that upper limb warm threshold Z-score was correlated with refractoriness at 2.5 ms (R = 0.97, p < 0.05), superexcitability (R = −0.98, p < 0.05). Upper limb cold threshold Z-score was correlated with superexcitability (R = 0.97, p < 0.05) and latency (R = −0.98, p < 0.05). Lower limb warm threshold Z-score was correlated with subexcitability (R = −0.98, p < 0.05).
Also, in the sensory axonal study of vitamin B12 deficiency patients, the present study found that upper limb cold threshold Z-score was correlated with peak response (R = 0.93, p < 0.05) and TEd (40–60 ms) (R = 0.89, p < 0.05). Lower limb warm threshold Z-score was correlated with refractoriness at 2.5 ms (R = 0.94, p < 0.05). Lower limb cold threshold Z-score was correlated with peak response (R = 0.95, p < 0.05), TEd (10–20 ms) (R = 0.9149, p < 0.05), TEd (40–60ms) (R = 0.91, p < 0.05), and TEd (peak) (R = 0.96, p < 0.05).
Discussion
In the present study, the spinal MRI, motor and sensory nerve excitability tests, and thermal QST revealed evidence of myeloneuropathy in both the N2O abuse and vitamin B12 deficiency patients. Nevertheless, the pattern of myeloneuropathy appears to be different between the two groups (Figure 4). Thermal QST revealed that temperature sensation could be affected by both N2O abuse and vitamin B12 deficiency.
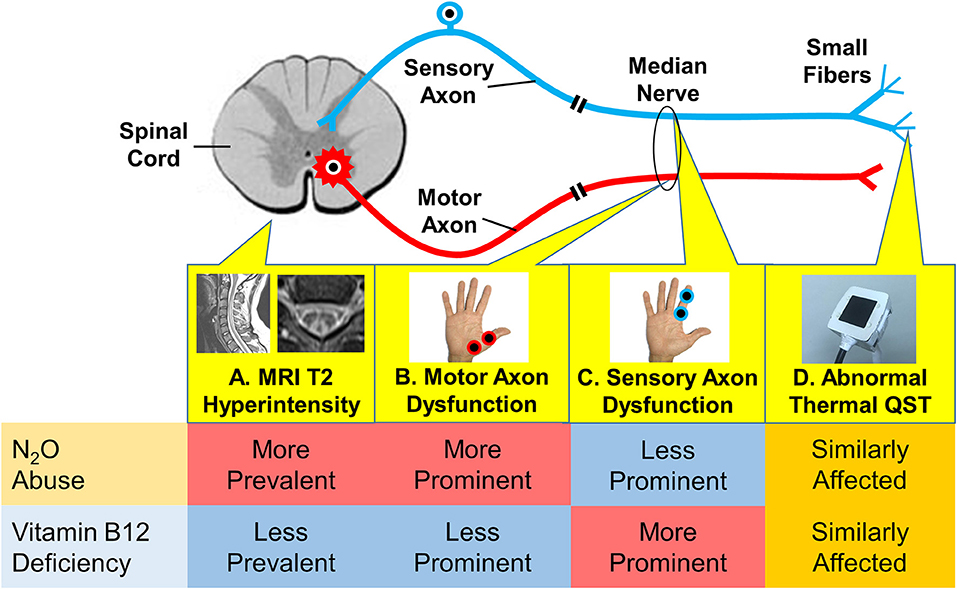
Figure 4. Different patterns of myeloneuropathy in N2O abuse and vitamin B12 deficiency, as revealed by different diagnostic modalities in the present study. (A) Spinal MRI revealed more prevalent T2 hyperintensity in the N2O abuse group. (B) The motor axonal study revealed more prominent dysfunction in the N2O abuse group. (C) The sensory axonal study revealed more prominent dysfunction in the vitamin B12 deficiency group. (D) Thermal QST (quantitative sensory testing) revealed that temperature sensation was similarly affected in both groups.
While spinal MRI abnormalities have been previously reported in both vitamin B12 deficiency (21) and N2O overexposure (12, 22, 23), we have not found studies that compare spinal MRI abnormalities in the two conditions; the present study revealed that spinal cord T2 hyperintense lesions were more prevalent in the N2O abuse group. Nerve excitability test can reveal evidence of axonal dysfunctions that cannot be detected by conventional NCS, allowing us to gain insights into the unique pathophysiology associated with N2O abuse.
A Different Pattern of Axonal Dysfunction in N2O Abuse and Vitamin B12 Deficiency
Compared to vitamin B12 deficiency patients, N2O abuse patients showed a unique nerve excitability pattern showing prominent motor superexcitability changes and less prominent sensory superexcitability changes. This provides evidence for the underlying pathological mechanism that affects the two conditions might be different, as discussed below.
Obvious Motor Axonal Dysfunction in N2O Abuse
The nerve excitability test in patients with N2O abuse showed decreased peak response, increased latency, decreased accommodation toward depolarizing current, and increased superexcitability. The increase in superexcitability may correspond to an alteration in fast K+ channels in the axonal paranodal region, while decreased accommodation toward depolarizing current signifies an alteration in nodal and internodal slow K+ channels (24). Similar changes in superexcitability and increased TE accommodation toward depolarizing current have been noted in other demyelinating diseases in previous studies. In particular, “fanning-out” of TE and increased superexcitability were seen in demyelinating diseases such as chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy and was thought to possibly be related to compensatory Na+/K+ pump action in measured nerve segments (25–27). Such changes observed in patients with N2O abuse may similarly be related to demyelination of proximal axons. The decrease in peak response noted in the patients may signify motor axonal loss.
Patients with vitamin B12 deficiency similarly showed motor axonal dysfunction, particularly, decreased accommodation toward depolarization. Although the changes are still in accordance with demyelination, the motor axonal damage appears to be less prominent than those in our N2O abuse patients, as there are no significant changes in the RC. Moreover, no significant reduction in peak response is observed.
The motor axonal dysfunction pattern in both patients with N2O abuse and vitamin B12 deficiency is compatible with motor demyelination. However, patients with N2O abuse appear to have more prominent dysfunction in motor axons. The finding is in accordance with the clinical findings, in which patients with N2O abuse show prominent motor deficits compared to those with vitamin B12 deficiency. Previous reports also described disproportionate motor amplitude reduction in the lower limbs in NCS of N2O abuse patients (1, 12).
Traditionally, neuropathy induced by N2O abuse was attributed to the effect of N2O on the inactivation of vitamin B12 through blockage of methionine synthase, which converts homocysteine to methionine via a methylation process. The blockage could lead to the reduction of the vitamin B12 level, which then leads to hypomyelination and abnormal myelination (9, 28). It would concurrently lead to elevation of the homocysteine level, which might cause inflammation, oxidative stress, and microvascular disease (9, 29–31). Nevertheless, previous reports have also mentioned that N2O might exert its neurotoxic effect independent of vitamin B12 deficiency, via mechanisms such as antagonism of N-methyl-D-aspartate (NMDA) receptors (6, 13). Certain reports reported ischemic pathological changes associated with nitrous oxide use (32).
While the exact pathophysiology of N2O-induced myeloneuropathy remains poorly understood, as mentioned in the discussion above, the prominent changes in motor superexcitability observed in the present study might be an opportunity to elucidate further whether N2O abuse causes unique pathophysiological changes in motor axons, especially in the paranodal region.
Prominent Sensory Axonal Dysfunction in Vitamin B12 Deficiency Patients
Although the results of the sensory nerve excitability test of patients with N2O abuse showed a decreased peak response, they showed only a trend of increased superexcitability and “fanning-out” toward depolarizing and hyperpolarizing current. These axonal changes are less pronounced than those of vitamin B12 deficiency patients, which not only showed peak reduction but also showed obvious signs of demyelination such as decreased accommodation toward depolarizing current, increased superexcitability, and decreased subexcitability. Decreased sensory SDTC in vitamin B12 deficiency patients, signifying alterations in persistent Na channels, is likewise compatible with hyperpolarization.
Sensory axonal dysfunction patterns in both N2O abuse and vitamin B12 deficiency patients are similarly compatible with demyelination. Prominent changes in patients with vitamin B12 deficiency reflected significant sensory axonal dysfunction. These findings are in accordance with the clinical situation, in which vitamin B12 deficiency patients have more significant sensory complaints than N2O abuse patients.
Previous nerve biopsy reports have shown that vitamin B12 deficiency could cause a combination of axonal degeneration and demyelination in vitamin B12 deficiency (33). A sural nerve biopsy report of N2O abuse revealed evidence for both axonal degeneration and demyelination. Specifically, it found prominent evidence for axonal degeneration such as axonal swelling, shrunken axon, and varying degrees of myelin ovoid formation. However, it also presented evidence for focal demyelination and subsequent remyelination, including the occasional focal area of myelin loss, focal sites of denuded myelin, and short internodal segments of myelin (34).
Decreased peak response, together with prominent TE and RC changes seen in motor axonal study of N2O abuse patients and sensory axonal study of vitamin B12 deficiency patients probably reflect a combined effect of axonal loss and focal demyelination, in accordance with previous pathology report (33, 34). Prominent motor superexcitability changes in N2O abuse patients might be related to toxicity involving paranodal region, possibly affecting fast K+ channel.
Less prominent TE and RC changes observed in the sensory axonal study of N2O abuse patient and motor axonal study of vitamin B12 deficiency patient probably indicate that focal demyelination and toxicity involving paranodal region has less effect on the sensory axon of N2O abuse patients and motor axon of vitamin B12 deficiency patient.
Potential Future Roles of Nerve Excitability Test in the Diagnosis of Myeloneuropathies
The ability of the nerve excitability test to detect axonal dysfunction in both N2O abuse and vitamin B12 deficiency suggests that the tool could play a role in the diagnosis of neuropathy in both conditions. A previous study has described the axonal dysfunction pattern in myelopathy and radiculopathy, and the present study has further elucidated axonal dysfunction pattern in N2O abuse and vitamin B12 deficiency myeloneuropathy, further clarifying axonal dysfunction patterns that could be observed when injury affects the spinal cord, root, and/or peripheral nerve (35). The present study has shown that the tool can distinguish motor and sensory nerve injury much more clearly than the NCS, and may pave the way for the nerve excitability test to become another useful neurophysiologic diagnostic tool in the evaluation of myeloneuropathies, alongside with NCS. In particular, for patients presenting with a myeloneuropathy with unclear history, a nerve excitability test result showing the unique motor predominant axonal dysfunction as unveiled by the present study could support the diagnosis of N2O abuse.
The fact that motor subexcitability and sensory TEd (peak) are correlated with N2O exposure duration, and that motor TEh(overshoot) are correlated with MRC strength score of tested muscle in N2O abuse, suggested that nerve excitability parameters may have the potential to monitor axonal damages caused by N2O exposure, and may further add to the value of the nerve excitability test as an evaluation tool for the myeloneuropathy. Correlation between vitamin B12 level with numerous sensory axonal study parameters suggested that the parameters reflect axonal dysfunction related to vitamin B12 deficiency. Correlational study results also showed that RC and TE parameters are correlated with sensory perception threshold in the patients, but the interpretation of the results should be very careful, as the nerve excitability test was not specifically designed to evaluate small fiber function.
In summary, the present study has revealed that the nerve excitability test could detect motor and sensory axonal dysfunction in both N2O abuse and vitamin B12 deficient patients. Follow-up studies with more cases would be beneficial to confirm the unique axonal dysfunction pattern in N2O abuse and to explore treatment strategies for the abuse of the inhalant based on its axonal pathophysiology.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
This study was carried out in accordance with the recommendations of the Joint Institutional Review Board, Taipei Medical University with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Joint Institutional Review Board, Taipei Medical University.
Author Contributions
JT and J-YS contributed to the study design. JT, H-JC, T-SC, H-YW, and J-YS contributed to the data collection. JT, T-SC, J-YS, and CL contributed to the data analysis and interpretation. JT, J-YS, and CL contributed to the manuscript preparation. All authors approved the final version of the manuscript.
Funding
This work was supported by Taipei Medical University, Wan Fang Hospital, Taipei, Taiwan (grant no. 105TMU-WFH- 02), Ministry of Science and Technology, Taiwan (MOST104-2314-B-038-012-MY3 and MOST107-2314-B-038-078), and Ministry of Education, Taiwan (DP2-107-21121-01-N-03).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Li HT, Chu CC, Chang KH, Liao MF, Chang HS, Kuo HC, et al. Clinical and electrodiagnostic characteristics of nitrous oxide-induced neuropathy in Taiwan. Clin Neurophysiol. (2016) 127:3288–93. doi: 10.1016/j.clinph.2016.08.005
2. Randhawa G, Bodenham A. The increasing recreational use of nitrous oxide: history revisited. Br J Anaesth. (2016) 116:321–4. doi: 10.1093/bja/aev297
3. Kaar SJ, Ferris J, Waldron J, Devaney M, Ramsey J, Winstock AR. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. (2016) 30:395–401. doi: 10.1177/0269881116632375
4. Barratt MJ, Ferris JA, Zahnow R, Palamar JJ, Maier LJ, Winstock AR. Moving on From Representativeness: testing the utility of the global drug survey. Subst Abuse. (2017) 11:1–17. doi: 10.1177/1178221817716391
5. The Lancet. Changing the conversation to make drug use safer. Lancet. (2018) 391:1965. doi: 10.1016/S0140-6736(18)31075-4
6. Garakani A, Jaffe RJ, Savla D, Welch AK, Protin CA, Bryson EO, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. (2016) 25:358–69. doi: 10.1111/ajad.12372
7. Chanarin I. Cobalamins and nitrous oxide: a review. J Clin Pathol. (1980) 33:909–16. doi: 10.1136/jcp.33.10.909
8. Van Amsterdam J, Nabben T, Van Den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. (2015) 73:790–6. doi: 10.1016/j.yrtph.2015.10.017
9. Green R, Allen LH, Bjorke-Monsen AL, Brito A, Gueant JL, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. (2017) 3:17040. doi: 10.1038/nrdp.2017.41
10. Morris N, Lynch K, Greenberg SA. Severe motor neuropathy or neuronopathy due to nitrous oxide toxicity after correction of vitamin B12 deficiency. Muscle Nerve. (2015) 51:614–6. doi: 10.1002/mus.24482
11. Kaski D, Kumar P, Murphy E, Warner TT. Iatrogenic B12-deficient peripheral neuropathy following nitrous oxide administration for functional tonic leg spasm: a case report. Clin Neurol Neurosurg. (2017) 160:108–10. doi: 10.1016/j.clineuro.2017.07.006
12. Johnson K, Mikhail P, Kim MG, Bosco A, Huynh W. Recreational nitrous oxide-associated neurotoxicity. J Neurol Neurosurg Psychiatry. (2018) 89:897–8. doi: 10.1136/jnnp-2017-317768
13. Maze M, Fujinaga M. Recent advances in understanding the actions and toxicity of nitrous oxide. Anaesthesia. (2000) 55:311–4. doi: 10.1046/j.1365-2044.2000.01463.x
14. Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. (1998) 21:137–58. doi: 10.1002/(SICI)1097-4598(199802)21:2andlt;137::AID-MUS1andgt;3.0.CO;2-C
15. Kiernan MC, Lin CS, Andersen KV, Murray NM, Bostock H. Clinical evaluation of excitability measures in sensory nerve. Muscle Nerve. (2001) 24:883–92. doi: 10.1002/mus.1085
17. Hannibal L, Lysne V, Bjorke-Monsen AL, Behringer S, Grunert SC, Spiekerkoetter U, et al. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front Mol Biosci. (2016) 3:27. doi: 10.3389/fmolb.2016.00027
18. Khan A, Shafiq I, Hassan Shah M. Prevalence of vitamin B12 deficiency in patients with type II diabetes mellitus on metformin: a study from khyber pakhtunkhwa. Cureus. (2017) 9:e1577. doi: 10.7759/cureus.1577
19. Kleyweg RP, Van Der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. (1991) 14:1103–9. doi: 10.1002/mus.880141111
20. Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. (1999) 41(Suppl. 1):8–13. doi: 10.1159/000052074
21. Marelli C, Salsano E, Politi LS, Labauge P. Spinal cord involvement in adult-onset metabolic and genetic diseases. J Neurol Neurosurg Psychiatry. (2018) 90:211–8. doi: 10.1136/jnnp-2018-318666
22. Beltramello A, Puppini G, Cerini R, El-Dalati G, Manfredi M, Roncolato G, et al. Subacute combined degeneration of the spinal cord after nitrous oxide anaesthesia: role of magnetic resonance imaging. J Neurol Neurosurg Psychiatry. (1998) 64:563–4. doi: 10.1136/jnnp.64.4.563
23. Sotirchos ES, Saidha S, Becker D. Nitrous oxide-induced myelopathy with inverted V-sign on spinal MRI. J Neurol Neurosurg Psychiatry. (2012) 83:915–6. doi: 10.1136/jnnp-2012-303105
24. Boerio D, Bostock H, Spescha R, Z'graggen WJ. Potassium and the excitability properties of normal human motor axons in vivo. PLoS ONE. (2014) 9:e98262. doi: 10.1371/journal.pone.0098262
25. Kiernan MC, Guglielmi JM, Kaji R, Murray NM, Bostock H. Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block. Brain. (2002) 125:664–75. doi: 10.1093/brain/awf041
26. Kaji R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies. Muscle Nerve. (2003) 27:285–96. doi: 10.1002/mus.10273
27. Sung JY, Tani J, Park SB, Kiernan MC, Lin CS. Early identification of 'acute-onset' chronic inflammatory demyelinating polyneuropathy. Brain. (2014) 137:2155–63. doi: 10.1093/brain/awu158
28. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. (1995) 45:1435–40. doi: 10.1212/WNL.45.8.1435
29. Maler JM, Seifert W, Huther G, Wiltfang J, Ruther E, Kornhuber J, et al. Homocysteine induces cell death of rat astrocytes in vitro. Neurosci Lett. (2003) 347:85–8. doi: 10.1016/S0304-3940(03)00655-4
30. Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci USA. (2008) 105:12474–9. doi: 10.1073/pnas.0805350105
31. Caterino M, Pastore A, Strozziero MG, Di Giovamberardino G, Imperlini E, Scolamiero E, et al. The proteome of cblC defect: in vivo elucidation of altered cellular pathways in humans. J Inherit Metab Dis. (2015) 38:969–79. doi: 10.1007/s10545-014-9806-4
32. Thompson AG, Leite MI, Lunn MP, Bennett DL. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. (2015) 15:207–9. doi: 10.1136/practneurol-2014-001071
33. Kalita J, Chandra S, Bhoi SK, Agarwal R, Misra UK, Shankar SK, et al. Clinical, nerve conduction and nerve biopsy study in vitamin B12 deficiency neurological syndrome with a short-term follow-up. Nutr Neurosci. (2014) 17:156–63. doi: 10.1179/1476830513Y.0000000073
34. Sahenk Z, Mendell JR, Couri D, Nachtman J. Polyneuropathy from inhalation of N2O cartridges through a whipped-cream dispenser. Neurology. (1978) 28:485–7. doi: 10.1212/WNL.28.5.485
Keywords: nerve excitability test, inhalant, nitrous oxide, vitamin B12, myeloneuropathy
Citation: Tani J, Weng H-Y, Chen H-J, Chang T-S, Sung J-Y and Lin CS-Y (2019) Elucidating Unique Axonal Dysfunction Between Nitrous Oxide Abuse and Vitamin B12 Deficiency. Front. Neurol. 10:704. doi: 10.3389/fneur.2019.00704
Received: 01 March 2019; Accepted: 14 June 2019;
Published: 09 July 2019.
Edited by:
Massimiliano Filosto, Civil Hospital of Brescia, ItalyReviewed by:
Fiore Manganelli, University of Naples Federico II, ItalyJames Howells, University of Sydney, Australia
Copyright © 2019 Tani, Weng, Chen, Chang, Sung and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-Ying Sung, c3VuZy5qaWF5aW5nJiN4MDAwNDA7dG11LmVkdS50dw==
 Jowy Tani
Jowy Tani Hsing-Yu Weng1,4
Hsing-Yu Weng1,4 Tsui-San Chang
Tsui-San Chang Jia-Ying Sung
Jia-Ying Sung Cindy Shin-Yi Lin
Cindy Shin-Yi Lin